Blood Procalcitonin Level as a Diagnostic Marker of Pediatric Bacterial Meningitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
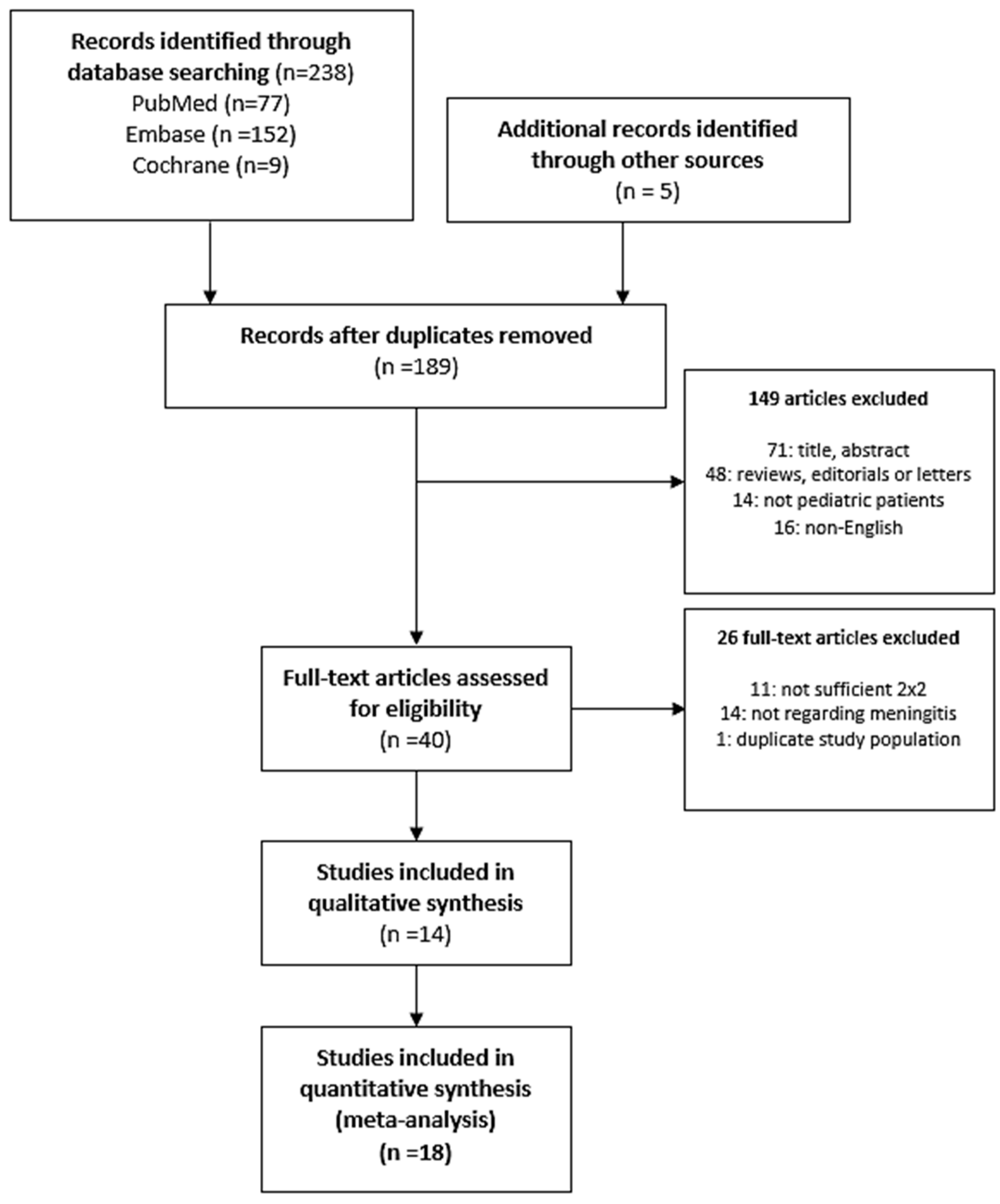
2.1. Search Strategy, Study Selection, and Eligibility Criteria
2.2. Data Extraction
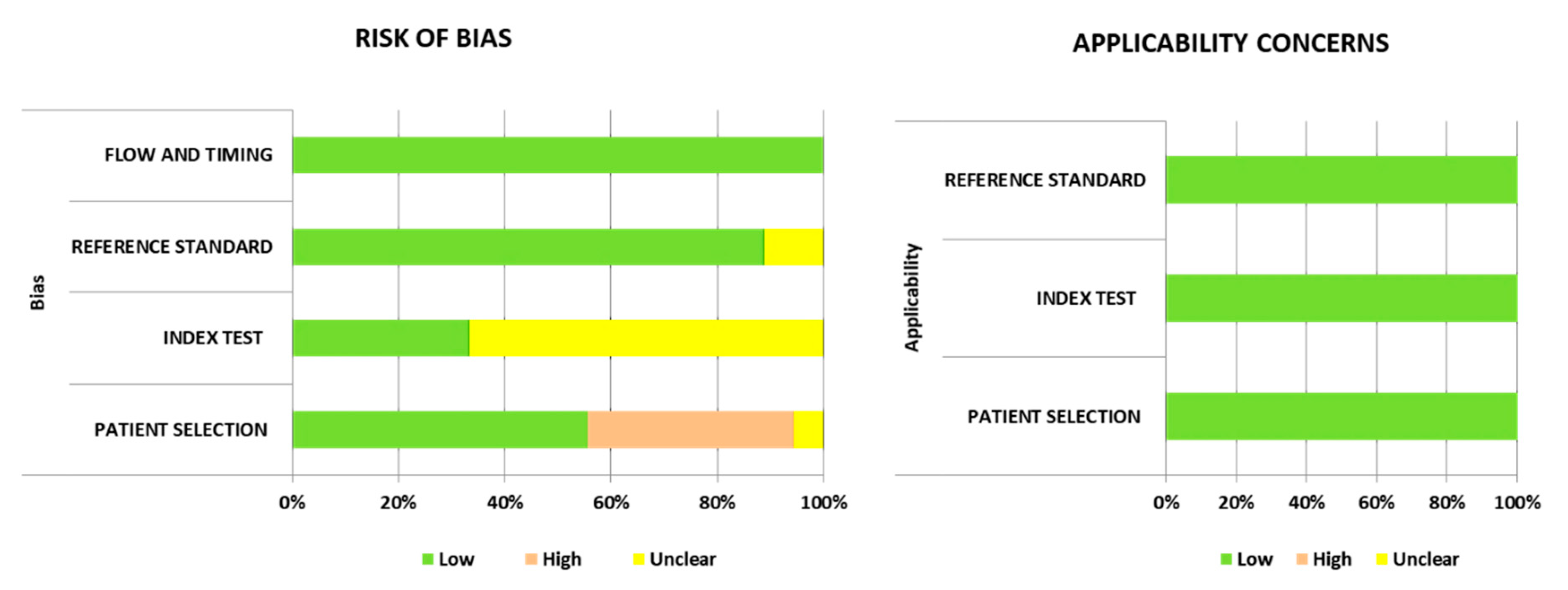
2.3. Quality Assessment
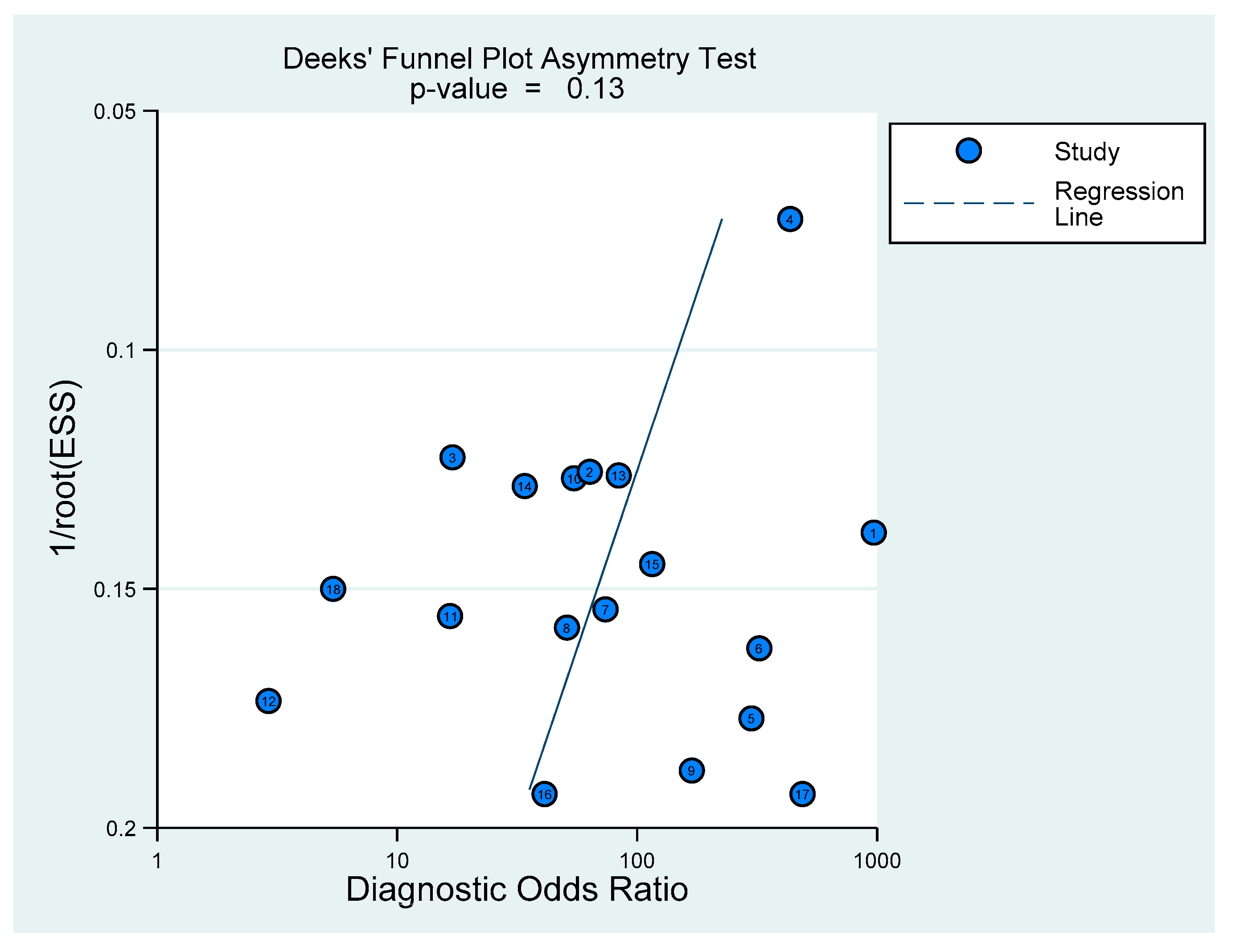
2.4. Statistical Analysis
3. Results
3.1. Characterization of the Studies
3.2. Quality Assessment of the Included Studies
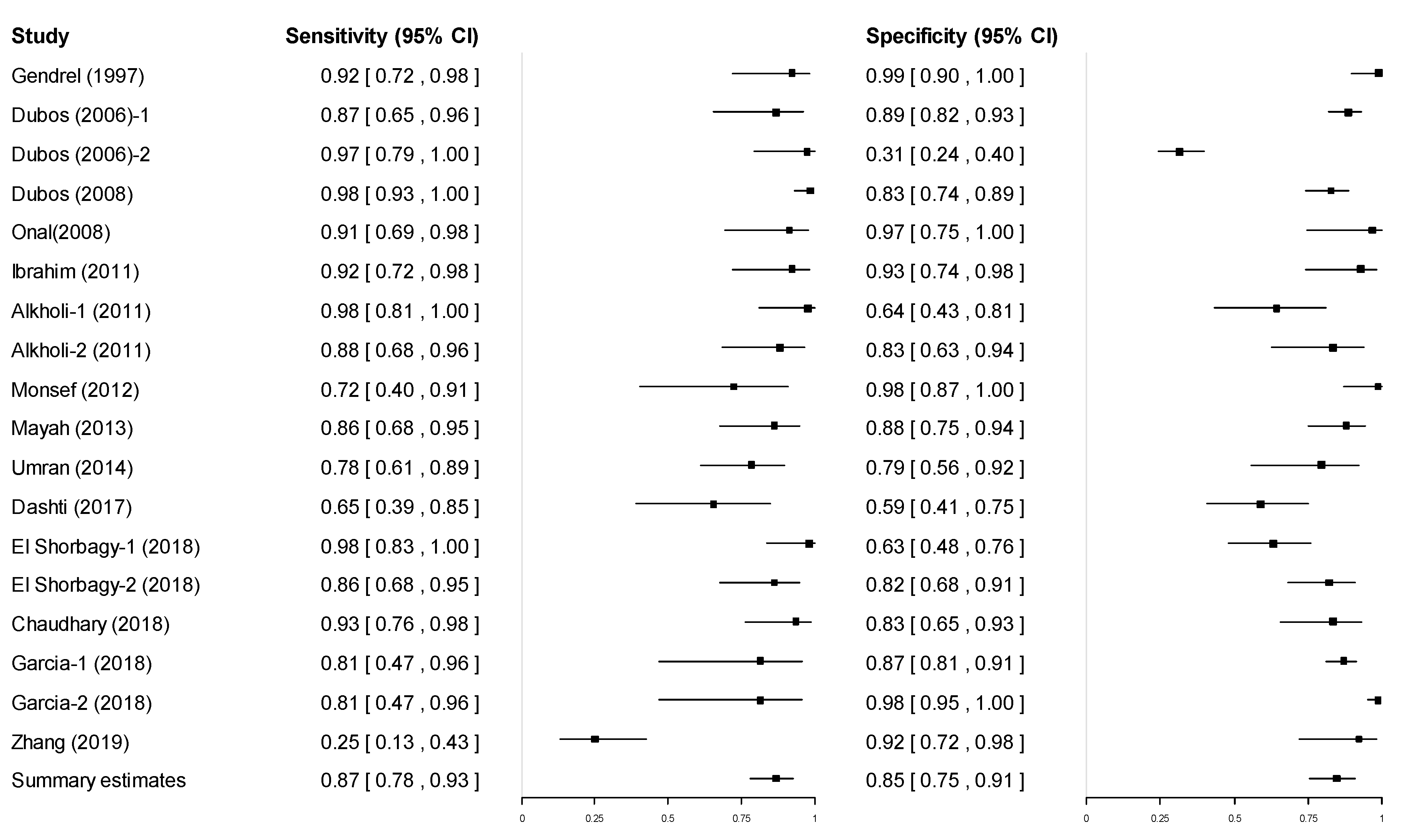
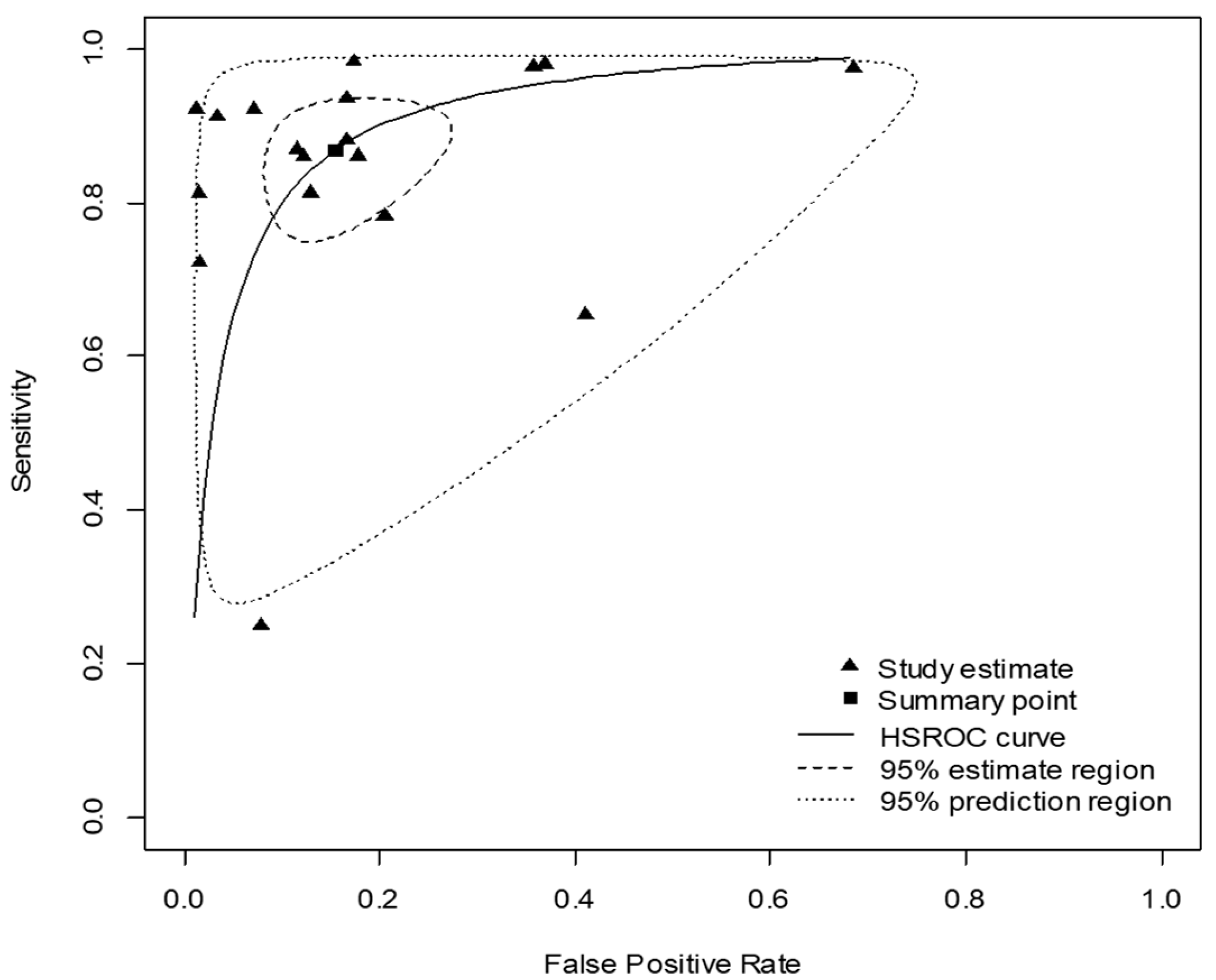
3.3. Pooled Diagnostic Accuracy of Procalcitonin
3.4. Subgroup Analysis according to the Cutoff Value
3.5. Comparison of Pooled Diagnostic Accuracy between Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.S. Acute bacterial meningitis in infants and children. Lancet Infect. Dis. 2010, 10, 32–42. [Google Scholar] [CrossRef]
- Hoffman, O.; Weber, R.J. Pathophysiology and treatment of bacterial meningitis. Ther. Adv. Neurol. Disord. 2009, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D. Meningitis. Pediatr Rev. 2015, 36, 514–524. [Google Scholar] [CrossRef]
- Thigpen, M.C.; Whitney, C.G.; Messonnier, N.E.; Zell, E.R.; Lynfield, R.; Hadler, J.L.; Harrison, L.H.; Farley, M.M.; Reingold, A.; Bennett, N.M.; et al. Bacterial Meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011, 364, 2016–2025. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Levine, O.S.; Schuchat, A.; Schwartz, B.; Wenger, J.D.; Elliott, J. Generic Protocol for Population-Based Surveillance of Haemophilus Influenzae Type B; World Health Organization: Geneva, Switzerland, 1996; Available online: https://apps.who.int/iris/handle/10665/64321 (accessed on 30 March 2020).
- Lee, T.J.; Aronson, P.L. To Spinal Tap or Not To Spinal Tap, That Is the Question. Hosp. Pediatr. 2018, 8, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; Smith, P.B.; Cotten, C.M.; Moody, M.A.; Clark, R.H.; Benjamin, D.K., Jr. Traumatic lumbar punctures in neonates: Test performance of the cerebrospinal fluid white blood cell count. Pediatr. Infect. Dis. J. 2008, 27, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.T.; Thao, N.T.; Diep, D.T.; Kikuchi, M.; Zamora, J.; Hirayama, K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: A systemic review and meta-analysis. Crit. Care 2010, 14, R240. [Google Scholar] [CrossRef] [PubMed]
- Sanaei Dashti, A.; Alizadeh, S.; Karimi, A.; Khalifeh, M.; Shoja, S.A. Diagnostic value of lactate, procalcitonin, ferritin, serum-c-reactive protein, and other biomarkers in bacterial and viral meningitis: A cross-sectional study. Medicine 2017, 96, e7637. [Google Scholar] [CrossRef] [PubMed]
- Julián-Jiménez, A.; Morales-Casado, M. Usefulness of blood and cerebrospinal fluid laboratory testing to predict bacterial meningitis in the emergency department. Neurología 2019, 34, 105–113. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Tseng, C.-P.; Tsay, P.-K.; Chang, S.-S.; Chiu, T.-F.; Chen, J.-C. Procalcitonin as a marker of bacterial infection in the emergency department: An observational study. Crit. Care 2004, 8, R12–R20. [Google Scholar] [CrossRef] [PubMed]
- Magrini, L.; Gagliano, G.; Travaglino, F.; Vetrone, F.; Marino, R.; Cardelli, P.; Salerno, G.; Di Somma, S. Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin. Chem. Lab. Med. 2014, 52, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.M.; Trompet, S.; Blauw, G.J.; Westendorp, R.G.J.; De Craen, A.J.M. White Blood Cell Count and C-Reactive Protein Are Independent Predictors of Mortality in the Oldest Old. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2010, 65, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.-O.; Axelsson, G.; Linne, T.; Aurelius, E.; Lindquist, L. Serum C-reactive Protein in the Differential Diagnosis of Acute Meningitis. Scand. J. Infect. Dis. 1993, 25, 625–630. [Google Scholar] [CrossRef]
- Dubos, F.; Korczowski, B.; Aygun, D.A.; Martinot, A.; Prat, C.; Galetto-Lacour, A.; Casado-Flores, J.; Taskin, E.; Leclerc, F.; Rodrigo, C.; et al. Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children: A european multicenter case cohort study. Arch. Pediatr. Adolesc. Med. 2008, 162, 1157–1163. [Google Scholar] [CrossRef]
- Samsudin, I.; Vasikaran, S.D. Clinical Utility and Measurement of Procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar]
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive. Care. 2017, 5, 51. [Google Scholar] [CrossRef]
- Covington, E.W.; Roberts, M.Z.; Dong, J. Procalcitonin Monitoring as a Guide for Antimicrobial Therapy: A Review of Current Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 569–581. [Google Scholar] [CrossRef]
- Schneider, H.-G.; Lam, Q.T. Procalcitonin for the clinical laboratory: A review. Pathol. 2007, 39, 383–390. [Google Scholar] [CrossRef]
- Henry, B.M.; Roy, J.; Ramakrishnan, P.K.; Vikse, J.; Tomaszewski, K.A.; Walocha, J.A. Procalcitonin as a serum biomarker for differentiation of bacterial meningitis from viral meningitis in children: Evidence from a meta-analysis. Clin. Pediatr. (Phila) 2016, 55, 749–764. [Google Scholar] [CrossRef]
- Vikse, J.; Henry, B.M.; Roy, J.; Ramakrishnan, P.K.; Tomaszewski, K.A.; Walocha, J.A. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2015, 38, 68–76. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Tacon, C.L.; Flower, O. Diagnosis and Management of Bacterial Meningitis in the Paediatric Population: A Review. Emerg. Med. Int. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Griffin, D.E. Chapter 20—bacterial infections. In Cerebrospinal Fluid in Clinical Practice; Irani, D.N., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 167–175. Available online: https://doi.org/10.1016/B978-141602908-3.50023-6 (accessed on 30 March 2020).
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.M.; The QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Gendrel, D.; Raymond, J.; Assicot, M.; Moulin, F.; Iniguez, J.; Lebon, P.; Bohuon, C. Measurement of Procalcitonin Levels in Children with Bacterial or Viral Meningitis. Clin. Infect. Dis. 1997, 24, 1240–1242. [Google Scholar] [CrossRef]
- Dubos, F.; Moulin, F.; Gajdos, V.; De Suremain, N.; Biscardi, S.; Lebon, P.; Raymond, J.; Breart, G.; Gendrel, D.; Chalumeau, M. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J. Pediatr. 2006, 149, 72–76. [Google Scholar] [CrossRef]
- Onal, H.; Onal, Z.; Ozdil, M.; Alhaj, S. A new parameter in the differential diagnosis of bacterial and viral meningitis. Neurosciences 2008, 13, 91–92. [Google Scholar] [PubMed]
- Ibrahim, K.A.; Abdel-Wahab, A.A.; Ibrahim, A.S. Diagnostic value of serum procalcitonin levels in children with meningitis: A comparison with blood leukocyte count and C-reactive protein. J. Pak. Med. Assoc. 2011, 61, 346–351. [Google Scholar] [PubMed]
- El-Azim, A.A.A.; Sultan, M.H.; Alkholi, U.M.; Al-Monem, N.A. Serum procalcitonin in viral and bacterial meningitis. J. Glob. Infect. Dis. 2011, 3, 14–18. [Google Scholar] [CrossRef]
- Monsef, A.; Eghbalian, F. Evaluation of Diagnostic Value of Procalcitonin as a Marker of Neonatal Bacterial Infections. Iran. J. Pediatr. 2012, 22, 314–318. [Google Scholar]
- El-Yamany, S.; Mayah, W.W.; Jiman-Fatani, A.; El Saadany, S.; Hassanien, M.; Hasan, A.; Abo-Hagar, H. Study of different diagnostic markers used to differentiate septic from aseptic meningitis. J. Microsc. Ultrastruct. 2013, 1, 35–42. [Google Scholar] [CrossRef]
- Umran, R.M.; Radhi, N.H. Diagnostic Value of Serum Procalcitonin Level in Differentiating Bacterial from Nonbacterial Meningitis in Children. Iran. J. Pediatr. 2014, 24, 739–744. [Google Scholar]
- El Shorbagy, H.H.; Barseem, N.F.; AbdelGhani, W.E.; Suliman, H.A.; Al-Shokary, A.H.; Elsadek, A.E.; Maksoud, Y.H.A.; Sabri, J.H. The value of serum procalcitonin in acute meningitis in children. J. Clin. Neurosci. 2018, 56, 28–33. [Google Scholar] [CrossRef]
- Chaudhary, S.; Bhatta, N.K.; Lamsal, M.; Chaudhari, R.K.; Khanal, B. Serum procalcitonin in bacterial & non-bacterial meningitis in children. BMC Pediatr. 2018, 18, 342. [Google Scholar] [CrossRef]
- Garcia, S.; Echevarri, J.; Arana-Arri, E.; Sota, M.; Benito, J.; Mintegi, S. Outpatient management of children at low risk for bacterial meningitis. Emerg. Med. J. 2018, 35, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, L.; Zhou, X.; Meng, J.; Wen, J.; Huang, R.; Gao, T.; Xu, L.; Zhu, L. Diagnostic Value of Procalcitonin for Bacterial Meningitis in Children: A Comparison Analysis Between Serum and Cerebrospinal Fluid Procalcitonin Levels. Clin. Pediatr. 2018, 58, 159–165. [Google Scholar] [CrossRef]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- Markanday, A. Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open Forum Infect. Dis. 2015, 2, ofv098. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- A Scirè, C.; Cavagna, L.; Perotti, C.; Bruschi, E.; Caporali, R.; Montecucco, C. Diagnostic value of procalcitonin measurement in febrile patients with systemic autoimmune diseases. Clin. Exp. Rheumatol. 2006, 24, 123–128. [Google Scholar]
- Park, A.; Anderson, D.; Battaglino, R.A.; Nguyen, N.; Morse, L.R. Ibuprofen use is associated with reduced C-reactive protein and interleukin-6 levels in chronic spinal cord injury. J. Spinal Cord Med. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uyl, D.D.; Van Raalte, D.H.; Nurmohamed, M.T.; Lems, W.F.; Bijlsma, J.W.J.; Hoes, J.N.; Dijkmans, B.A.C.; Diamant, M. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: Balance between diabetogenic effects and inflammation reduction. Arthritis Rheum. 2012, 64, 639–646. [Google Scholar] [CrossRef]
- Lee, H. Procalcitonin as a biomarker of infectious diseases. Korean J. Intern. Med. 2013, 28, 285–291. [Google Scholar] [CrossRef]
- Mount, H.R.; Boyle, S.D. Aseptic and Bacterial Meningitis: Evaluation, Treatment, and Prevention. Am. Fam. Physician 2017, 96, 314–322. [Google Scholar] [PubMed]
- Bilavsky, E.; Leibovitz, E.; Elkon-Tamir, E.; Fruchtman, Y.; Ifergan, G.; Greenberg, D. The diagnostic accuracy of the ‘classic meningeal signs’ in children with suspected bacterial meningitis. Eur. J. Emerg. Med. 2013, 20, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Uchihara, T.; Tsukagoshi, H. Jolt Accentuation of Headache: The Most Sensitive Sign of CSF Pleocytosis. Headache: J. Head Face Pain 1991, 31, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, L.E.; Kuppermann, N.; Neuman, M.I. Risk Factors for Traumatic or Unsuccessful Lumbar Punctures in Children. Ann. Emerg. Med. 2007, 49, 762–771. [Google Scholar] [CrossRef]
- Glatstein, M.M.; Zucker-Toledano, M.; Arik, A.; Scolnik, D.; Oren, A.; Reif, S. Incidence of traumatic lumbar puncture: Experience of a large, tertiary care pediatric hospital. Clin. Pediatr. (Phila) 2011, 50, 1005–1009. [Google Scholar] [CrossRef]
- Kessler, D.; Pahalyants, V.; Kriger, J.; Behr, G.; Dayan, P. Preprocedural Ultrasound for Infant Lumbar Puncture: A Randomized Clinical Trial. Acad. Emerg. Med. 2018, 25, 1027–1034. [Google Scholar] [CrossRef]
- Elenius, V.; Peltola, V.; Ruuskanen, O.; Ylihärsilä, M.; Waris, M. Plasma procalcitonin levels in children with adenovirus infection. Arch. Dis. Child. 2011, 97, 582–583. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Müller, B. Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med. Wkly. 2005, 135, 451–460. [Google Scholar] [PubMed]
- Hsiao, A.L.; Baker, M.D. Fever in the new millennium: A review of recent studies of markers of serious bacterial infection in febrile children. Curr. Opin. Pediatr. 2005, 17, 56–61. [Google Scholar] [CrossRef]
- Hu, R.; Gong, Y.; Wang, Y. Relationship of Serum Procalcitonin Levels to Severity and Prognosis in Pediatric Bacterial Meningitis. Clin. Pediatr. 2015, 54, 1141–1144. [Google Scholar] [CrossRef]
- Enguix, A.; Rey, C.; Concha, A.; Medina, A.; Coto, D.; Diéguez, M.A. Comparison of procalcitonin with C-reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensiv. Care Med. 2001, 27, 211–215. [Google Scholar] [CrossRef]
- Nabulsi, M.; Hani, A.; Karam, M. Impact of C-reactive protein test results on evidence-based decision-making in cases of bacterial infection. BMC Pediatr. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ishida, T.; Tokumasu, H.; Washio, Y.; Yamazaki, A.; Ito, Y.; Tachibana, H. Impact of procalcitonin-guided therapy for hospitalized community-acquired pneumonia on reducing antibiotic consumption and costs in Japan. J. Infect. Chemother. 2017, 23, 142–147. [Google Scholar] [CrossRef]
- Mewes, J.C.; Pulia, M.S.; Mansour, M.K.; Broyles, M.R.; Nguyen, H.B.; Steuten, L.M. The cost impact of PCT-guided antibiotic stewardship versus usual care for hospitalised patients with suspected sepsis or lower respiratory tract infections in the US: A health economic model analysis. PLoS ONE 2019, 14, e0214222. [Google Scholar] [CrossRef]
- Collins, C.D.; Brockhaus, K.; Sim, T.; Suneja, A.; Malani, A.N. Analysis to determine cost-effectiveness of procalcitonin-guided antibiotic use in adult patients with suspected bacterial infection and sepsis. Am. J. Heal. Pharm. 2019, 76, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Roubille, M.; Szymanowicz, A.; Cartier, B.; Albinet, H.; Carlier, A.; Goux, A.; Lefevre, F.; Pellae, I.; Rozand, I.; Billion, P.; et al. Study on turnaround time of biological analysis in urgent need in hospital laboratories. Ann. Biol. Clin. (Paris) 2010, 68, 741–746. [Google Scholar]
- Steinbach, G.; Rau, B.; Debard, A.-L.; Javourez, J.-F.; Bienvenu, J.; Ponzio, A.; Bonfà, A.; Hubl, W.; Demant, T.; Külpmann, W.-R.; et al. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor® System. Clin. Chem. Lab. Med. 2004, 42, 440–449. [Google Scholar] [CrossRef]
- Fortunato, A. A new sensitive automated assay for procalcitonin detection: LIAISON® BRAHMS PCT® II GEN. Pr. Lab. Med. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year), Reference | Country | BM (n) | Non-BM (n) | Sample Type | Cutoff (ng/mL) | PCT Assay | Time of PCT Assessment |
|---|---|---|---|---|---|---|---|
| Gendrel (1997) [27] | France | 18 | 41 | Plasma | 5 | LUMItest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Dubos-1 (2006) [28] | France | 18 | 134 | Serum | 0.5 | LUMItest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Dubos-2 (2006) [28] | France | 18 | 134 | Serum | 0.2 | LUMItest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Dubos (2008) [16] | Switzerland, France, Spain, Turkey, Poland | 90 | 100 | Serum | 0.5 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Onal (2008) [29] | Turkey | 16 | 14 | Plasma | 0.5 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Ibrahim (2011) [30] | KSA | 18 | 20 | Serum | 0.5 | Immunoluminometric assay (Brahms Diagnostica, Berlin, Germany) | On admission |
| Alkholi-1 (2011) [31] | Egypt | 20 | 20 | Serum | 2 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On diagnosis |
| Alkholi-2 (2011) [31] | Egypt | 20 | 20 | Serum | 10 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On diagnosis |
| Monsef (2012) [32] | Iran | 8 | 32 | Serum | 0.5 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | Before antibiotic therapy |
| Mayah (2013) [33] | Egypt | 24 | 44 | Serum | 3.3 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On admission |
| Umran (2014) [34] | Iraq | 29 | 16 | Serum | 0.05 | ELIZA M6 (NA, USA) | On admission |
| Sanaei Dashti (2017) [10] | Iran | 12 | 27 | Serum | 0.6 | Human PCT ELISA (BT Laboratory, Shanghai, China) | On admission |
| El Shorbagy-1 (2018) [35] | KSA | 24 | 41 | Serum | 2 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On diagnosis |
| El Shorbagy-2 (2018) [35] | KSA | 24 | 41 | Serum | 10 | Lumitest PCT (Brahms Diagnostica, Berlin, Germany) | On diagnosis |
| Chaudhary (2018) [36] | Nepal | 22 | 26 | Serum | 0.5 | Maglumi PCT (Snibe Diagnostics, Shenzhen, China) | On admission |
| Garcia-1 (2018) [37] | Spain | 7 | 165 | NA | 0.5 | NA | At the time of ED visit |
| Garcia-2 (2018) [37] | Spain | 7 | 165 | NA | 2 | NA | At the time of ED visit |
| Zhang (2019) [38] | China | 29 | 18 | Serum | 5.91 | VIDAS BRAHMS PCT (Biomerieux, Marcy l’Etoile, France) | On admission |
| Cutoff | Number of Studies | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR− (95% CI) | DOR (95% CI) | AUC |
|---|---|---|---|---|---|---|---|
| ≤0.5 pg/mL | 9 | 0.899 (0.81–0.949) | 0.844 (0.702–0.96) | 5.763 (2.718–23.725) | 0.12 (0.271–0.053) | 48.157 (10.043–446.588) | 0.935 |
| >0.5 pg/mL | 9 | 0.831 (0.647–0.93) | 0.851 (0.706–0.931) | 5.577 (2.201–13.478) | 0.199 (0.5–0.075) | 28.084 (4.401–179.261) | 0.908 |
| Biomarkers | Number of Studies | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR− (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|
| CRP | 10 | 0.797 (0.741–0.844) | 0.725 (0.665–0.777) | 2.894 (2.213–3.781) | 0.28 (0.39–0.201) | 10.334 (5.679–18.808) |
| WBCs | 5 | 0.659 (0.504–0.786) | 0.713 (0.587–0.813) | 2.294 (1.22–4.195) | 0.479 (0.845–0.263) | 4.794 (1.443–15.925) |
| CSF WBCs | 4 | 0.733 (0.601–0.834) | 0.669 (0.58–0.748) | 2.217 (1.43–3.309) | 0.399 (0.689–0.223) | 5.556 (2.076–14.87) |
| CSF neutrophils | 4 | 0.793 (0.377–0.96) | 0.749 (0.519–0.892) | 3.158 (0.784–8.895) | 0.277 (1.201–0.045) | 11.403 (0.652–199.271) |
| CSF protein | 4 | 0.838 (0.699–0.92) | 0.658 (0.55–0.753) | 2.452 (1.552–3.718) | 0.247 (0.548–0.107) | 9.934 (2.833–34.833) |
| CSF glucose | 3 | 0.563 (0.172–0.889) | 0.193 (0.145–0.253) | 0.698 (0.201–1.189) | 2.264 (5.718–0.44) | 0.308 (0.035–2.701) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Roh, Y.-H.; Yoon, S.-H. Blood Procalcitonin Level as a Diagnostic Marker of Pediatric Bacterial Meningitis: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 846. https://doi.org/10.3390/diagnostics11050846
Kim H, Roh Y-H, Yoon S-H. Blood Procalcitonin Level as a Diagnostic Marker of Pediatric Bacterial Meningitis: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(5):846. https://doi.org/10.3390/diagnostics11050846
Chicago/Turabian StyleKim, Heeyeon, Yun-Ho Roh, and Seo-Hee Yoon. 2021. "Blood Procalcitonin Level as a Diagnostic Marker of Pediatric Bacterial Meningitis: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 5: 846. https://doi.org/10.3390/diagnostics11050846
APA StyleKim, H., Roh, Y.-H., & Yoon, S.-H. (2021). Blood Procalcitonin Level as a Diagnostic Marker of Pediatric Bacterial Meningitis: A Systematic Review and Meta-Analysis. Diagnostics, 11(5), 846. https://doi.org/10.3390/diagnostics11050846






