Risk Minimization of Hemolytic Disease of the Fetus and Newborn Using Droplet Digital PCR Method for Accurate Fetal Genotype Assessment of RHD, KEL, and RHCE from Cell-Free Fetal DNA of Maternal Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and DNA Isolation
2.3. Determination of Fetal Blood Genotype by ddPCR
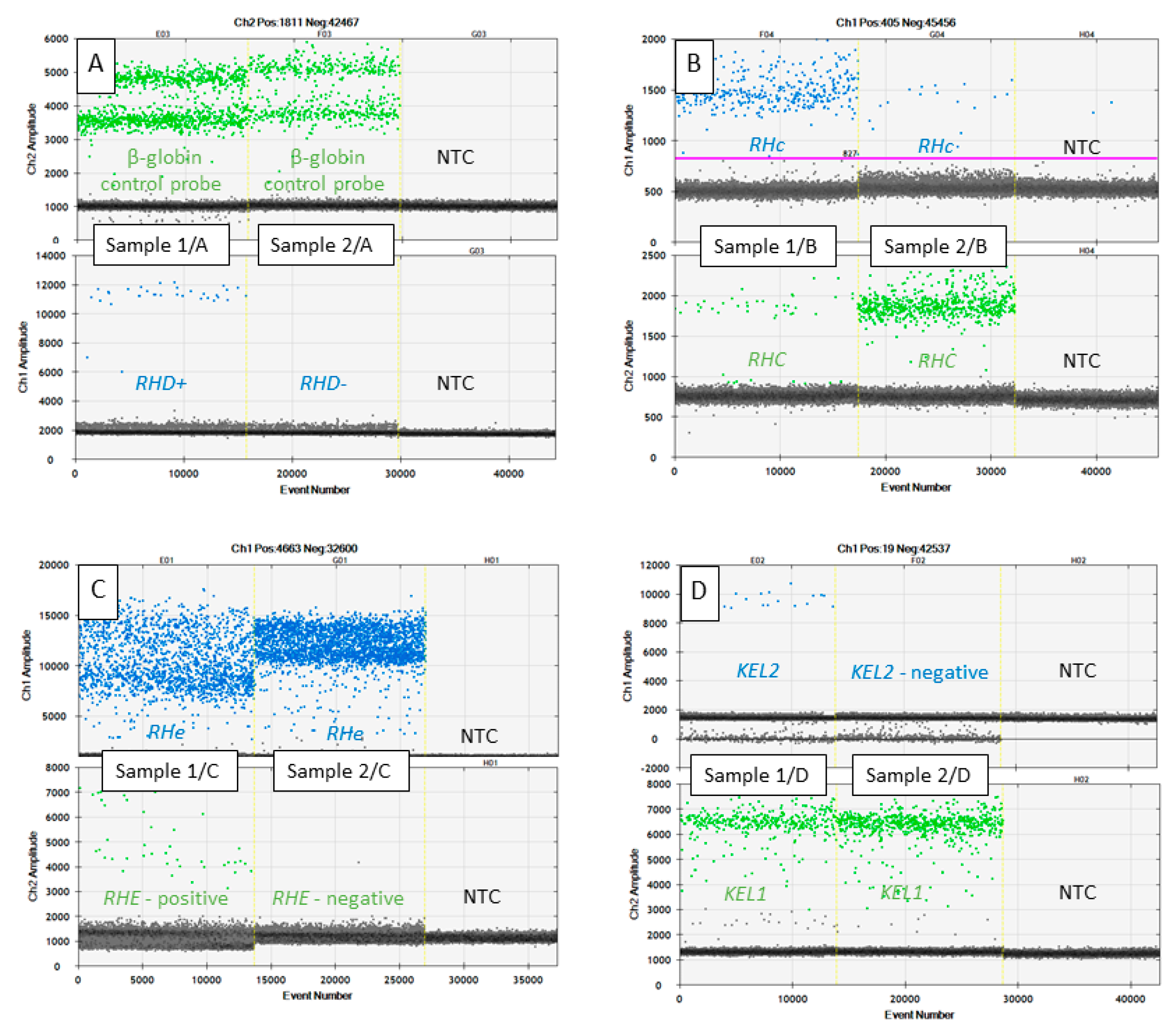
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| β-globin | hemoglobin subunit beta |
| cfDNA | cell free deoxyribonucleic acid |
| cffDNA | cell free fetal deoxyribonucleic acid |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| ddPCR | droplet digital polymerase chain reaction |
| DNA | deoxyribonucleic acid |
| dUTP | 2´-deoxyuridine, 5´-triphosphate |
| EDTA | ethylenediaminetetraacetic acid |
| FSP | fetus specific positives |
| HDFN | Hemolytic disease of the fetus and newborn |
| KEL | Kell metallo-endopeptidase |
| MSP | maternal specific positives |
| NF1 | neurofibromin 1 |
| NGS | next generation sequencing |
| NTC | non-template control |
| PCR | polymerase chain reaction |
| Rh | Rhesus |
| RHCE | Rh blood group CcEe antigens |
| RHD | Rh blood group D antigen |
| SNP | single nucleotide polymorphisms |
| SRY | sex-determining region |
| Tm | melting temperature |
References
- Avent, N.D.; Reid, M. The Rh blood group system. Blood 2000, 95, 375–387. [Google Scholar] [CrossRef]
- Bowman, J.M. RhD hemolytic disease of the newborn. N. Engl. J. Med. 1998, 339, 1775–1777. [Google Scholar] [CrossRef]
- Eder, A. Update on HDFN: New information on long-standing controversies. Immunohematology 2006, 22, 188–195. [Google Scholar]
- Urbaniak, S.; Greiss, S. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000, 14, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.G.; Anstee, D.J. (Eds.) Hemolytic disease of the fetus and the newborn. In Mollison’s Blood Transfusion in Clinical Medicine, 12th ed.; Blackwell Scientific: Hoboken, NJ, USA, 2014; pp. 499–549. [Google Scholar]
- de Haas, M.; Thurik, F.F.; Koelewijn, J.M.; van der Schoot, C.E. Haemolytic disease of the fetus and newborn. Vox Sang. 2015, 109, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J. Fetal anemia due to non-Rhesus-D red-cell alloimmunization. Semin. Fetal Neonatal Med. 2008, 13, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Quinley, E.D. Haemolytic disease of the newborn. In Immunohaematology: Principles and Practice; Lippincott: New York, NY, USA, 1993; pp. 277–308. [Google Scholar]
- Moise, K.J. Hemolytic disease of the fetus and newborn. In Maternal-Fetal Medicine: Principles and Practice, 6th ed.; Greene, M.F., Creasy, R.K., Resnik, R., Iams, J.D., Lockwood, C.J., Moore, T.R., Eds.; Saunders: Philadelphia, PA, USA, 2008; pp. 477–503. [Google Scholar]
- Hendrickson, J.E.; Delaney, M. Hemolytic Disease of the Fetus and Newborn: Modern Practice and Future Investigations. Transfus. Med. Rev. 2016, 30, 159–164. [Google Scholar] [CrossRef]
- Maheshwari, A.; Carlo, W.A. Hemolytic disease of the Newborn (erythroblastosis fetalis). In Nelson Textbook of Pediatrics, 19th ed.; Kliegman, R.M., Stanton, B.F., Schor, N.F., St Geme, J.W., III, Behrman, R.E., Eds.; Thomas Press India Ltd.: New Delhi, India, 2012; pp. 615–619. [Google Scholar]
- Maitra, A. Disease of infancy and childhood. In Robbins and Cortan Pathologic Basis of Disease, 8th ed.; Kumar, V., Abbas, A.K., Fausto, N., Aster, J.C., Eds.; Elsevier Inc.: New Delhi, India, 2010; pp. 447–486. [Google Scholar]
- Kennedy, M.S. Perinatal issues in transfusion practices. In Technical Manual, 17th ed.; Roback, J.D., Grossman, B.J., Harris, T., Hillyer, C.D., Eds.; AABB: Bethesda, MD, USA, 2011; pp. 631–645. [Google Scholar]
- Stephen, J.; Cairns, L.S.; Pickford, W.J.; Vickers, M.A.; Urbaniak, S.J.; Barker, R.N. Identification, immunomodulatory activity, and immunogenicity of the major helper T-cell epitope on the K blood group antigen. Blood 2012, 119, 5563–5574. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G. Kell and Kx Blood Group Systems. In Human Blood Groups, 3rd ed.; Wiley-Blackwell: Oxford, UK, 2013; Chapter 7. [Google Scholar]
- Wagner, F.F.; Flegel, W.A. RHD gene deletion occurred in the Rhesus box. Blood 2000, 95, 3662–3668. [Google Scholar] [CrossRef]
- Daniels, G. Human Blood Groups, 2nd ed.; Blackwell Science: Oxford, UK, 2002; ISBN 978-1-405-14007-2. [Google Scholar]
- Colin, Y.; Chérif-Zahar, B.; Le Van Kim, C.; Raynal, V.; Van Huffel, V.; Cartron, J.P. Genetic basis of the RhD−positive and RhD−negative blood group polymorphism as determined by Southern analysis. Blood 1991, 78, 2747–2752. [Google Scholar] [CrossRef]
- Mouro, I.; Colin, Y.; Chérif-Zahar, B.; Cartron, J.P.; Le Van Kim, C. Molecular genetic basis of the human Rhesus blood group system. Nat. Genet. 1993, 5, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E.; Lomas-Francis, C. The Blood Group Antigen Facts Book, 2nd ed.; Elsevier Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Reid, M.E.; Denomme, G.A. DNA-based methods in the immunohematology reference laboratory. Transfus. Apher. Sci. 2011, 44, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Arnoni, C.P.; Muniz, J.G.; de Paula, T.A.; Person, R.D.; Gazito, D.; Baleotti WJr Barreto, J.A.; Castilho, L.; Latini, F.R. An easy and efficient strategy for KEL genotyping in a multiethnic population. Rev. Bras. Hematol. Hemoter. 2013, 35, 99–102. [Google Scholar] [CrossRef]
- Lee, S.; Wu, X.; Reid, M.; Zelinski, T.; Redman, C. Molecular basis of the Kell (K1) phenotype. Blood 1995, 85, 912–916. [Google Scholar] [CrossRef]
- Poole, J.; Warke, N.; Hustinx, H.; Taleghani, B.M.; Martin, P.; Finning, K.; Crew, V.K.; Green, C.; Bromilow, I.; Daniels, G. A KEL gene encoding serine at position 193 of the Kell glycoprotein results in expression of KEL1 antigen. Transfusion 2006, 46, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Molecular basis of Kell blood group phenotypes. Vox Sang. 1997, 73, 1–11, Erratum in: Vox Sang. 1998, 74, 58. [Google Scholar] [CrossRef]
- Bohmova, J.; Lubusky, M.; Holuskova, I.; Studnickova, M.; Kratochvilova, R.; Krejcirikova, E.; Durdova, V.; Kratochvilova, T.; Dusek, L.; Prochazka, M.; et al. Two Reliable Methodical Approaches for Non-Invasive RHD Genotyping of a Fetus from Maternal Plasma. Diagnostics 2020, 10, 564. [Google Scholar] [CrossRef]
- Böhmova, J.; Vodicka, R.; Lubusky, M.; Holuskova, I.; Studnickova, M.; Kratochvilova, R.; Krejcirikova, E.; Janikova, M.; Durdová, V.; Dolezalová, T.; et al. Clinical Potential of Effective Noninvasive Exclusion of KEL1-Positive Fetuses in KEL1-Negative Pregnant Women. Fetal Diagn Ther. 2016, 40, 48–53. [Google Scholar] [CrossRef]
- Durdova, V.; Bohmova, J.; Kratochvilova, T.; Vodicka, R.; Holuskova, I.; Langova, K.; Lubusky, M. The effectiveness of KEL and RHCE fetal genotype assessment in alloimmunized women by minisequencing. Ceska Gynekol. 2020, 85, 164–173. [Google Scholar]
- Barrett, A.N.; Xiong, L.; Tan, T.Z.; Advani, H.V.; Hua, R.; Laureano-Asibal, C.; Soong, R.; Biswas, A.; Nagarajan, N.; Choolani, M. Measurement of fetal fraction in cell-free DNA from maternal plasma using a panel of insertion/deletion polymorphisms. PLoS ONE 2017, 12, e0186771. [Google Scholar] [CrossRef]
- Schlütter, J.M.; Hatt, L.; Bach, C.; Kirkegaard, I.; Kølvraa, S.; Uldbjerg, N. The cell-free fetal DNA fraction in maternal blood decreases after physical activity. Prenat. Diagn. 2014, 34, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Moturi, S.; Angkachatchai, V.; Mueller, R.; DeSantis, G.; van den Boom, D.; Ehrich, M. Optimizing blood collection, transport and storage conditions for cell free DNA increases access to prenatal testing. Clin. Biochem. 2013, 46, 1099–1104. [Google Scholar] [CrossRef]
- Yang, W.C.; Zhu, L.; Qiu, Y.M.; Zhou, B.X.; Cheng, J.L.; Wei, C.L.; Chen, H.C.; Li, L.Y.; Fu, X.D.; Fu, J.J. Isolation and analysis of cell-free fetal DNA from maternal peripheral blood in Chinese women. Genet. Mol. Res. 2015, 14, 18078–18089. [Google Scholar] [CrossRef] [PubMed]
- Vodicka, R.; Vrtel, R.; Dusek, L.; Prochazka, M.; Schneiderova, E.; Vrbicka, D.; Krejcirikova, E.; Dhaifalah, I.; Santava, A.; Santavy, J. Refined fluorescent STR quantification of cell-free fetal DNA during pregnancy in physiological and Down syndrome fetuses. Prenat. Diagn. 2008, 28, 425–433. [Google Scholar] [CrossRef]
- Svobodová, I.; Pazourková, E.; Hořínek, A.; Novotná, M.; Calda, P.; Korabečná, M. Performance of Droplet Digital PCR in Non-Invasive Fetal RHD Genotyping-Comparison with a Routine Real-Time PCR Based Approach. PLoS ONE 2015, 10, e0142572. [Google Scholar] [CrossRef]
- Sillence, K.A.; Roberts, L.A.; Hollands, H.J.; Thompson, H.P.; Kiernan, M.; Madgett, T.E.; Welch, C.R.; Avent, N.D. Fetal Sex and RHD Genotyping with Digital PCR Demonstrates Greater Sensitivity than Real-time PCR. Clin. Chem. 2015, 61, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Ouzegdouh Mammasse, Y.; Chenet, C.; Drubay, D.; Martageix, C.; Cartron, J.P.; Vainchenker, W.; Petermann, R. A new efficient tool for non-invasive diagnosis of fetomaternal platelet antigen incompatibility. Br. J. Haematol. 2020, 190, 787–798. [Google Scholar] [CrossRef]
- Finning, K.; Martin, P.; Summers, J.; Daniels, G. Fetal genotyping for the K (Kell) and Rh C, c, and E blood groups on cell-free fetal DNA in maternal plasma. Transfusion 2007, 47, 2126–2133. [Google Scholar] [CrossRef]
- Cro’, F.; Lapucci, C.; Vicari, E.; Salsi, G.; Rizzo, N.; Farina, A. An innovative test for non-invasive Kell genotyping on circulating fetal DNA by means of the allelic discrimination of K1 and K2 antigens. Am. J. Reprod. Immunol. 2016, 76, 499–503. [Google Scholar] [CrossRef]
- O’Brien, H.; Hyland, C.; Schoeman, E.; Flower, R.; Daly, J.; Gardener, G. Non-invasive prenatal testing (NIPT) for fetal Kell, Duffy and Rh blood group antigen prediction in alloimmunised pregnant women: Power of droplet digital PCR. Br. J. Haematol. 2020, 189, e90–e94. [Google Scholar] [CrossRef]
- Wienzek-Lischka, S.; Krautwurst, A.; Fröhner, V.; Hackstein, H.; Gattenlöhner, S.; Bräuninger, A.; Axt-Fliedner, R.; Degenhardt, J.; Deisting, C.; Santoso, S.; et al. Noninvasive fetal genotyping of human platelet antigen-1a using targeted massively parallel sequencing. Transfusion 2015, 55, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Caswell, R.C.; Snowsill, T.; Houghton, J.A.L.; Chakera, A.J.; Shepherd, M.H.; Laver, T.W.; Knight, B.A.; Wright, D.; Hattersley, A.T.; Ellard, S. Noninvasive Fetal Genotyping by Droplet Digital PCR to Identify Maternally Inherited Monogenic Diabetes Variants. Clin. Chem. 2020, 66, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Pacault, M.; El Khattabi, L.A.; Vaucouleur, N.; Orhant, L.; Bienvenu, T.; Girodon, E.; Vidaud, D.; Leturcq, F.; Costa, C.; et al. Non-invasive prenatal diagnosis of paternally inherited disorders from maternal plasma: Detection of NF1 and CFTR mutations using droplet digital PCR. Clin. Chem. Lab. Med. 2018, 56, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Debrand, E.; Lykoudi, A.; Bradshaw, E.; Allen, S.K. A Non-Invasive Droplet Digital PCR (ddPCR) Assay to Detect Paternal CFTR Mutations in the Cell-Free Fetal DNA (cffDNA) of Three Pregnancies at Risk of Cystic Fibrosis via Compound Heterozygosity. PLoS ONE 2015, 10, e0142729. [Google Scholar] [CrossRef]
| Pregnant Woman Blood Antigen | n = 53 | Confirmation Method | Newborn Phenotype | Gestation Week | Gestation Week | Age | Age | BMI | BMI | Ethnic Group of Participants | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | Median | Mean | Median | Mean | ||||||||
| “D-antigen” negative pregnant women (genotype RHdd) | 10 | Real-Time PCR | 5 RhD+ 5 RhD− | 10–18 | 12 | 12.5 | 18–43 | 29 | 30 | 17–36 | 23 | 24.3 | Caucasian |
| “C-antigen” pregnant women (genotype RHCC) | 11 | Minisequencing | 4 RhC 7 Rhc | ||||||||||
| “c-antigen” pregnant women (genotype RHcc) | 6 | Minisequencing | 4 RhC 2 Rhc | ||||||||||
| “e-antigen” pregnant women (genotype RHee) | 16 | Minisequencing | 12 Rhe 4 RhE | ||||||||||
| “k-antigen” pregnant women (genotype KEL2/KEL2) | 10 | Minisequencing | 5 Kell− 5 Kell+ | ||||||||||
| Gene, SNP rs Number | Allele | Primer F (5′–3′) | Primer R (5′–3′) | Probe (5′–3′) | PCR Product bp | Specificity to cffDNA | Optimal Annealing Temperature | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Fluorophore | Sequence of the Probe | Quencher | 55 °C ** | 60 °C | ||||||
| RHD | RHD | GGGTGTTGTAACCGAGTGCTG | CTCCAAGCAGACCCAGCAAG | 56-FAM | CCCACAGCTCCATCATGGGCTACAA | 3IAkFQ | 72 | * YES | YES | YES |
| GGGTGTTGTAACCGAGTGCTG | CCGGCTCCGACGGTATC | 134 | YES | YES | YES | |||||
| β-globin | --- | GTGCATCTGACTCCTGAGGAGA | CCTTGATACCAACCTGCCCAG | 5HEX | AAGGTGAACGTGGATGAAGTTGGTGG | 3IAkFQ | 74 | * YES | YES | YES |
| RHCE, rs609320 | RHE | ATTCTTCCTTTGGATTGGAC | CTTGTGGATGTTCTGGC | 5HEX | TCAGCAGAGGAGAGTTG | 3IAkFQ | 64 | * YES | YES | YES |
| TGGCATTCTTCCTTTGGATTGG | CTCAGACCTTTGGAGCAGGAGT | 134 | YES | YES | YES | |||||
| RHe | ATTCTTCCTTTGGATTGGAC | CTTGTGGATGTTCTGGC | 56-FAM | TCAGCAGAGCAGAGTTG | 3IAkFQ | 64 | * YES | YES | YES | |
| TGGCATTCTTCCTTTGGATTGG | CTCAGACCTTTGGAGCAGGAGT | 134 | YES | YES | YES | |||||
| RHCE, rs676785 | RHC | AATACCTGAACAGTGTGATG | CTGCTGGACGGCTTC | 5HEX | CCTTCCCAGAAGGGAAC | 3IAkFQ | 74 | * YES | YES | NO |
| CCAGCCACCATCCCAATACC | TGTGCAGTGGGCAATCCTG | 94 | YES | YES | NO | |||||
| RHc | AATACCTGAACAGTGTGATG | CTGCTGGACGGCTTC | 56-FAM | GATGACCACCTTCCCAGGAGGGAA | 3IAkFQ | 74 | * YES | YES | NO | |
| CCAGCCACCATCCCAATACC | TGTGCAGTGGGCAATCCTG | 94 | YES | YES | NO | |||||
| KEL, rs8176058 | KEL1 | ddPCR Mutation Assay: KEL p.T193M, Human | 56-FAM | ddPCR Mutation Assay: KEL p.T193M, Human | 3IAkFQ | 65 | * YES | YES | NO | |
| GGTAAATGGACTTCCTTAAAC | CTGAAGAAAGGGAAATGG | 56-FAM | TAACCGAATGCTGAGACTTCTGATGAGTCAG | 3IAkFQ | 77 | NO | NO | NO | ||
| KEL2 | ddPCR Mutation Assay: KEL p.T193M, Human | 5HEX | ddPCR Mutation Assay: KEL p.T193M, Human | 3IAkFQ | 65 | * YES | YES | NO | ||
| GGTAAATGGACTTCCTTAAAC | CTGAAGAAAGGGAAATGG | 5HEX | TAACCGAACGCTGAGACTTCTGATGAGTCAG | 3IAkFQ | 77 | NO | NO | NO | ||
| Maternal Genotype (n) | Fetal Genotype */Phenotype ** (n) | FAM (Blue) Channel Positive Droplets Mean (Min; Max) | VIC (Green) Channel Positive Droplets Mean (Min; Max) | Negative Droplets Mean (Min; Max) | Fetal Fraction Percentage Mean (Min; Max) *** |
|---|---|---|---|---|---|
| RHD− (10) | RHD+/RhD+ (5) | 47.6 (28; 91) | 1540 (467; 2875) | 13,761 (11,583; 16,561) | 9% (5.6%; 25%) |
| RHD−/RhD− (5) | 0.6 (0; 2) | 1457 (562; 2767) | 14,240 (10,471; 17,560) | ||
| RHee (16) | RHee (12)/Rhee(12) | 792 (295; 1809) | 0.4 (0; 1) | 11,735 (10,417; 13,687) | |
| RhEe (4)/RhEe (4) | 1450 (289; 2854) | 25.6 (8; 49) | 12,479 (9096; 14,939) | 7% (3.5%;11%) | |
| RHCC (11) | RHCc (4)/RhCc (4) | 21 (18; 26) | 672 (522; 805) | 13,706 (11,718; 17,142) | 8% (6.6–10%) |
| RHCC (7)/RhCC (7) | 1 (0;3) | 938 (239; 2474) | 12,669 (10,528; 13,717) | ||
| RHcc (6) | RHCc (2)/RhCc (2) | 379 and 517 | 44 and 54 | 26% and 23% | |
| RHcc (4)/Rhcc(4) | 695 (390; 1284) | 1.4 (0; 5) | |||
| KEL2 (10) | KEL1 (5)/K (5) | 22 (17; 31) | 760 (376; 1341) | 14,237 (13,558; 15,069) | 7% (5.5%; 11%) |
| KEL2 (5)/k (5) | 625 (440; 922) | 14,328 (13,598; 15,321) | |||
| NTC (23) | NTC (23) | 0.55 (0; 3) | 0.22 (0; 4) | 15,646 (12,448; 17,735) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodicka, R.; Bohmova, J.; Holuskova, I.; Krejcirikova, E.; Prochazka, M.; Vrtel, R. Risk Minimization of Hemolytic Disease of the Fetus and Newborn Using Droplet Digital PCR Method for Accurate Fetal Genotype Assessment of RHD, KEL, and RHCE from Cell-Free Fetal DNA of Maternal Plasma. Diagnostics 2021, 11, 803. https://doi.org/10.3390/diagnostics11050803
Vodicka R, Bohmova J, Holuskova I, Krejcirikova E, Prochazka M, Vrtel R. Risk Minimization of Hemolytic Disease of the Fetus and Newborn Using Droplet Digital PCR Method for Accurate Fetal Genotype Assessment of RHD, KEL, and RHCE from Cell-Free Fetal DNA of Maternal Plasma. Diagnostics. 2021; 11(5):803. https://doi.org/10.3390/diagnostics11050803
Chicago/Turabian StyleVodicka, Radek, Jana Bohmova, Iva Holuskova, Eva Krejcirikova, Martin Prochazka, and Radek Vrtel. 2021. "Risk Minimization of Hemolytic Disease of the Fetus and Newborn Using Droplet Digital PCR Method for Accurate Fetal Genotype Assessment of RHD, KEL, and RHCE from Cell-Free Fetal DNA of Maternal Plasma" Diagnostics 11, no. 5: 803. https://doi.org/10.3390/diagnostics11050803
APA StyleVodicka, R., Bohmova, J., Holuskova, I., Krejcirikova, E., Prochazka, M., & Vrtel, R. (2021). Risk Minimization of Hemolytic Disease of the Fetus and Newborn Using Droplet Digital PCR Method for Accurate Fetal Genotype Assessment of RHD, KEL, and RHCE from Cell-Free Fetal DNA of Maternal Plasma. Diagnostics, 11(5), 803. https://doi.org/10.3390/diagnostics11050803






