Fecal Metabolomics Reveals Distinct Profiles of Kidney Transplant Recipients and Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. GC–MS Sample Preparation and Metabolites Extraction
2.3. GC–MS Analysis and Metabolites Detection
2.4. Identification and Comparison of Volatile Compounds
2.5. Data Analysis
2.6. Selection of Biomarkers
3. Results
3.1. Clinical Data of Kidney Allografts Patients
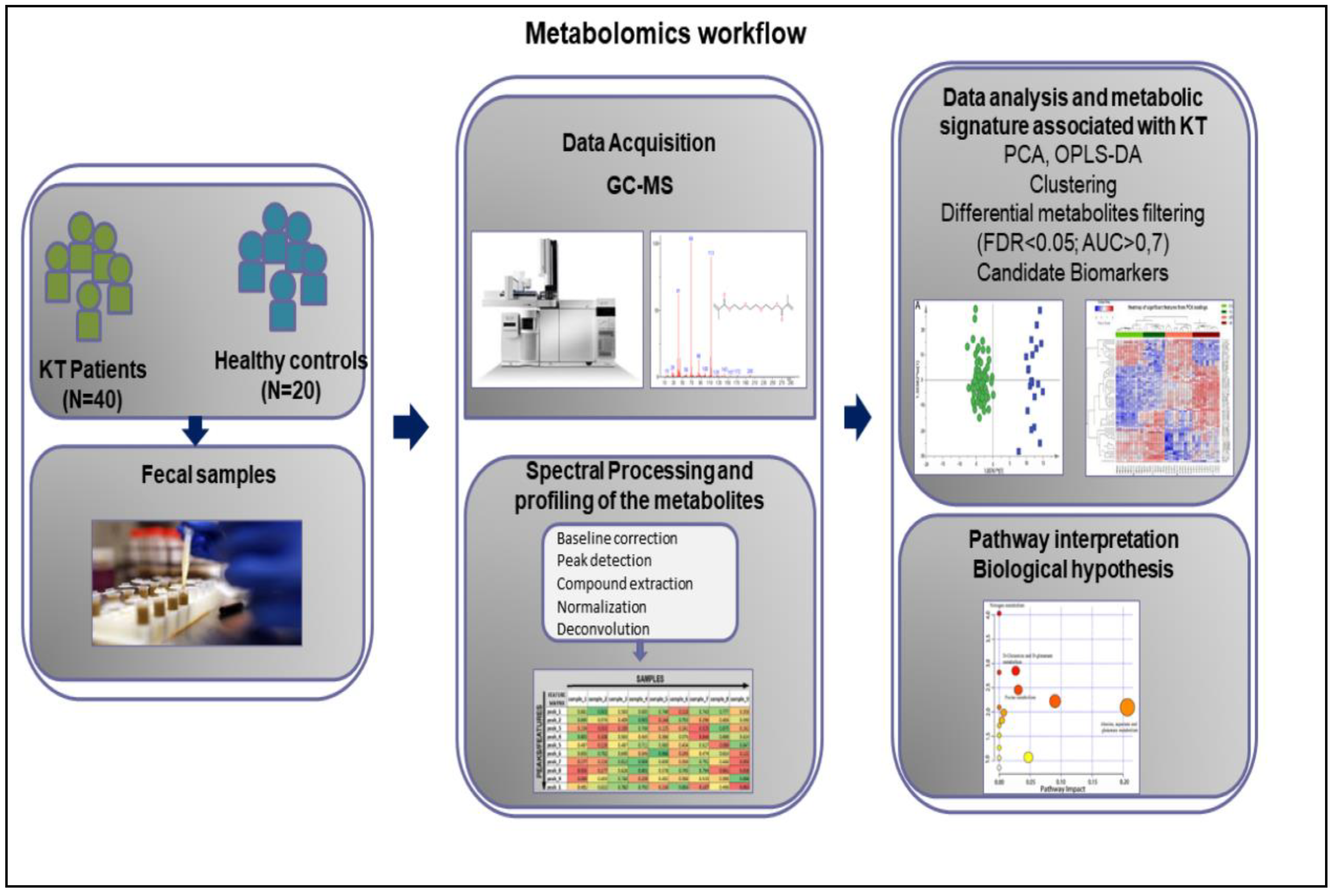
3.2. Metabolomics Workflow
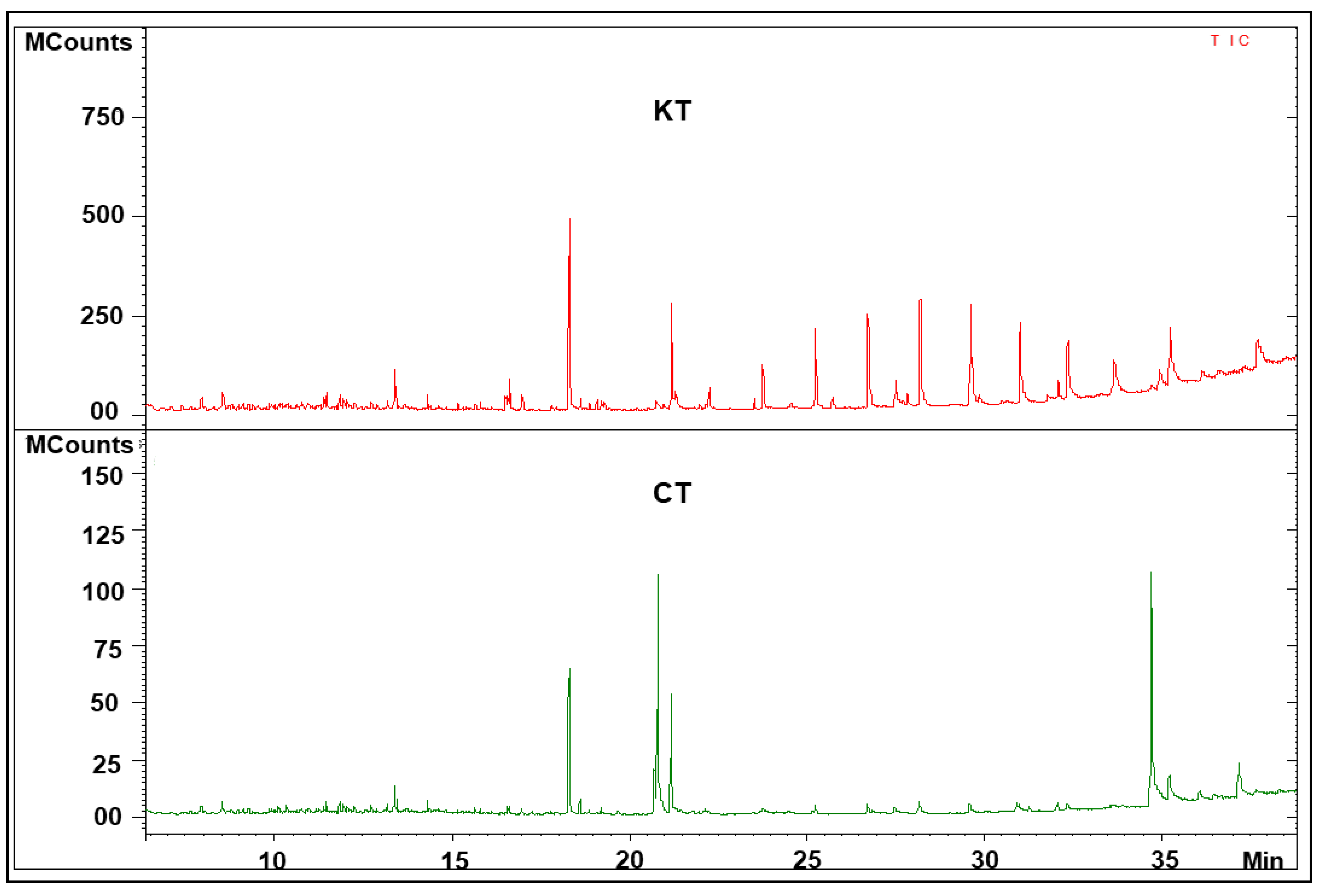
3.3. Analysis of Fecal Metabolic Profiling by GC–MS
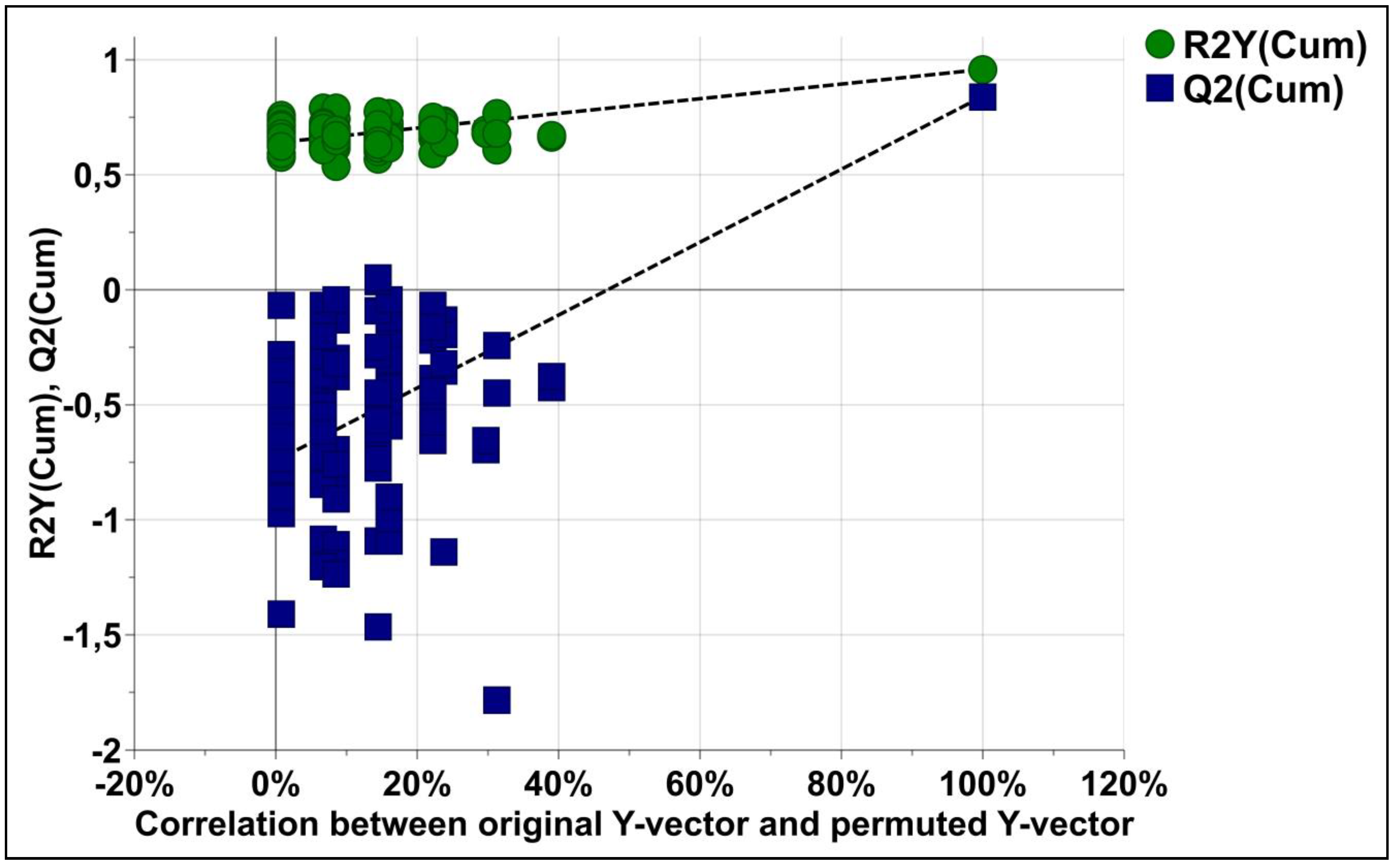
3.4. Fecal Metabolic Profiles of KT Patients and Healthy Individuals Are Different
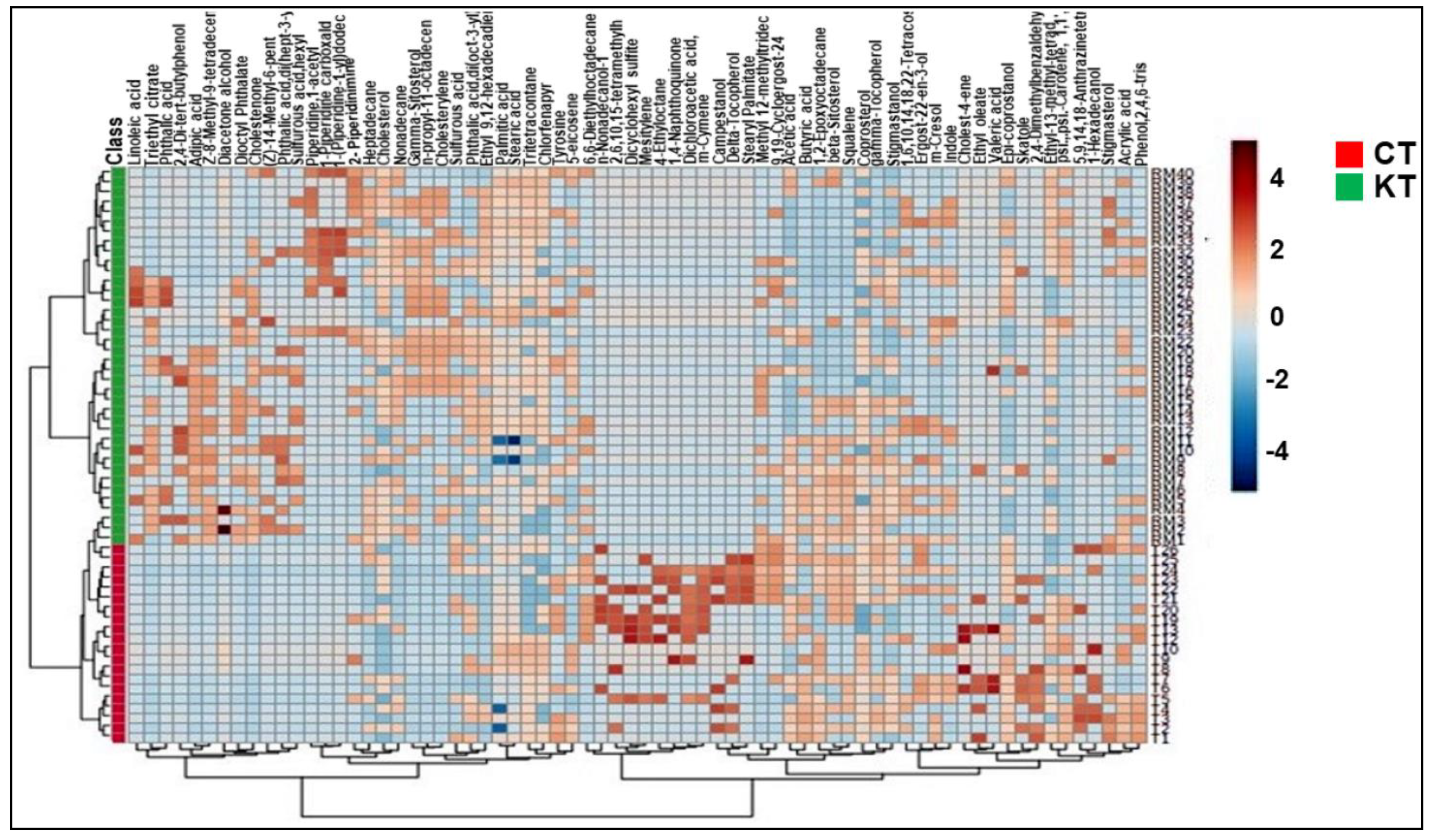
3.5. Identification of Potential Biomarkers of Kidney Transplants Status and Biological Explanation
4. Discussion
4.1. Relevant Metabolites Highlighted
4.2. Gut Microbiota-Derived Metabolites
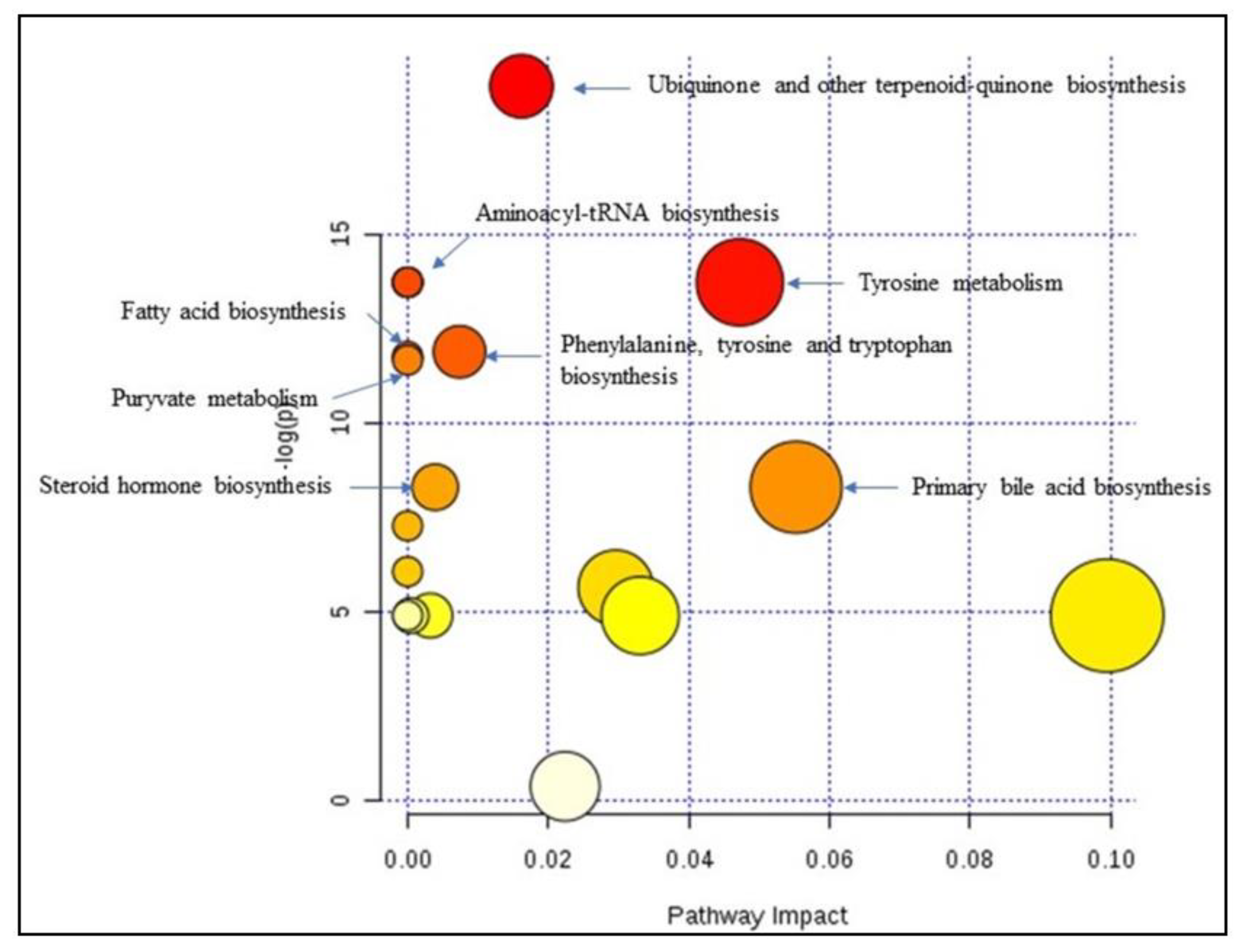
4.3. Relevant Pathways Highlighted
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Consent to Participate
Consent for Publication
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholas, S.B.; Kalantar-Zadeh, K.; Norris, K.C. Socioeconomic Disparities in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2015, 22, 6–15. [Google Scholar] [CrossRef]
- Fishbane, S.; Hazzan, A.D.; Halinski, C.; Mathew, A.T. Challenges and opportunities in late-stage chronic kidney disease. Clin. Kidney J. 2015, 8, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Satanovskij, R.; Angermann, S.; Hasenau, A.-L.; Stecher, L.; Heemann, U. Plasma Uromodulin Correlates with Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine (Baltimore) 2016, 95, 3011. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef]
- Perkins, B.A.; Ficociello, L.H.; Roshan, B.; Warram, J.H.; Krolewski, A.S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010, 77, 57–64. [Google Scholar] [CrossRef]
- Williams, W.W.; Taheri, D.; Tolkoff-Rubin, N.; Colvin, R.B. Clinical role of the renal transplant biopsy. Nat. Rev. Nephrol. 2012, 8, 110–121. [Google Scholar] [CrossRef]
- Roig, B.; Rodríguez-Balada, M.; Samino, S.; Lam, E.W.-F.; Guaita-Esteruelas, S.; Gomes, A.R.; Correig, X.; Borràs, J.; Yanes, O.; Gumà, J. Metabolomics reveals novel blood plasma biomarkers associated to the BRCA1-mutated phenotype of human breast cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Cheng, J.; Lan, W.; Zheng, G.; Gao, X. Metabolomics: A High-Throughput Platform for Metabolite Profile Exploration. In Computational System Biology; Huang, T., Ed.; Springer: New York, NY, USA, 2018; pp. 265–292. [Google Scholar] [CrossRef]
- Barrios, C.; Spector, T.D.; Menni, C. Blood, urine and faecal metabolite profiles in the study of adult renal disease. Arch. Biochem. Biophys. 2016, 589, 81–92. [Google Scholar] [CrossRef]
- Kalantari, S.; Nafar, M. An update of urine and blood metabolomics in chronic kidney disease. Biomark. Med. 2019, 13, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, H.; Maker, G.; Trengove, R. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, D.-Q.; Chen, L.; Liu, J.-R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Hecking, M.; Kainz, A.; Werzowa, J.; Haidinger, M.; Doller, D.; Tura, A.; Karaboyas, A.; Horl, W.H.; Wolzt, M.; Sharif, A.; et al. Glucose metabolism after renal transplantation. Diabetes Care 2013, 36, 2763–2771. [Google Scholar] [CrossRef]
- Egestad, B.; Pettersson, P.; Sjövall, J.; Rafter, J.; Hyvönen, K.; Gustafsson, J. Studies on the chromatographic fractionation of metabolites of benzo[a]pyrene in faeces and urine from germfree and conventional rats. Biomed. Chromatogr. 1987, 2, 120–134. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Chen, X.; Chen, C.; Xia, Y.; Qi, H.; Baker, P.N.; Zhang, H.; Han, T.-L. Evaluating different extraction solvents for GC-MS based metabolomic analysis of the fecal metabolome of adult and baby giant pandas. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: The combination of targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016, 114, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, X.; Zhou, Q.; Villanueva, N.; Nian, W.; Liu, X.; Huan, T. Metabolomics-Based Discovery of Molecular Signatures for Triple Negative Breast Cancer in Asian Female Population. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, 1–128. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S.; Valencia, A. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2011, 27, 2342–2344. [Google Scholar] [CrossRef]
- Kienana, M.; Lydie, N.-D.; Jean-Michel, H.; Binta, D.; Matthias, B.; Patrick, E.; Hélène, B.; Chantal, G. Elucidating time-dependent changes in the urinary metabolome of renal transplant patients by a combined (1) H NMR and GC-MS approach. Mol. Biosyst. 2015, 11, 2493–2510. [Google Scholar] [CrossRef]
- Kisielnicka, E.; Zdrojewski, Z.; Wróblewska, M.; Kortas, B.; Rutkowski, B. Lipid disturbances in a two-year follow-up after successful kidney transplantation. Transplant. Proc. 2000, 32, 1358–1362. [Google Scholar] [CrossRef]
- Chmielewski, M.; Zdrojewski, Z.; Rutkowski, B. Benefits and menaces related to the use of statins in patients after renal transplantation. Ann. Transplant. 2002, 7, 6–10. [Google Scholar] [PubMed]
- Draper, N.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef]
- Izem, L.; Morton, R.E. Cholesteryl ester transfer protein biosynthesis and cellular cholesterol homeostasis are tightly interconnected. J. Biol. Chem. 2001, 276, 26534–26541. [Google Scholar] [CrossRef] [PubMed]
- Holdaas, H.; Fellström, B.; Jardine, A.G.; Holme, I.; Nyberg, G.; Fauchald, P.; Grönhagen-Riska, P.; Madsen, S.; Neumayer, H.-H.; Cole, E.; et al. Pedersen, Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators, Effect of fluvastatin on cardiac outcomes in renal transplant recipients: A multicentre, randomised, placebo-controlled trial. Lancet 2003, 361, 2024–2031. [Google Scholar] [CrossRef]
- Ceglarek, U.; Kresse, K.; Becker, S.; Fiedler, G.M.; Thiery, J.; Quante, M.; Wieland, R.; Bartels, M.; Aust, G. Circulating sterols as predictors of early allograft dysfunction and clinical outcome in patients undergoing liver transplantation Metabolomics. Off. J. Metabolomic. Soc. 2016, 12, 182. [Google Scholar] [CrossRef]
- Bonneau, E.; Tétreault, N.; Robitaille, R.; Boucher, A.; De Guire, V. Metabolomics: Perspectives on potential biomarkers in organ transplantation and immunosuppressant toxicity. Clin. Biochem. 2016, 49, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prasad, G.V.R. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 125–134. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid Metabolism and Toxicity in the Heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef]
- Eide, I.A.; Dörje, C.; Svensson, M.; Jenssen, T.; Hammarstrøm, C.; Scott, H.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Hartmann, A.; et al. Development of Kidney Transplant Fibrosis Is Inversely Associated with Plasma Marine Fatty Acid Level. J. Ren. Nutr. 2018, 28, 118–124. [Google Scholar] [CrossRef]
- Szczuko, M.; Kaczkan, M.; Drozd, A.; Maciejewska, D.; Palma, J.; Owczarzak, A.; Marczuk, N.; Rutkowski, N.; Małgorzewicz, S. Comparison of Fatty Acid Profiles in a Group of Female Patients with Chronic Kidney Diseases (CKD) and Metabolic Syndrome (MetS)–Similar Trends of Changes, Different Pathophysiology. Int. J. Mol. Sci. 2019, 20, 1719. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Browne, D.; Williams, M.A.; Maxwell, A.P.; McGuinness, B.; Passmore, P.; Silvestri, S.; Woodside, J.V.; McKay, G.J. Serum xanthophyll carotenoids are associated with estimated glomerular filtration rate in an aged cohort. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. Med. Plants 2019, 333–359. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Souai, N.; Zidi, O.; Mosbah, A.; Kosai, I.; El Manaa, J.; Mokhart, N.B.; Asimakis, E.; Stathopoluou, P.; Cherif, A.; Tsiamis, G.; et al. Impact of the Post-Transplant Period and Lifestyle Diseases on Human Gut Microbiota in Kidney Graft Recipients. Microorganisms 2020, 8, 1724. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacteriumprausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases, Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Mantle, D.; Hargreaves, I. Coenzyme Q10 and Degenerative Disorders Affecting Longevity: An Overview. Antioxidants 2019, 8, 44. [Google Scholar] [CrossRef]
- Koople, J.D. Phenylalanine and Tyrosine Metabolism in Chronic Kidney Failure. J. Nutr. 2007, 137. [Google Scholar] [CrossRef]
- Boirie, Y.; Albright, R.; Bigelow, N.; Nair, K.S. Impairment of phenylalanine conversion to tyrosine in end-stage renal disease causing tyrosine deficiency. Kidney Int. 2004, 66, 591–596. [Google Scholar] [CrossRef]
- Debnath, S.; Velagapudi, C.; Redus, L.; Thameem, F.; Kasinath, B.; Hura, C.E.; Lorenzo, C.; Abboud, H.E.; O’Connor, J.C. Tryptophan Metabolism in Patients with Chronic Kidney Disease Secondary to Type 2 Diabetes: Relationship to Inflammatory Markers. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef]
- Aregger, F.; Uehlinger, D.E.; Fusch, G.; Bahonjic, A.; Pschowski, R.; Walter, M.; Schefold, J.C. Increased urinary excretion of kynurenic acid is associated with non-recovery from acute kidney injury in critically ill patients. BMC Nephrol. 2018, 19. [Google Scholar] [CrossRef]
- Jimenez, F.; Monte, M.J.; El-Mir, M.Y.; Pascual, M.J.; Marin, J.J.G. Chronic renal failure-induced changes in serum and urine bile acid profiles. Dig. Dis. Sci. 2002, 7, 2398–2406. [Google Scholar] [CrossRef]
- Staels, B.; Fonseca, V.A. Bile Acids and Metabolic Regulation. Diabetes Care 2009, 32, 237–245. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, Y.; Huang, J.; Yu, D.; Li, X.; Hu, H.; Ge, T.; Li, D.; et al. Gut Microbiota Dysbiosis Is Associated with Altered Bile Acid Metabolism in Infantile Cholestasis. MSystems 2019, 4. [Google Scholar] [CrossRef]

| Subjects | Age (Y) Mean (SD) | Gender | Diet | BMI | Immunosuppressive Therapy | Period (Y) After Tx Mean (SD) |
|---|---|---|---|---|---|---|
| Patients | 42 (6) | 28 M/12 F | Low Salt | 23.7 (5) | Str/Fk/MMF | 6 (5) |
| Controls | 44 (5) | 10 M/10 F | Balanced | 20 (4) | - | - |
| Chemical Classes | Compounds | m/z | Chemical Structure |
|---|---|---|---|
| Alkanes | Tritetracontane | 57.99 / 71.64 /43.47 | MF: C43H88 MW: 605.2 g/mol |
| 1,6,10,14,18,22-Tetracosahexaen-3-ol, 2,6,10,15,19,23-hexamethyl | 96/81/41 | MF: C30H50O MW: 426.7 g/mol | |
| 6,6-Diethylhoctadecane | 57/71/85 | MF: C22H46 MW: 310.6 g/mol | |
| nonadecane | 57.99 / 43.82 / 71.66 | MF: C19H40 MW: 268.5 g/mol | |
| Heptadecane | 57.99 / 43.65 / 71.64 | MF: C17H36 MW:240.5 g/mol | |
| 4-Ethyloctane | 57/43/41 | MF: C10H22 MW: 142.28 g/mol | |
| 1,2-Epoxyoctadecane | 82/55/71 | MF: C18H36O MW: 268.5 g/mol | |
| Amines | 1-Piperidine carboxaldehyde | 113.08/ 56.05/ 84.04 | MF: C6H11NO MW: 113.16 g/mol |
| Piperidine,1-acetyl | 84/43/127 | MF: C7H13NO MW: 127.18 g/mol | |
| 1-(Piperidine-1-yl)dodecan-1-ol | 127.22/140.85/84.14 | MF: C17H33NO MW: 267.4 g/mol | |
| Alkaloids | 2- Piperidinimine | 98.06/ 70.55/43.78 | MF: C5H10N2 MW: 98.15 g/mol |
| Amino Acid | Tyrosine | 218.18/ 71.85/ 43.41 | MF: C9H11NO3 MW: 181.19 g/mol |
| Benzenoids | Phthalic acid,di(oct-3-yl) ester | 149/ 167/ 150 | MF: C24H38O4 MW: 390.6 g/mol |
| 2,4-Dimethylbenzaldehyde | 134.07/ 133.06/ 105.06 | MF: C9H10O MW: 134.17 g/mol | |
| Dioctyl Phthalate | 149.99/ 391.66/ 261.40 | MF: C24H38O4 MW: 390.6 g/mol | |
| Phthalic acid,di(hept-3-yl) | 149.02/ 167.03/ 150 | MF: C22H34O4 MW: 362.5 g/mol | |
| Phthalic acid | 149.1/ 139.2/ 121.3 | MF: C8H6O4 MW: 166.13 g/mol | |
| Mesitylene | 105.99/ 120.86/77.98 | MF: C9H12 MW: 120.19 g/mol | |
| Carboxylic Acids | Acrylic acid | 72.2/27.23/55.17 | MF:C3H4O2 MW: 72.06 g/mol |
| Triethyl Citrate | 157/ 203/115 | MF: C12H20O7 MW: 276.28 g/mol | |
| Carotenoids | Squalene | 69.06/81.06/41.03 | MF: C30H50 MW: 410.7 g/mol |
| psi.,.psi.-Carotene, 1,1′,2,2′-tetrahydro-1,1′-bis[(trimethylsilyl)oxy] | 73.99/ 69.53/ 91.51 | MF: C42H64O2 MW: 600.49 g/mol | |
| Cumenes | m-Cymene | 93.02/ 135.11/ 121.08 | MF: C10H14 MW: 134.22 g/mol |
| Dialkyldisulfides | Dicyclohexyl sulfite | 83/55/41 | MF: C12H22O3S MW: 246.37 g/mol |
| Dicarboxylic Acids | Di2-ethylhexyladipate | 129/371/259 | MF: C22H42O4 MW: 370.6 g/mol |
| Long-Chain Fatty Acids | Methyl 12-methyltridecanoate | 74/55/75 | MF: C15H30O2 MW: 242.4 g/mol |
| Stearic acid | 117/129/132 | MF: C18H36O2 MW: 284.5 g/mol | |
| Palmitic acid | 237.3/255.3/227.1 | MF: C16H32O2 MW: 256.42 g/mol | |
| Linoleic acid | 263.1/256.1/95 | MF: C18H32O2 MW: 280.4 g/mol | |
| Short Chain Fatty Acids | Acetic acid | 43/55/60 | MF: C2H4O2 MW: 60.05 g/mol |
| Butyric acid | 43.05/87.04/29.04 | MF: C4H8O2 MW: 88.11 g/mol | |
| Fatty Acids | Adipic acid | 129.1/115.1/119.2 | MF: C6H10O4 MW: 146.14 g/mol |
| Dichloroacetic acid, 4-hexadecyl ester | 55.99/69.79/83.76 | MF: C18H34Cl2O2 MW: 352.19 g/mol | |
| Valeric acid | 60.02/29.04/27.03 | MF: C5H10O2 MW: 102.13 g/mol | |
| Z-8-Methyl-9-tetradecenoic acid | 55/41/43 | MF: C15H28O2 MW: 240.38 g/mol | |
| Ethyl-14-methyl-hexadecanoate | 88/55/57 | MF: C19H38O2 MW: 298.5 g/mol | |
| Fatty Acids Esters | Ethyl oleate | 43.99/55.68/69.67 | MF: C20H38O2 MW: 310.29 g/mol |
| Ethyl 9,12-hexadecadienoate | 67/81/55 | MF: C18H32O2 MW: 280.4 g/mol | |
| Ethyl-13-methyl-tetradecanoate | 88/101/55 | MF: C17H34O2 MW: 270.5 g/mol | |
| Ethyl hexadecanoate | 257.5/219.3/237.3 | MF: C18H36O2 MW: 284.5 g/mol | |
| n-propyl-11-octadecenoate | 55/69/83 | MF: C21H40O2 MW: 324.5 g/mol | |
| Stearyl Palmitate | 257/57/43 | MF: C34H68O2 MW: 508.9 g/mol | |
| Fatty Alcohol | 1-Hexadecanol | 55.99/69.82/83.76 | MF: C16H34O MW: 242.26 g/mol |
| n-Nonadecanol-1 | 83/55/97 | MF: C19H40O MW: 284.5 g/mol | |
| Fatty Aldehydes | (Z)-14-Methyl-6-pentadecenoic acid | 73.05/89.04/43.7 | MF: C16H30O2 MW: 254.41 g/mol |
| Hydrocarbons | 5-eicosene | 55.99/57.77/43.69 | MF: C20H40 MW: 280.31 g/mol |
| Indoles | Indole | 117.05/89.02/101.03 | MF: C8H7N MW: 117.15 g/mol |
| Skatole | 131.07/103.04/77.03 | MF: C9H9N MW: 131.17 g/mol | |
| Ketones | Diacetone alcohol | 43.02/59.05/57.03 | MF: C6H12O2 MW: 116.16 g/mol |
| Non-metal sulfates | Sulfurous acid,hexyl octyl ester | 57/85/43 | MF: C14H30O3S MW: 278.45 g/mol |
| Sulfurous acid | 81.97/53.9/64.96 | MF: H2SO3 MW: 82.08 g/mol | |
| Not Attributed | 3,9.beta:14,15-Diepoxypregn-16-en-20-one,3,11.beta.,18-triacetoxy- | 429.98/43.67/430.73 | MF: C27H34O9 MW: 502.6 g/mol |
| Alanine, N-methyl-n-propoxycarbonyl-, isohexyl ester | 144/102/43 | MF: C14H27NO4 MW: 273.37 g/mol | |
| 5,9,14,18-Anthrazinetetrone, 6,15-dihydro-8-hydroxy- | 458.09/49.55 | MF: C28H14N2O5 MW: 458.4 g/mol | |
| Phenols | m-Cresol | 81.03/91.05/67.01 | MF: C7H8O MW: 108.14 g/mol |
| Phenol,2,4,6-tris(1-methylethyl) | 205.78/220.12/206.34 | MF: C15H24O MW: 220.35 g/mol | |
| Phenylpropanes | 2,4-Di-tert-butylphenol | 191.99/57.32/41.16 | MF: C14H22O MW: 206.17 g/mol |
| Prenol lipids | 2,6,10,15-tetramethylheptadecane | 57.06/71.09/43.99 | MF: C21H44 MW: 296.6 g/mol |
| Quinones | 1,4-Naphthoquinone,6-acetyl | 217.99/232.91/189.44/43.27 | MF: C12H8O5 MW: 232.04 g/mol |
| Sterols | Coprosterol | 388.32/44.05/233.15 | MF: C27H48O MW: 388.7 g/mol |
| beta-Sitosterol | 43.99/ 55.35/41.33 | MF: C29H50O MW: 414.39 g/mol | |
| Cholesterol | 43.99/55.89/57. 74 | MF: C27H46O MW: 386.35 g/mol | |
| Ergost-22-en-3-ol (3alpha,5beta,22E) | 55.04/69.06/81.04 | MF: C28H48O MW: 400.5 g/mol | |
| Stigmastanol | 43.99/107.71/215.7 | MF: C29H52O MW: 416.4 g/mol | |
| Cholestenone | 124/43/55 | MF: C27H44O MW: 384.6 g/mol | |
| Gamma-Sitosterol | 43/55/41 | MF: C29H50O MW: 414.7 g/mol | |
| 4,6-cholestadienol | 43.99/143.7/135.63 | MF: C27H44O MW: 384.34 g/mol | |
| Stigmasterol | 255/83/159 | MF: C29H48O MW: 412.7 g/mol | |
| Cholesterylene | 368/81/147 | MF: C27H44 MW:368.6g/mol | |
| Steroids | Epi-coprostanol | 215/55/43 | MF: C27H48O MW: 388.7 g/mol |
| Campestanol | 215/233/234 | MF: C28H50O MW: 402.7 g/mol | |
| Cholest-4-ene | 370.99/108.92/43.39 | MF: C27H46 MW: 370.36 g/mol | |
| Stanol | 9,19-Cycloergost-24(28)-en-3-ol,4,14-dimethyl-, (3β,4α,5α)- | 412.36/369.33/43.05 | MF: C30H50O MW:426.71 g/mol |
| Tocopherols | gamma-Tocopherol | 151.99/416.72/ 417.21 | MF:C28H48O2 MW: 416.37 g/mol |
| Delta-Tocopherol | 402/138/177 | MF: C27H46O2 MW: 402.7 g/mol |
| Compound | FDR | FC | AUC a | Sensitivity b | Specificity c | Pathways |
|---|---|---|---|---|---|---|
| Cholestenone | 0.00168 | 1.7408 × 10−5 | 0.86579 | 0.77 | 0.951 | Steroid degradation |
| Triethyl Citrate | 0.00147 | 4.4656 × 10−6 | 0.84013 | 0.725 | 0.921 | Not identified |
| Gamma-Sitosterol | 0.0161 | 3.4307 × 10−5 | 0.81711 | 0.709 | 0.916 | Not identified |
| Cholesterylene | 0.0153 | 2.3588 | 0.79868 | 0.684 | 0.906 | Steroid hormone biosynthesis |
| Ethyl 9,12-hexadecadienoate | 0.0133 | 1.0012 × 10−5 | 0.78158 | 0.654 | 0.906 | Primary bile acid biosynthesis; Steroid hormone biosynthesis |
| Adipic acid | 0.00158 | 21.854 | 0.77961 | 0.649 | 0.891 | Caprolactam degradation |
| Nonyl dichloroacetate | 0.00769 | 9.9808 × 10−6 | 0.76974 | 0.657 | 0.876 | Not identified |
| Dioctyl Phthalate | 0.0022 | 9.0143 × 10−6 | 0.76908 | 0.641 | 0.875 | Not identified |
| Delta-Tocopherol | 0.0131 | 20654.0 | 0.75395 | 0.607 | 0.896 | Ubiquinone and other terpenoid-quinone biosynthesis |
| Cholesterol | 0.00675 | 4.46284 | 0.74803 | 0.609 | 0.866 | Steroid hormone biosynthesis; Primary bile acid biosynthesis |
| Tyrosine | 0.00985 | 57.844 | 0.74408 | 0.619 | 0.859 | Ubiquinone and other terpenoid-quinone biosynthesis; Tyrosinemetabolism, Phenylalanine, tyrosine and tryptophan biosynthesis; Aminoacyl-tRNA biosynthesis |
| m-cymene | 0.036354 | 105790.0 | 0.74276 | 0.699 | 0.866 | Not identified |
| Indole | 0.04437 | 45722.0 | 0.72763 | 0.671 | 0.87 | Phenylalanine, tyrosine and tryptophan biosynthesis |
| Epi-coprostanol | 0.0337 | 10665.0 | 0.71645 | 0.685 | 0.838 | Not identified |
| nonadecane | 0.0118 | 0.29481 | 0.71645 | 0.599 | 0.845 | Not identified |
| Sulfurous acid,hexyl octyl ester | 0.00715 | 0.12937 | 0.7125 | 0.566 | 0.831 | Cysteine and methionine metabolism |
| gamma-tocopherol | 0.00421 | 2.1326 | 0.71118 | 0.644 | 0.778 | Ubiquinone and other terpenoid-quinone biosynthesis |
| Stigmasterol | 0.02382 | 6.593 × 10−5 | 0.70724 | 0.656 | 0.764 | Steroid biosynthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouidhi, S.; Zidi, O.; Alhujaily, M.; Souai, N.; Mosbah, A.; Belali, T.M.; Ghedira, K.; El Kossai, I.; El Manaa, J.; Mnif, W.; et al. Fecal Metabolomics Reveals Distinct Profiles of Kidney Transplant Recipients and Healthy Controls. Diagnostics 2021, 11, 807. https://doi.org/10.3390/diagnostics11050807
Kouidhi S, Zidi O, Alhujaily M, Souai N, Mosbah A, Belali TM, Ghedira K, El Kossai I, El Manaa J, Mnif W, et al. Fecal Metabolomics Reveals Distinct Profiles of Kidney Transplant Recipients and Healthy Controls. Diagnostics. 2021; 11(5):807. https://doi.org/10.3390/diagnostics11050807
Chicago/Turabian StyleKouidhi, Soumaya, Oumaima Zidi, Muhanad Alhujaily, Nessrine Souai, Amor Mosbah, Tareg M. Belali, Kais Ghedira, Imene El Kossai, Jamelddine El Manaa, Wissem Mnif, and et al. 2021. "Fecal Metabolomics Reveals Distinct Profiles of Kidney Transplant Recipients and Healthy Controls" Diagnostics 11, no. 5: 807. https://doi.org/10.3390/diagnostics11050807
APA StyleKouidhi, S., Zidi, O., Alhujaily, M., Souai, N., Mosbah, A., Belali, T. M., Ghedira, K., El Kossai, I., El Manaa, J., Mnif, W., & Cherif, A. (2021). Fecal Metabolomics Reveals Distinct Profiles of Kidney Transplant Recipients and Healthy Controls. Diagnostics, 11(5), 807. https://doi.org/10.3390/diagnostics11050807









