Abstract
Endemic mycoses including Histoplasma, Blastomyces, Coccidioides, Paracoccidioides, and Talaromyces are dimorphic fungi that can cause a variety of clinical manifestations, including respiratory infections. Their pulmonary presentations are variable, and diagnosis is often delayed as they can mimic other infectious and non-infectious causes of pulmonary disease. Delay in diagnosis can lead to unnecessary antibiotic use, repeat hospitalizations, and increased morbidity and mortality. The diagnosis of endemic fungal pulmonary infections often relies on multiple diagnostic tests including culture, tissue histopathology, antigen assays, and antibody assays. Due to the increased use of immunosuppressive agents and the widening geographic ranges where these infections are being found, the prevalence of endemic fungal infections is increasing. Physicians need to be aware of the clinical manifestations of pulmonary infections due to endemic fungal in order to ensure that the proper diagnostic work up is obtained promptly. A high index of suspicion is particularly important in patients with suspected pulmonary infections who have failed to improve despite antibiotics in the appropriate setting. We present a review diagnostic testing for pulmonary infections due to endemic mycoses.
1. Introduction
Fungal pneumonia caused by endemic fungi including Histoplasma, Blastomyces, Coccidioides, Paracoccidioides, and Talaromyces can be challenging to diagnose. These mycoses are termed endemic as they classically occur in particular geographic regions [1]. Endemic fungi are dimorphic, existing as molds at cooler environmental temperatures and yeast within the warmer temperatures of the human body. The endemic fungi can cause a variety of syndromes, but all carry the potential to cause respiratory infections since inhalation is a major mode of disease acquisition in humans. Furthermore, rates of pneumonia due to endemic fungi are increasing due to increased use of immunosuppressive therapies [2]. Diagnosis of pneumonia due to endemic fungi can be challenging as the clinical presentations are varied and non-specific, meaning this type of pneumonia may be mistaken for a variety of infectious or non-infectious causes of lung disease [1,3]. The diagnosis is frequently delayed, particularly when occurring outside of traditional endemic areas as physicians may not be familiar with the disease manifestations [1].
Multiple diagnostic tests may be required for diagnosis including tissue histopathology, culture, or specific fungal antigen or antibody detection assays in the appropriate clinical scenario. One non-specific fungal diagnostic test may be useful in the diagnosis of Coccidioides, Histoplasma, Talaromyces, and Paracoccidioides is detection of 1,3-β-d-glucan, a component of the cell wall of many fungi [4,5,6]. The 1,3-β-d-glucan assay is less useful to detect Blastomyces given its yeast phase produces low levels of 1,3-β-d-glucan [4]. 1,3-β-d-glucan assay is a nonspecific test, therefore additional testing is needed to differentiate between fungi when the test is positive. Accordingly, the main role for 1,3-β-d-glucan testing in the diagnosis of endemic fungal pneumonia is as a screening test, or a non-specific test to help one further narrow the planned workup. We present a review of diagnostic testing for pneumonia due to endemic fungi. Given that geographic location and clinical presentations are crucial to proper diagnosis, these factors are described as well.
2. Histoplasmosis
Histoplasmosis is caused by Histoplasma capsulatum var capsulatum and Histoplasma capsulatum var. duboisii [7]. Classically, H capsulatum is thought of as endemic to the Ohio and Mississippi River Valleys in the United States, as well as parts of Central and South America [2,7,8,9,10]. More recently it has become clear that Histoplasma occurs frequently in many parts of the world including: Central and Eastern North America, the majority of Central and South America, much of sub-Saharan Africa, large portions of southeast Asia and small areas within Australia and Europe [7]. H duboisii has primarily been described in West Africa.
Infection is acquired through inhalation of spores from soil that is contaminated with bird or bat droppings [9,10]. Histoplasma can cause a wide variety of clinical manifestations including a spectrum of pulmonary diseases ranging from acute to chronic presentations [9,11]. Table 1 describes signs, symptoms, imaging and lab findings, and epidemiology of histoplasmosis and other endemic fungi.

Table 1.
Signs, symptoms, imaging and lab findings, and epidemiology of pulmonary infections due to endemic mycoses *.
Acute pulmonary histoplasmosis (APH) typically presents with fever, chills, shortness of breath, and resembles community acquired pneumonia [2,3,9,11,12]. APH can range from a mild self-limiting illness to acute respiratory distress syndrome [2,9,11,13]. Subacute pulmonary histoplasmosis (SPH) has a more insidious onset over at least one month and may develop after a smaller inoculum exposure [2,3,9,11]. Chronic pulmonary histoplasmosis (CPH) is classically seen in older males with underlying lung disease [2,3,10,11]. CPH has a similar presentation to tuberculosis with fever, night sweats, weight loss, cough, and dyspnea over at least three months [9,10,11,12]. H capsulatum may also cause pulmonary nodules, mediastinal adenitis, mediastinal granulomas, and mediastinal fibrosis [1,2]. Progressive disseminated histoplasmosis is a form of histoplasmosis that result from hematogenous spread and can impact multiple organ symptoms including the respiratory tract and cause severe disease [11].
In APH, imaging frequently shows diffuse bilateral patchy opacities with hilar and mediastinal adenopathy while diffuse reticulonodular or miliary infiltrates can be seen less commonly [2,3,11,13,14]. In CPH patchy infiltrates can progress to large, destructive cavities; hilar and mediastinal lymphadenopathy are uncommon compared to APH [10,11].
Identification of H capsulatum on histopathology and culture is the classical diagnostic standard [2,10,15]. The narrow based budding ovoid Histoplasma yeast (2–4 μM in diameter) is visualized via direct microscopic examination or the use of Gomori methenamine silver, Giemsa, periodic acid-Schiff, or hematoxylin eosin stains of specimens such as respiratory samples, lymph node tissue, or lung tissue (Figure 1A) [1,2,8,9,11,16,17,18]. Cytopathologic examination of bronchoalveolar lavage (BAL) fluid is positive in up to 50% of cases [2,3,8]. Histopathologic examination can reveal both caseating and non-caseating granulomas [8,9]. Despite the potential to improve diagnosis, pathological examination is not feasible in most patients as it requires invasive procedures, such as bronchoscopy or biopsies [8]. In general, it is more useful in disseminated histoplasmosis compared to pulmonary histoplasmosis and is more likely to be positive in SPH or CPH compared to APH [11,19]. Histoplasma can take 2–8 weeks to grow on culture which is similarly more likely to be positive in SPH or CPH compared to APH [2,3,8,9,10,16,19]. Overall sensitivity of culture of sputum or bronchoscopy specimens is 48–75% in pulmonary histoplasmosis [3,11]. Table 2 shows the performance of various tests for histoplasmosis and the other endemic fungi.

Table 2.
Comparison of diagnostic tests utilized in the diagnosis of pulmonary infections due to endemic mycosis.
Antigen detection can provide rapid diagnosis of pulmonary histoplasmosis. Numerous commercial and in-house tests are available, however, agreement between tests is not uniform [12,15,24]. Most though not all antigen tests use an enzyme immunoassay (EIA) [12,15,21]. Antigen is generally more likely to be positive in APH compared with SPH and CPH although CPH commonly yields positive results as well [2,3,11,19]. In a multicenter evaluation by Hage et al., antigenuria was detected in 83% of acute cases, 30% of subacute cases, and 87.5% of chronic pulmonary histoplasmosis, with the highest antigen concentrations in acute cases—combined urine and serum antigen testing improved yield [19]. In another large study of APH, antigen was detected in serum and urine in 65% and 69% of cases, respectively [13]. This same study found that antigen was more likely to be detected in patients who require hospitalization, likely reflecting higher fungal burden in more severe disease [13]. Antigen testing of BAL can further aid in diagnosis of pulmonary histoplasmosis, particularly in CPH or diffuse pulmonary disease complicating disseminated histoplasmosis. Hage et al. found that among 31 patients with histoplasmosis and pulmonary involvement, antigen detection in BAL had 93.5% sensitivity, 97.8% specificity, 68.8% positive predictive value, and 99.6% negative predictive value [20]. Overall, 21 of the 31 patients in this study were immunocompromised with disseminated histoplasmosis including disseminated pulmonary disease [20]. One limitation of Histoplasma antigen testing is its cross reactivity with other mycoses, such as Blastomyces spp, Talaromyces marneffei, Paracoccidioides, Coccidioides, and Aspergillus spp. [2,3,10,11,12,14,15,19,21,22,24].
The IMMY Histoplasma EIA, is a commercially available, FDA approved EIA for detection of Histoplasma antigen in urine [12,15]. In a study by Theel et al. the IMMY EIA had 97.6% agreement with MiraVista Diagnostic’s EIA and a specificity and sensitivity of 99.8% and 64.5%, respectively [15]. As opposed to MiraVista’s EIA which is done at a central laboratory, health centers can perform IMMY’s test given its FDA approval. MiraVista recently developed a lateral flow assay (LFA) for serum antigen detection [66]. In patients with HIV and disseminated histoplasmosis, sensitivity was 96% and specificity 94% [66]. This test is not FDA approved.
Antibody testing is also used to diagnose histoplasmosis. Because antibodies take time to develop after acute infection, they are more useful in SPH and CPH than APH and negative initial antibody testing should be repeated in one to two months if suspicion is high [2,3,8,9,10,11,13,14,17]. Additionally, antibody testing may be negative in immunocompromised patients and may cross react with other endemic mycoses such as Blastomyces, Paracoccidioides, and Coccidioides [2,8,9,11]. Common methodologies include immunodiffusion (ID), complement fixation (CF), or EIA [2,8,9,11]. ID detects H and M bands, H bands are more rare but when found indicate acute infection whereas M bands are more common and may persist for years [2,8,11]. A fourfold rise in CF titers or a single titer of 1:32 or higher is indicative of active infection [2,8,11]. Compared to CF, ID is slightly more specific and less sensitive [10]. One multicenter evaluation found 67% seropositivity (by CF or ID) in APH, compared to 95% in SPH and 83% in CPH [19]. In one study of patients with APH, MiraVista Diagnostics’ IgM, and IgG EIA exhibited 89% sensitivity and 92% specificity [14]. Combining antigen and antibody testing may improve sensitivity for diagnosis of APH, potentially as high as 96% [13,14]. Similar sensitivity has been found using the combination of BAL antigen detection and BAL cytopathology [3].
Nucleic acid amplification tests (NAAT) such as polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP) have been utilized for the identification of H. capsulatum, however these have variable sensitivities and none are commercially available [2,67,68,69,70,71,72,73,74]. NAATs are less likely to have false positive results due to other endemic fungi compared to antigen and antibody testing [67]. A reference database has been created to identify H. capsulatum via matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) but little data on performance is available [75,76]. Similarly, while metagenomic next generation sequencing (mNGS) has been used on BAL fluid to diagnose H. capsulatum causing chronic progressive pulmonary lesions and epiglottis lesions, broader performance data are not available [77,78]. Finally, there may be a role for panfungal PCR to diagnose histoplasmosis, but so far use has been exploratory [79].
Pulmonary infection is more common due to H. capuslatum var capsulatum than H. capsulatum var. duboisii [7]. In a summary of 94 reported cases, only 10 were suspected to have pulmonary involvement [80]. H capsulatum var. duboisii frequently causes lymphadenopathy, bone, cutaneous, sub-cutaneous, and disseminated disease, and may occur decades after leaving the endemic area [80]. H capsulatum var. duboisii diagnosis has not been well studied and so diagnostic test performance characteristics are less well understood. Histopathology, cytology are commonly used while confirmation with culture or PCR are less common and antibody testing is even more rare [80]. Histoplasma antigen testing has not been utilized [80].
3. Blastomycosis
Blastomyces dermatitidis, Blastomyces gilchristii and the recently characterized Blastomyces helicus and percursus can cause pneumonia in both immunocompetent and immunocompromised individuals [33,81,82,83]. Blastomyces spp are likely endemic throughout the midwestern United States and eastern North America, much of Africa and the majority of India [7,25,33,81,82,84]. Blastomycosis occurs due to inhalation of Blastomyces spores commonly found in sandy soils with an acidic pH, near a water source within forested areas with vegetative decay [1,25,33,81]. Activities associated with infection include landscaping, construction, fishing, and hunting [26,33,85]. Blastomyces infection causes nonspecific symptoms and diagnosis is frequently delayed, leading to excess healthcare visits and courses of antibiotics [25,33]. Pulmonary infection due to Blastomyces may cause mild or severe acute infections including acute respiratory distress syndrome, and chronic presentations that mimic tuberculosis or malignancy [1,25,28,33,83,84].
Definitive diagnosis of pulmonary blastomycosis is achieved by identification of Blastomyces through direct visualization of yeast or growth on culture. Staining of sputum with potassium hydroxide, calcofluor white, Gomori methenamine silver or periodic acid-schiff can help visualize the yeast form of Blastomyces [25,26,33,83]. Blastomyces yeast is 8–20 μM with broad based budding and a doubly refractile cell wall (Figure 1B) [30,33,34]. Direct identification of Blastomyces in clinical specimens can lead to diagnosis rapidly and before culture or other testing methods [25,33,83]. The potassium hydroxide smear is an appealing test as it takes only 15–30 min, but its sensitivity varies from 50–90% [25]. Fungal culture is the gold standard for diagnosing Blastomyces as it is highly specific, though growth typically 5–14 days and may take up to five weeks [25,28,33,34,83]. In one study, culture from sputum, tracheal secretions, or bronchial washings had sensitivities of 75%, 100%, and 67%, respectively, while combining results from multiple sources improved yield [28].
EIA detection of Blastomyces galactomannan antigen is also utilized [25,29]. Urine antigen testing has a sensitivity of up to 93% and specificity of 79% in pulmonary and extrapulmonary blastomycosis and serial urine antigen measurements are sometimes used to monitor treatment response [25,27,29,31,32,33]. Serum antigen testing can be done but sensitivity is sub-optimal (57% even with EDTA-heat treatment, improved from 36% without) however BAL fluid antigen testing sensitivity may be as high as 82% in pulmonary blastomycosis [3,26,29,31,32]. Limitations of antigen testing include delayed results due to transit times required for shipping to reference laboratories and cross-reactivity with other endemic fungi such as Histoplasma, Paracoccidioides, and Talaromyces [1,25,27,31,32,33,34].
CF and ID testing for Blastomyces have variable reported sensitivity and specificity [28,33,34,35,36]. A more sensitive (88%) EIA that detects antibody against the BAD1 protein also has higher specificity (94–99%) but is not commercially available [36]. As is typical for antibody testing in infectious diseases, utility is lower in early disease prior to antibody production and in some immune suppressed patients due to inability to produce antibodies [33,36]. PCR assays have been developed but are not commercially available and may be limited by polymorphisms within target regions [86,87,88]. Interestingly, one real time PCR test has been designed to test for both H. Capsulatum and B. dermatitidis with a sensitivity of 86% and specificity of 99% for B. dermatitidis [67]. mNGS has been used to diagnosis blastomycosis from a transbronchial biopsy and BAL fluid but diagnostic performance characteristics are unknown [89]. Whole genome sequencing may have a role for identification, but experience is limited at this time [90].
4. Coccidiomycosis
Coccidiomycosis colloquially known as “Valley Fever” is caused by Coccidioides immitis and Coccidioides posadasii [44,45,91,92,93]. C. immitis predominates in central California but has been found as far north as Washington, whereas C. posadasii is the predominant species in South and Central America, Mexico, Arizona, Texas, and Utah [7,44,91,92,94]. The primary route of infection is via inhalation of arthroconidia [40,44,45,91]. Many individuals who are exposed do not develop clinical symptoms [41,44,45,92,95]. The most common presentation is acute pulmonary infection similar to community acquired pneumonia with fever, headache, cough, fatigue, and pleuritic chest pain among the common symptoms though presentations may be more severe in immune compromised persons [40,41,45,93,96]. Additionally, Coccidioides more frequently causes associated hilar and paratracheal adenopathy on imaging compared to bacterial pneumonia and may cause chronic progressive cavitary lesions or pulmonary nodules [40,41,45,96].
The standard for diagnosis of coccidioidomycosis is culture or identification of spherules on histological examination of clinical specimens [40,45,91,96]. Spherules range in diameter from 10 to 200 μm, are filled with 2–5 μm diameter endospores and can be visualized with Papanicolaou, calcofluor white stain, potassium hydroxide, periodic acid Schiff, Grocott-methenamine silver, or hematoxylin-eosin stains (Figure 1C) [40,41,43,45,97]. Clinical specimens may lack intact spherules and instead have numerous endospores which can be mistaken for Histoplasma, Blastomyces, or Cryptococcus [91]. Coccidioides can typically be grown on routine culture in one week where a chemiluminescent DNA probe (Accuprobe, GenProbe, San Diego, CA, USA) can then be used for rapid identification, however, the laboratory should be informed of suspicion for coccidioidomycosis prior to sending the specimen given that arthroconidia are easily aerosolized [40,41,42,43,97].
Serologic testing via tube precipitin (TP) and CF are commonly used due to ease of specimen collection and laboratory safety [40,45,91,97,98]. TP tests for IgM, whereas CF tests for IgG [91]. Immunodiffusion (ID) can also be used to test for both IgM and IgG and may be referred to as IDTP and IDCF as they use the same antigen preparations as TP and CF [91,97,98]. CF and ID are often only available at reference laboratories [45,46]. Commercial EIAs for both IgG and IgM are now available, are more sensitive than traditional antibody tests, and can detect antibody earlier in the disease course [40,44,45,47,48,91,99]. A positive IgG on EIA should be sent to a reference lab for confirmation and quantification with CF [40,45,97]. An isolated positive EIA IgM should be confirmed with repeat EIA testing or another method as false positive results have been noted duet to blastomycosis and other fungal infections [43,46,47,49,97]. CF titers can be utilized to monitor treatment response and can provide prognostic information [41,96]. As with other antibody tests, those for Coccidioides may be negative early in disease (repeat testing should be done if suspicion is high) and may be falsely negative in immune suppressed patients; combining antibody tests may improve sensitivity [37,41,42,45,46,91,96,97].
An EIA for detection of Coccidioides antigen in urine and serum is now commercially available [39,40,41,42,91]. One study found 71% sensitivity using urine antigen testing in severe disease in a mostly immunocompromised population–further study is needed in isolated pulmonary diseases where one would expect relatively decreased sensitivity [42]. Skin testing for Coccidioides was available until the late 1990s, in 2011 a newly formulated skin test (Spherusol, Nielson Biosciences, San Diego California) was approved by the FDA [100]. Spherusol can be utilized to test for cellular immunity to Coccidioides due to prior infection but its role in diagnosis of active infection is uncertain [50,100]. Coccidioides specific PCR is infrequently used for diagnosis and panfungal PCR’s role is exploratory at best at this point while use of MALDI-TOF MS for identification of coccidioidomycosis is similarly rare although commercial MALDI-TOF MS panels are now available for Coccidioides immitis and C posadasii [79,101,102,103,104]. Whole genome sequencing may also be an option for identification of Coccidioides, particularly for cluster investigations [105].
5. Paracoccidiomycosis
Paracoccidiomycosis is caused by Paracoccidioides brasiliensis or Paracoccidioides lutzii and is endemic in parts of South, Central, and North America, but is most common in Brazil [7,52,54,55,106]. Paracoccidioides lutzii has only been reported in limited regions of Brazil fairly recently while P. braziliensis has been reported in Argentina, Venezuela, and Mexico as well—most descriptions of clinical disease refer to P braziliensis [7]. Paracoccidioides infection is associated with exposure to contaminated soil and occurs after inhalation of fungal conidia [54,56,106,107]. Paracoccidioides can cause infection in both immunocompromised and immunocompetent individuals [54]. The acute or subacute form is typically seen in children or adults less than 30 years old and may cause fever, weight loss, and lymphadenopathy. Disseminated disease commonly includes gastrointestinal, cutaneous, or osteoarticular involvement [52,54,55,106]. Pulmonary disease is rare and is more common in the common (adult) form which occurs due to reactivation or reinfection and causes symptoms similar to tuberculosis (cough, dyspnea, weight loss, anorexia) [54,55,106]. In patients with HIV, paracoccidioidomycosis progresses more rapidly and is more likely to disseminate [54,55,106]. A relatively large descriptive study of P. lutzii cases describes 34 cases, all of the ‘chronic’ or adult form of which 28 (82.4%) had pulmonary involvement [108].
Traditionally diagnosis is achieved by identification of the characteristic yeast form in tissue or clinical specimens [51,52,54,56,106,107]. The yeast are large mother cells surrounded by multiple narrow-necked budding daughter cells resembling a “pilot wheel” or mother cells with only two daughter cells resembling a “Mickey mouse head” (Figure 1D) [52,106,107]. In one study, cytopathological examination on sputum had sensitivities of ~60% for both acute and subacute and chronic paracoccidiomycosis [53]. Culture is also possible, however less clinically useful given culture growth takes 2–4 weeks [55,106,107]. Typical methods of identification in clinical specimens or culture are not able to differentiate P. brasiliensis from P. lutzii, at this point genotyping is required to do so [108].
Antibody detection through double immunodiffusion (DID), immunoblots (IB), latex agglutination (LA), counterimmunoelectrophoresis (CIE), and enzyme linked immunosorbent assay (ELISA) is available at reference laboratories for diagnosis of P brasiliensis, with DID being utilized most frequently [52,53,54,56,57,107]. The sensitivity and specificity of DID is high, 90% and 100%, respectively, in one study [53]. DID titers may be used to monitor response to treatment with higher levels typical in acute and subacute and disseminated forms [52,53,54,106]. Antibody testing, particularly ELISA may cross-react with Histoplasma and other fungi [52,56,58,106,107]. DID, IB, ELISA, and LA most commonly detect antibodies to the gp43 antigen which is specific for P. brasiliensis complex, thus, they do not detect P. lutzii infection or some P. brasiliensis strains where the gp43 antigen is not expressed [54,56,107,109]. Antigen, PCR, and MALDI-TOF MS testing are not currently available outside research settings but may hold promise in the future [52,57,58,107,110,111].
6. Talaromycosis
Talaromyces marneffei, formerly known as Penicillium marneffei, is endemic to South and Southeast Asia [7,59,112,113,114]. The primary route of acquisition is pulmonary with subsequent hematogenous spread [112]. Talaromyces infection has been associated with exposure to soil in the rainy season [113]. T. marneffei causes infection in immunocompromised patients, classically people living with HIV, but more recently patients with other forms of immunosuppression have been reported as well [112,115,116]. Talaromycosis in AIDS presents as a disseminated illness with weight loss, fever, anemia, lymphadenopathy, hepatosplenomegaly, respiratory signs, and skin lesions [64,112,113]. Immunosuppressed patient without HIV commonly present with fever, cutaneous lesions, hepatomegaly, and lymphadenopathy, and are more likely to have bone and joint infection compared to HIV infected individuals [117].
Traditionally, diagnosis of T. marneffei has relied on fungal cultures from clinical specimens including bone marrow, skin biopsies, blood, sputum, BAL, and cerebrospinal fluid (CSF), but growth may take up to four weeks and reported performance has been highly variable [60,62,64,65,112,113,116]. Growth of T. marneffei on Sabouraud’s agar produces a characteristic soluble red pigment [117,118]. Blood cultures are only positive when Talaromycosis has progressed to advanced disease and can miss 30% and 50% of HIV infected and HIV non-infected patients respectively [62]. T. marneffei can also be diagnosed by visualization of intracellular and extracellular round to oval yeast cells (3–8 μm in diameter) from clinical specimens (Figure 1E) [59,112]. Cytology has been reported to have sensitivity of 46% in one small study [63].
Antigen detection is a promising field to aid in earlier diagnosis of Talaromyces with impressive progress in recent years [65,116,119,120,121]. A lateral flow immunochromatographic assay utilizing a monoclonal antibody, 4D1, conjugated with gold colloid as a signal generator and lined with T. marneffei cytoplasmic yeast antigen detected antigen in urine with a sensitivity of 87.9% and specificity of 100% in persons with confirmed T. marneffei infection by blood culture [64]. This assay was able to provide results in 20 min and did not require specialized equipment or skilled personnel [64]. Recently, a novel EIA using Mp1p antigen (a cell wall mannoprotein and important virulence factor for T. marneffei) has been developed [62,122,123]. In a large study of 372 HIV positive patients with culture proven (blood or other sterile body fluid) T. marneffei and 517 controls, the Mp1p EIA showed 86.3% sensitivity compared to 72.8% for blood culture [62]. Specificity for the EIA was 98.1% and time to test result was six hours versus 6.6 (± 3) days for blood culture [62]. Among 269 patients with paired urine and plasma samples tested by EIA, sensitivity increased to 88.8%, with a p value of <0.001 or 0.02 compared to plasma or urine alone [62]. These results will need to be duplicated in other populations, including immune suppressed persons without HIV but are obviously quite impressive. Although the test is not yet commercially available, a recent report describing two patients with HIV and talaromycosis diagnosed by the Mp1p EIA with negative blood cultures demonstrates the utility of the assay in concordance with the larger study results [124].
Antibody assays have been developed for diagnosis for talaromycosis; however, their clinical utility has been limited by cross reactivity, varied sensitivity and specificity, and inability of patients within the most affected populations to form antibodies due to underlying immune deficits [65,115,116,120,125]. Several traditional PCR methodologies have shown promise to detect T. marneffei infection but are not commercially available and are limited by variable performance [126,127,128,129]. mNGS has been used to diagnose T. marneffei in a HIV negative patient with disseminated disease including skin, bone marrow, CSF, and BAL involvement but its performance characteristics for talaromycosis are unknown [130].
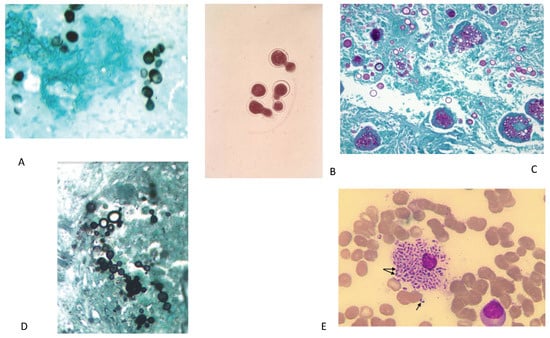
Figure 1.
(A) Gomori methenamine silver stain showing small oval budding yeast form of H. capsulatum yeast. (B) KOH wet mount showing B. dermatitidis yeast with characteristic broad-based budding. (C) PAS stain C. immitis/posadasii endospore containing spherules with round thick walls in the tissue. Figure 1A–C from Wheat LJ, Goldman M, Hage CA, Knox KS, Cryptococcosis and the Endemic Mycoses In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, Siegel MD, Fishman’s Pulmonary Diseases and Disorders. 5th ed. McGraw-Hill Education: 2015. Figure 134-3 [131]. (D) Typical multi-budding yeast cells (black staining) with a ‘ships-wheel’ appearance in a tissue sample of Paracoccidioides (Grocott-Gomori methenamine silver). Figure 1D from Restrepo-Moreno A, Tobόn-Orozco, AM, González-Marín A, Paracoccidioidomycosis. In: Bennett JE, Dolin R, Blaser MJ, Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier: 2020. Figure 267.6B [132]. (E) Wright’s stain of bone marrow aspirate of patient showing numerous small, non-budding oval yeast cells measuring 5–6 um inside an engorged histiocytes. The arrows show the actively dividing yeast cells, revealing a midline septum characteristic of Talaromyces marneffei. Figure 1E from Trieu Ly V, Tat Than N, Chan J, Day JN, Perfect J, Ngoc Nga, C, Van Vinh Chau N, Le T. Occult Talaromyces marneffei Infection Unveiled by the Novel Mp1P Antigen Detection Assay. Open Forum Infectious Diseases. 2020. (PMID 33269295) [124].
7. Conclusions
Diagnosis of pulmonary infections due to endemic fungi can be challenging, as they frequently mimic other diseases. The areas of endemicity for many of these fungi are expanding, with climate change likely playing a role—this further complicates rapid diagnosis as knowledge about these infections may not be as high outside of their traditional endemic areas. Diagnosis is frequently delayed which can lead to unnecessary antibiotic exposure, repeated hospitalizations, and increased morbidity and mortality. While diagnosis has traditionally relied on culture or visualization of fungi, time to test results with culture, and insensitivity of visualization tests can limit utility. Thus, combined approaches are often necessary and rapid diagnosis frequently relies on antigen testing, with antibody testing also having a role in some cases. Cross-reactivity is a common limitation for both antibody and antigen testing but varies by the specific test and fungus. Important advances in antigen testing for talaromycosis and histoplasmosis have occurred in recent years. Molecular testing including mNGS may have a role going forward, as may MALDI-TOF MS (for identification of culture isolates), however further studies and logistical streamlining will be needed before wider adoption can be a consideration. It is important for physicians to be aware of the pulmonary manifestations of endemic fungi, as well as the changing epidemiology for many of these infections. Despite advances in diagnostic testing, a test never sent is of zero utility. A physician’s knowledge is the first step in proper diagnosis of these infections and endemic mycoses should be considered among the causes of pulmonary infections, particularly when a patient with a putative diagnosis of bacterial pneumonia does not respond to antibacterial therapy.
Author Contributions
V.P. assisted with literature review, drafting the manuscript as well as formatting and editing. C.S. assisted with literature review, drafting and editing the manuscript. D.M. assisted with literature review, drafting, and editing the manuscript. L.Z. assisted with literature review, drafting, and editing the manuscript. N.C.B. designed the project and assisted with literature review, editing, and drafting the manuscript, and is the guarantor of the content. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health, K23NS110470.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Salzer, H.J.F.; Burchard, G.; Cornely, O.A.; Lange, C.; Rolling, T.; Schmiedel, S.; Libman, M.; Capone, D.; Le, T.; Dalcolmo, M.P.; et al. Diagnosis and Management of Systemic Endemic Mycoses Causing Pulmonary Disease. Respiration 2018, 96, 283–301. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef]
- Hage, C.A.; Knox, K.S.; Davis, T.E.; Wheat, L.J. Antigen detection in bronchoalveolar lavage fluid for diagnosis of fungal pneumonia. Curr. Opin. Pulm. Med. 2011, 17, 167–171. [Google Scholar] [CrossRef]
- Tran, T.; Beal, S.G. Application of the 1,3-beta-D-Glucan (Fungitell) Assay in the Diagnosis of Invasive Fungal Infections. Arch. Pathol. Lab. Med. 2016, 140, 181–185. [Google Scholar] [CrossRef]
- Melo, A.S.A.; Santos, D.; Lima, S.L.; Rodrigues, A.M.; de Camargo, Z.P.; Finkelman, M.; Colombo, A.L. Evaluation of (1 --> 3)-beta-D-glucan assay for diagnosing paracoccidioidomycosis. Mycoses 2020, 63, 38–42. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Sakamoto, Y.; Lee, K.; Amano, Y.; Tachikawa, N. Penicillium marneffei Infection with beta-D-glucan Elevation: A Case Report and Literature Review. Intern. Med. 2016, 55, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Azar, M.M.; Bahr, N.; Loyd, J.; Wheat, L.J. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin. Respir. Crit. Care Med. 2015, 36, 729–745. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, D.S.; McKinsey, J.P. Pulmonary histoplasmosis. Semin. Respir. Crit. Care Med. 2011, 32, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kosmidis, C.; Rozaliyani, A.; Wahyuningsih, R.; Denning, D.W. Chronic Pulmonary Histoplasmosis-A Scoping Literature Review. Open Forum Infect. Dis. 2020, 7, ofaa119. [Google Scholar] [CrossRef]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.R.; Graf, E.H.; Griffin, A.T. Urine antigen tests for the diagnosis of respiratory infections: Legionellosis, histoplasmosis, pneumococcal pneumonia. Clin. Lab. Med. 2014, 34, 219–236. [Google Scholar] [CrossRef]
- Swartzentruber, S.; Rhodes, L.; Kurkjian, K.; Zahn, M.; Brandt, M.E.; Connolly, P.; Wheat, L.J. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin. Infect. Dis. 2009, 49, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Herman, K.M.; Hage, C.A.; Fuller, D.; Wheat, L.J. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin. Infect. Dis. 2016, 62, 896–902. [Google Scholar] [CrossRef]
- Theel, E.S.; Jespersen, D.J.; Harring, J.; Mandrekar, J.; Binnicker, M.J. Evaluation of an enzyme immunoassay for detection of Histoplasma capsulatum antigen from urine specimens. J. Clin. Microbiol. 2013, 51, 3555–3559. [Google Scholar] [CrossRef]
- Fandino-Devia, E.; Rodriguez-Echeverri, C.; Cardona-Arias, J.; Gonzalez, A. Antigen Detection in the Diagnosis of Histoplasmosis: A Meta-analysis of Diagnostic Performance. Mycopathologia 2016, 181, 197–205. [Google Scholar] [CrossRef]
- Azar, M.M.; Malo, J.; Hage, C.A. Endemic Fungi Presenting as Community-Acquired Pneumonia: A Review. Semin. Respir. Crit. Care Med. 2020, 41, 522–537. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Ribes, J.A.; Wengenack, N.L.; Baddour, L.M.; Assi, M.; McKinsey, D.S.; Hammoud, K.; Alapat, D.; Babady, N.E.; Parker, M.; et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 2011, 53, 448–454. [Google Scholar] [CrossRef]
- Hage, C.A.; Davis, T.E.; Fuller, D.; Egan, L.; Witt, J.R., 3rd; Wheat, L.J.; Knox, K.S. Diagnosis of histoplasmosis by antigen detection in BAL fluid. Chest 2010, 137, 623–628. [Google Scholar] [CrossRef]
- Connolly, P.A.; Durkin, M.M.; Lemonte, A.M.; Hackett, E.J.; Wheat, L.J. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 2007, 14, 1587–1591. [Google Scholar] [CrossRef]
- Swartzentruber, S.; LeMonte, A.; Witt, J.; Fuller, D.; Davis, T.; Hage, C.; Connolly, P.; Durkin, M.; Wheat, L.J. Improved detection of Histoplasma antigenemia following dissociation of immune complexes. Clin. Vaccine Immunol. 2009, 16, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Haydour, Q.; Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Knox, K.S.; Kolls, J.K.; Wengenack, N.L.; Prokop, L.J.; et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann. Am. Thorac. Soc. 2019, 16, 1179–1188. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, G.S.; Lee, C.H.; Hage, C.A. Evaluation of two new enzyme immunoassay reagents for diagnosis of histoplasmosis in a cohort of clinically characterized patients. Med. Mycol. 2015, 53, 868–873. [Google Scholar] [CrossRef]
- Alpern, J.D.; Bahr, N.C.; Vazquez-Benitez, G.; Boulware, D.R.; Sellman, J.S.; Sarosi, G.A. Diagnostic Delay and Antibiotic Overuse in Acute Pulmonary Blastomycosis. Open Forum. Infect. Dis. 2016, 3, ofw078. [Google Scholar] [CrossRef] [PubMed]
- Carlos, W.G.; Rose, A.S.; Wheat, L.J.; Norris, S.; Sarosi, G.A.; Knox, K.S.; Hage, C.A. Blastomycosis in indiana: Digging up more cases. Chest 2010, 138, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.; Witt, J.; Lemonte, A.; Wheat, B.; Connolly, P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 2004, 42, 4873–4875. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, M.A.; Prakash, U.B. Pulmonary blastomycosis: An appraisal of diagnostic techniques. Chest 2002, 121, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Hage, C.A.; Bariola, J.R.; Bensadoun, E.; Rodgers, M.; Bradsher, R.W., Jr.; Wheat, L.J. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin. Vaccine Immunol. 2012, 19, 53–56. [Google Scholar] [CrossRef]
- Patel, A.J.; Gattuso, P.; Reddy, V.B. Diagnosis of blastomycosis in surgical pathology and cytopathology: Correlation with microbiologic culture. Am. J. Surg. Pathol. 2010, 34, 256–261. [Google Scholar] [CrossRef]
- Frost, H.M.; Novicki, T.J. Blastomyces Antigen Detection for Diagnosis and Management of Blastomycosis. J. Clin. Microbiol. 2015, 53, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Bariola, J.R.; Hage, C.A.; Durkin, M.; Bensadoun, E.; Gubbins, P.O.; Wheat, L.J.; Bradsher, R.W., Jr. Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagn Microbiol. Infect. Dis. 2011, 69, 187–191. [Google Scholar] [CrossRef]
- McBride, J.A.; Gauthier, G.M.; Klein, B.S. Clinical Manifestations and Treatment of Blastomycosis. Clin. Chest Med. 2017, 38, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Saccente, M.; Woods, G.L. Clinical and laboratory update on blastomycosis. Clin. Microbiol. Rev. 2010, 23, 367–381. [Google Scholar] [CrossRef]
- Klein, B.S.; Vergeront, J.M.; Kaufman, L.; Bradsher, R.W.; Kumar, U.N.; Mathai, G.; Varkey, B.; Davis, J.P. Serological tests for blastomycosis: Assessments during a large point-source outbreak in Wisconsin. J. Infect. Dis. 1987, 155, 262–268. [Google Scholar] [CrossRef]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Brandhorst, T.T.; Hage, C.A.; Connolly, P.A.; Leland, D.S.; Davis, T.E.; Klein, B.S.; Wheat, L.J. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin. Vaccine Immunol. 2014, 21, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kassis, C.; Durkin, M.; Holbrook, E.; Myers, R.; Wheat, L. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin. Infect. Dis. 2021, 72, 968–975. [Google Scholar] [CrossRef]
- Saubolle, M.A.; McKellar, P.P.; Sussland, D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J. Clin. Microbiol. 2007, 45, 26–30. [Google Scholar] [CrossRef]
- Durkin, M.; Estok, L.; Hospenthal, D.; Crum-Cianflone, N.; Swartzentruber, S.; Hackett, E.; Wheat, L.J. Detection of Coccidioides antigenemia following dissociation of immune complexes. Clin. Vaccine Immunol. 2009, 16, 1453–1456. [Google Scholar] [CrossRef]
- Gabe, L.M.; Malo, J.; Knox, K.S. Diagnosis and Management of Coccidioidomycosis. Clin. Chest Med. 2017, 38, 417–433. [Google Scholar] [CrossRef]
- Twarog, M.; Thompson, G.R., 3rd. Coccidioidomycosis: Recent Updates. Semin. Respir. Crit. Care Med. 2015, 36, 746–755. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef] [PubMed]
- Saubolle, M.A. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef]
- Kollath, D.R.; Miller, K.J.; Barker, B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence 2019, 10, 222–233. [Google Scholar] [CrossRef]
- Malo, J.; Luraschi-Monjagatta, C.; Wolk, D.M.; Thompson, R.; Hage, C.A.; Knox, K.S. Update on the diagnosis of pulmonary coccidioidomycosis. Ann. Am. Thorac. Soc. 2014, 11, 243–253. [Google Scholar] [CrossRef]
- Blair, J.E.; Coakley, B.; Santelli, A.C.; Hentz, J.G.; Wengenack, N.L. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006, 162, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Currier, J.T. Significance of isolated positive IgM serologic results by enzyme immunoassay for coccidioidomycosis. Mycopathologia 2008, 166, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Jaskowski, T.D.; Mouritsen, C.L.; Hill, H.R. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J. Clin. Microbiol. 1995, 33, 940–943. [Google Scholar] [CrossRef]
- Kaufman, L.; Sekhon, A.S.; Moledina, N.; Jalbert, M.; Pappagianis, D. Comparative evaluation of commercial Premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. J. Clin. Microbiol. 1995, 33, 618–619. [Google Scholar] [CrossRef]
- Johnson, R.; Kernerman, S.M.; Sawtelle, B.G.; Rastogi, S.C.; Nielsen, H.S.; Ampel, N.M. A reformulated spherule-derived coccidioidin (Spherusol) to detect delayed-type hypersensitivity in coccidioidomycosis. Mycopathologia 2012, 174, 353–358. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.V.; Pecanha Pietrobom, P.M.; Rosa Junior, M.; Baptista, R.M.; Pecanha, P.M. New Insights on Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 53–68. [Google Scholar] [CrossRef]
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [PubMed]
- Moreto, T.C.; Marques, M.E.; de Oliveira, M.L.; Moris, D.V.; de Carvalho, L.R.; Mendes, R.P. Accuracy of routine diagnostic tests used in paracoccidioidomycosis patients at a university hospital. Trans. R Soc. Trop. Med. Hyg. 2011, 105, 473–478. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Miller, R.F.; Huang, L. Approach to Fungal Infections in Human Immunodeficiency Virus-Infected Individuals: Pneumocystis and Beyond. Clin. Chest Med. 2017, 38, 465–477. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.F.; de Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Advances and challenges in paracoccidioidomycosis serology caused by Paracoccidioides species complex: An update. Diagn Microbiol. Infect. Dis. 2016, 84, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.L.; Figueroa, J.I.; Hamilton, A.J.; Ortiz, B.; Robledo, M.A.; Hay, R.J.; Restrepo, A. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: New strategies for detection of circulating antigens. J. Clin. Microbiol. 1997, 35, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, S.H.; Colombo, A.L.; Blotta, M.H.; Lopes, J.D.; Queiroz-Telles, F.; Pires de Camargo, Z. Detection of circulating gp43 antigen in serum, cerebrospinal fluid, and bronchoalveolar lavage fluid of patients with paracoccidioidomycosis. J. Clin. Microbiol. 2003, 41, 3675–3680. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Khamwan, C.; Baosoung, V.; Nelson, K.E.; Sirisanthana, T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994, 344, 110–113. [Google Scholar] [CrossRef]
- Wu, T.C.; Chan, J.W.; Ng, C.K.; Tsang, D.N.; Lee, M.P.; Li, P.C. Clinical presentations and outcomes of Penicillium marneffei infections: A series from 1994 to 2004. Hong Kong Med. J. 2008, 14, 103–109. [Google Scholar]
- Wong, S.Y.; Wong, K.F. Penicillium marneffei Infection in AIDS. Patholog. Res. Int. 2011, 2011, 764293. [Google Scholar] [CrossRef]
- Thu, N.T.M.; Chan, J.F.W.; Ly, V.T.; Ngo, H.T.; Hien, H.T.A.; Lan, N.P.H.; Chau, N.V.V.; Cai, J.P.; Woo, P.C.Y.; Day, J.N.; et al. Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of talaromycosis. Clin. Infect. Dis. 2020, ciaa826. [Google Scholar] [CrossRef] [PubMed]
- Jan, I.S.; Chung, P.F.; Wang, J.Y.; Weng, M.H.; Hung, C.C.; Lee, L.N. Cytological diagnosis of Penicillium marneffei infection. J. Formos. Med. Assoc. 2008, 107, 443–447. [Google Scholar] [CrossRef][Green Version]
- Pruksaphon, K.; Intaramat, A.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Development and characterization of an immunochromatographic test for the rapid diagnosis of Talaromyces (Penicillium) marneffei. PLoS ONE 2018, 13, e0195596. [Google Scholar] [CrossRef] [PubMed]
- Prakit, K.; Nosanchuk, J.D.; Pruksaphon, K.; Vanittanakom, N.; Youngchim, S. A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 647–656. [Google Scholar] [CrossRef]
- Caceres, D.H.; Gomez, B.L.; Tobon, A.M.; Chiller, T.M.; Lindsley, M.D. Evaluation of a Histoplasma antigen lateral flow assay for the rapid diagnosis of progressive disseminated histoplasmosis in Colombian patients with AIDS. Mycoses 2020, 63, 139–144. [Google Scholar] [CrossRef]
- Babady, N.E.; Buckwalter, S.P.; Hall, L.; Le Febre, K.M.; Binnicker, M.J.; Wengenack, N.L. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J. Clin. Microbiol. 2011, 49, 3204–3208. [Google Scholar] [CrossRef]
- Gago, S.; Esteban, C.; Valero, C.; Zaragoza, O.; Puig de la Bellacasa, J.; Buitrago, M.J. A multiplex real-time PCR assay for identification of Pneumocystis jirovecii, Histoplasma capsulatum, and Cryptococcus neoformans/Cryptococcus gattii in samples from AIDS patients with opportunistic pneumonia. J. Clin. Microbiol. 2014, 52, 1168–1176. [Google Scholar] [CrossRef]
- Zatti, M.D.S.; Arantes, T.D.; Fernandes, J.A.L.; Bay, M.B.; Milan, E.P.; Naliato, G.F.S.; Theodoro, R.C. Loop-mediated Isothermal Amplification and nested PCR of the Internal Transcribed Spacer (ITS) for Histoplasma capsulatum detection. PLoS Negl. Trop. Dis. 2019, 13, e0007692. [Google Scholar] [CrossRef] [PubMed]
- Dantas, K.C.; Freitas, R.S.; da Silva, M.V.; Criado, P.R.; Luiz, O.D.C.; Vicentini, A.P. Comparison of diagnostic methods to detect Histoplasma capsulatum in serum and blood samples from AIDS patients. PLoS ONE 2018, 13, e0190408. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.F.; Munoz, C.O.; Caceres, D.H.; Tobon, A.M.; Loparev, V.; Clay, O.; Chiller, T.; Litvintseva, A.; Gade, L.; Gonzalez, A.; et al. Standardization and validation of real time PCR assays for the diagnosis of histoplasmosis using three molecular targets in an animal model. PLoS ONE 2017, 12, e0190311. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, I.; Dalla Lana, D.F.; Pasqualotto, A.C. The Role of Molecular Tests in the Diagnosis of Disseminated Histoplasmosis. J. Fungi (Basel) 2019, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. J. Fungi (Basel) 2019, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.M.; Zhou, Y.; Theodoro, R.C.; Abrams, B.; Balajee, S.A.; Litvintseva, A.P. Development of a loop-mediated isothermal amplification method for detection of Histoplasma capsulatum DNA in clinical samples. J. Clin. Microbiol. 2014, 52, 483–488. [Google Scholar] [CrossRef]
- Panda, A.; Ghosh, A.K.; Mirdha, B.R.; Xess, I.; Paul, S.; Samantaray, J.C.; Srinivasan, A.; Khalil, S.; Rastogi, N.; Dabas, Y. MALDI-TOF mass spectrometry for rapid identification of clinical fungal isolates based on ribosomal protein biomarkers. J. Microbiol. Methods 2015, 109, 93–105. [Google Scholar] [CrossRef]
- Valero, C.; Buitrago, M.J.; Gago, S.; Quiles-Melero, I.; Garcia-Rodriguez, J. A matrix-assisted laser desorption/ionization time of flight mass spectrometry reference database for the identification of Histoplasma capsulatum. Med. Mycol. 2018, 56, 307–314. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Chen, G.; Liu, X.; Ding, L. Metagenomic next-generation sequencing identified Histoplasma capsulatum in the lung and epiglottis of a Chinese patient: A case report. Int. J. Infect. Dis. 2020, 101, 33–37. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Ling, H.; Dong, X.; Zhang, Y.; Li, J.; Zhang, Y.; Song, J.; Liu, W.J.; Li, Y.; et al. Identification of Histoplasma causing an unexplained disease cluster in Matthews Ridge, Guyana. Biosaf. Health 2019, 1, 150–154. [Google Scholar] [CrossRef]
- Frickmann, H.; Loderstaedt, U.; Racz, P.; Tenner-Racz, K.; Eggert, P.; Haeupler, A.; Bialek, R.; Hagen, R.M. Detection of tropical fungi in formalin-fixed, paraffin-embedded tissue: Still an indication for microscopy in times of sequence-based diagnosis? Biomed. Res. Int. 2015, 2015, 938721. [Google Scholar] [CrossRef]
- Develoux, M.; Amona, F.M.; Hennequin, C. Histoplasmosis caused by Histoplasma capsulatum var. duboisii: A comprehensive review of cases from 1993 to 2019. Clin. Infect. Dis. 2020, ciaa1304. [Google Scholar] [CrossRef]
- McBride, J.A.; Gauthier, G.M.; Klein, B.S. Turning on virulence: Mechanisms that underpin the morphologic transition and pathogenicity of Blastomyces. Virulence 2019, 10, 801–809. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. J. Fungi (Basel) 2019, 5, 67. [Google Scholar] [CrossRef]
- Bradsher, R.W., Jr. The endemic mimic: Blastomycosis an illness often misdiagnosed. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 188–202. [Google Scholar]
- Litvinjenko, S.; Lunny, D. Blastomycosis hospitalizations in northwestern Ontario: 2006–2015. Can. Commun. Dis. Rep. 2017, 43, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [CrossRef] [PubMed]
- Sidamonidze, K.; Peck, M.K.; Perez, M.; Baumgardner, D.; Smith, G.; Chaturvedi, V.; Chaturvedi, S. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J. Clin. Microbiol. 2012, 50, 1783–1786. [Google Scholar] [CrossRef]
- Bialek, R.; Cirera, A.C.; Herrmann, T.; Aepinus, C.; Shearn-Bochsler, V.I.; Legendre, A.M. Nested PCR assays for detection of Blastomyces dermatitidis DNA in paraffin-embedded canine tissue. J. Clin. Microbiol. 2003, 41, 205–208. [Google Scholar] [CrossRef]
- Meece, J.K.; Anderson, J.L.; Klein, B.S.; Sullivan, T.D.; Foley, S.L.; Baumgardner, D.J.; Brummitt, C.F.; Reed, K.D. Genetic diversity in Blastomyces dermatitidis: Implications for PCR detection in clinical and environmental samples. Med. Mycol. 2010, 48, 285–290. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Luo, Z.; Deng, S.; Li, Q. A young male with chronic nonproductive cough diagnosed with blastomycosis in China: A case report. BMC Pulm. Med. 2020, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Maphanga, T.G.; Birkhead, M.; Munoz, J.F.; Allam, M.; Zulu, T.G.; Cuomo, C.A.; Schwartz, I.S.; Ismail, A.; Naicker, S.D.; Mpembe, R.S.; et al. Human Blastomycosis in South Africa Caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967 to 2014. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef]
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Sondermeyer Cooksey, G.L.; et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Marsden-Haug, N.; Goldoft, M.; Ralston, C.; Limaye, A.P.; Chua, J.; Hill, H.; Jecha, L.; Thompson, G.R., 3rd; Chiller, T. Coccidioidomycosis acquired in Washington State. Clin. Infect. Dis. 2013, 56, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Jude, C.M.; Nayak, N.B.; Patel, M.K.; Deshmukh, M.; Batra, P. Pulmonary coccidioidomycosis: Pictorial review of chest radiographic and CT findings. Radiographics 2014, 34, 912–925. [Google Scholar] [CrossRef]
- Parish, J.M.; Blair, J.E. Coccidioidomycosis. Mayo Clin. Proc. 2008, 83, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ampel, N.M. The diagnosis of coccidioidomycosis. F1000 Med. Rep. 2010, 2. [Google Scholar] [CrossRef]
- Galgiani, J.N.; Grace, G.M.; Lundergan, L.L. New serologic tests for early detection of coccidioidomycosis. J. Infect. Dis. 1991, 163, 671–674. [Google Scholar] [CrossRef]
- Wieden, M.A.; Lundergan, L.L.; Blum, J.; Delgado, K.L.; Coolbaugh, R.; Howard, R.; Peng, T.; Pugh, E.; Reis, N.; Theis, J.; et al. Detection of coccidioidal antibodies by 33-kDa spherule antigen, Coccidioides EIA, and standard serologic tests in sera from patients evaluated for coccidioidomycosis. J. Infect. Dis. 1996, 173, 1273–1277. [Google Scholar] [CrossRef]
- Wack, E.E.; Ampel, N.M.; Sunenshine, R.H.; Galgiani, J.N. The Return of Delayed-Type Hypersensitivity Skin Testing for Coccidioidomycosis. Clin. Infect. Dis. 2015, 61, 787–791. [Google Scholar] [CrossRef]
- Johnson, S.M.; Simmons, K.A.; Pappagianis, D. Amplification of coccidioidal DNA in clinical specimens by PCR. J. Clin. Microbiol. 2004, 42, 1982–1985. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides species in clinical specimens by real-time PCR. J. Clin. Microbiol. 2007, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J.; Slechta, E.S.; Barker, A.P.; Miranda, E.; Babady, N.E.; Tang, Y.W.; Gibas, C.; Wiederhold, N.; Sutton, D.; Hanson, K.E. Multicenter Evaluation of the Vitek MS v3.0 System for the Identification of Filamentous Fungi. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi (Basel) 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Oltean, H.N.; Etienne, K.A.; Roe, C.C.; Gade, L.; McCotter, O.Z.; Engelthaler, D.M.; Litvintseva, A.P. Utility of Whole-Genome Sequencing to Ascertain Locally Acquired Cases of Coccidioidomycosis, Washington, USA. Emerg. Infect. Dis. 2019, 25, 501–506. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Escuissato, D.L. Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2011, 32, 764–774. [Google Scholar] [CrossRef]
- Pinheiro, B.G.; Hahn, R.C.; Camargo, Z.P.; Rodrigues, A.M. Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives. J. Fungi (Basel) 2020, 6, 293. [Google Scholar] [CrossRef]
- Hahn, R.C.; Rodrigues, A.M.; Della Terra, P.P.; Nery, A.F.; Hoffmann-Santos, H.D.; Gois, H.M.; Fontes, C.J.F.; de Camargo, Z.P. Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2019, 13, e0007437. [Google Scholar] [CrossRef]
- de Camargo, Z.P. Serology of paracoccidioidomycosis. Mycopathologia 2008, 165, 289–302. [Google Scholar] [CrossRef]
- Nobrega de Almeida, J., Jr.; Del Negro, G.M.; Grenfell, R.C.; Vidal, M.S.; Thomaz, D.Y.; de Figueiredo, D.S.; Bagagli, E.; Juliano, L.; Benard, G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for differentiation of the dimorphic fungal species Paracoccidioides brasiliensis and Paracoccidioides lutzii. J. Clin. Microbiol. 2015, 53, 1383–1386. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Pinheiro, B.G.; Teixeira-Ferreira, A.; Hahn, R.C.; de Camargo, Z.P. Immunoproteomic Analysis Reveals Novel Candidate Antigens for the Diagnosis of Paracoccidioidomycosis Due to Paracoccidioides lutzii. J. Fungi (Basel) 2020, 6, 357. [Google Scholar] [CrossRef] [PubMed]
- Vanittanakom, N.; Cooper, C.R., Jr.; Fisher, M.C.; Sirisanthana, T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 2006, 19, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Wolbers, M.; Chi, N.H.; Quang, V.M.; Chinh, N.T.; Lan, N.P.; Lam, P.S.; Kozal, M.J.; Shikuma, C.M.; Day, J.N.; et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin. Infect. Dis. 2011, 52, 945–952. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.Q.; Pan, M.L.; Zeng, W.; Tang, S.D.; Tan, C.M. Determinants of prognosis in Talaromyces marneffei infections with respiratory system lesions. Chin. Med. J. (Engl.) 2019, 132, 1909–1918. [Google Scholar] [CrossRef]
- Wong, S.S.; Wong, K.H.; Hui, W.T.; Lee, S.S.; Lo, J.Y.; Cao, L.; Yuen, K.Y. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J. Clin. Microbiol. 2001, 39, 4535–4540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pruksaphon, K.; Intaramat, A.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Diagnostic laboratory immunology for talaromycosis (penicilliosis): Review from the bench-top techniques to the point-of-care testing. Diagn Microbiol. Infect. Dis. 2020, 96, 114959. [Google Scholar] [CrossRef]
- Kawila, R.; Chaiwarith, R.; Supparatpinyo, K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: A retrospective study. BMC Infect. Dis. 2013, 13, 464. [Google Scholar] [CrossRef]
- Hien, T.V.; Loc, P.P.; Hoa, N.T.; Duong, N.M.; Quang, V.M.; McNeil, M.M.; Dung, N.T.; Ashford, D.A. First cases of disseminated penicilliosis marneffei infection among patients with acquired immunodeficiency syndrome in Vietnam. Clin. Infect. Dis. 2001, 32, e78–e80. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Chen, X.; Hao, W.; Yang, M.; Cai, J.; Wang, Y.; Yuan, G.; Che, X. A double-antigen sandwich ELISA for detecting Penicillium marneffei Mp1p-specific antibody. Nan Fang Yi Ke Da Xue Xue Bao 2013, 33, 439–443. [Google Scholar] [PubMed]
- Cao, L.; Chan, K.M.; Chen, D.; Vanittanakom, N.; Lee, C.; Chan, C.M.; Sirisanthana, T.; Tsang, D.N.; Yuen, K.Y. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J. Clin. Microbiol. 1999, 37, 981–986. [Google Scholar] [CrossRef]
- Pornprasert, S.; Dettrairat, S.; Vongchan, P.; Apichatpiyakul, C. Production of a monoclonal antibody against a yeast secreted antigen of Penicillium marneffei. Southeast Asian J. Trop. Med. Public Health 2005, 36, 966–969. [Google Scholar]
- Cao, L.; Chan, C.M.; Lee, C.; Wong, S.S.; Yuen, K.Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 1998, 66, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Lau, C.C.; Tung, E.T.; Chong, K.T.; Yang, F.; Zhang, H.; Lo, R.K.; Cai, J.P.; Au-Yeung, R.K.; et al. Mp1p Is a Virulence Factor in Talaromyces (Penicillium) marneffei. PLoS Negl. Trop. Dis. 2016, 10, e0004907. [Google Scholar] [CrossRef]
- Ly, V.T.; Thanh, N.T.; Thu, N.T.M.; Chan, J.; Day, J.N.; Perfect, J.; Nga, C.N.; Vinh Chau, N.V.; Le, T. Occult Talaromyces marneffei Infection Unveiled by the Novel Mp1p Antigen Detection Assay. Open Forum. Infect. Dis. 2020, 7, ofaa502. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, D.L.; Lee, C.; Chan, C.M.; Chan, K.M.; Vanittanakom, N.; Tsang, D.N.; Yuen, K.Y. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 1998, 36, 3028–3031. [Google Scholar] [CrossRef]
- Hien, H.T.A.; Thanh, T.T.; Thu, N.T.M.; Nguyen, A.; Thanh, N.T.; Lan, N.P.H.; Simmons, C.; Shikuma, C.; Chau, N.V.V.; Thwaites, G.; et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses 2016, 59, 773–780. [Google Scholar] [CrossRef]
- Ning, C.; Lai, J.; Wei, W.; Zhou, B.; Huang, J.; Jiang, J.; Liang, B.; Liao, Y.; Zang, N.; Cao, C.; et al. Accuracy of rapid diagnosis of Talaromyces marneffei: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195569. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, X.; Calderone, R.; Zhang, J.; Ma, J.; Cai, W.; Xi, L. Whole blood Nested PCR and Real-time PCR amplification of Talaromyces marneffei specific DNA for diagnosis. Med. Mycol. 2016, 54, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Y.; Wu, F.; Mo, D.; Liang, G.; Yan, R.; Khader, J.A.; Wu, N.; Cao, C. Evaluation of quantitative real-time PCR and Platelia galactomannan assays for the diagnosis of disseminated Talaromyces marneffei infection. Med. Mycol. 2020, 58, 181–186. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Ai, J.W.; Xu, B.; Cui, P.; Cheng, Q.; Wu, H.; Qian, Y.Y.; Zhang, H.C.; Zhou, X.; Xing, L.; et al. Rapid and precise diagnosis of disseminated T.marneffei infection assisted by high-throughput sequencing of multifarious specimens in a HIV-negative patient: A case report. BMC Infect. Dis. 2018, 18, 379. [Google Scholar] [CrossRef]
- Grippi, M.A.; Elias, J.A.; Fishman, J.A.; Pack, A.I.; Senior, R.M.; Kotloff, R. Fishman’s Pulmonary Diseases and Disorders, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).