A Novel, Inexpensive In-House Immunochromatographic Strip Test for Cryptococcosis Based on the Cryptococcal Glucuronoxylomannan Specific Monoclonal Antibody 18B7
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of C. neoformans Capsular Antigens and Other Pathogenic Fungal Antigen
2.1.1. Fungal Isolates
2.1.2. Purification of Capsular Antigens of C. neoformans
2.1.3. Crude Fungal Cytoplasmic Protein Antigen Extraction
2.2. Production and Purification of Monoclonal Antibody 18B7 (mAb18B7)
2.3. Determination of the Immunoreactivity of mAb18B7 by Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.1. Determination of the Cross-Reactivity of mAb 18B7 with Other Fungal Pathogens by Indirect ELISA
2.3.2. Determination of the Immunoreactivity by Inhibition ELISA
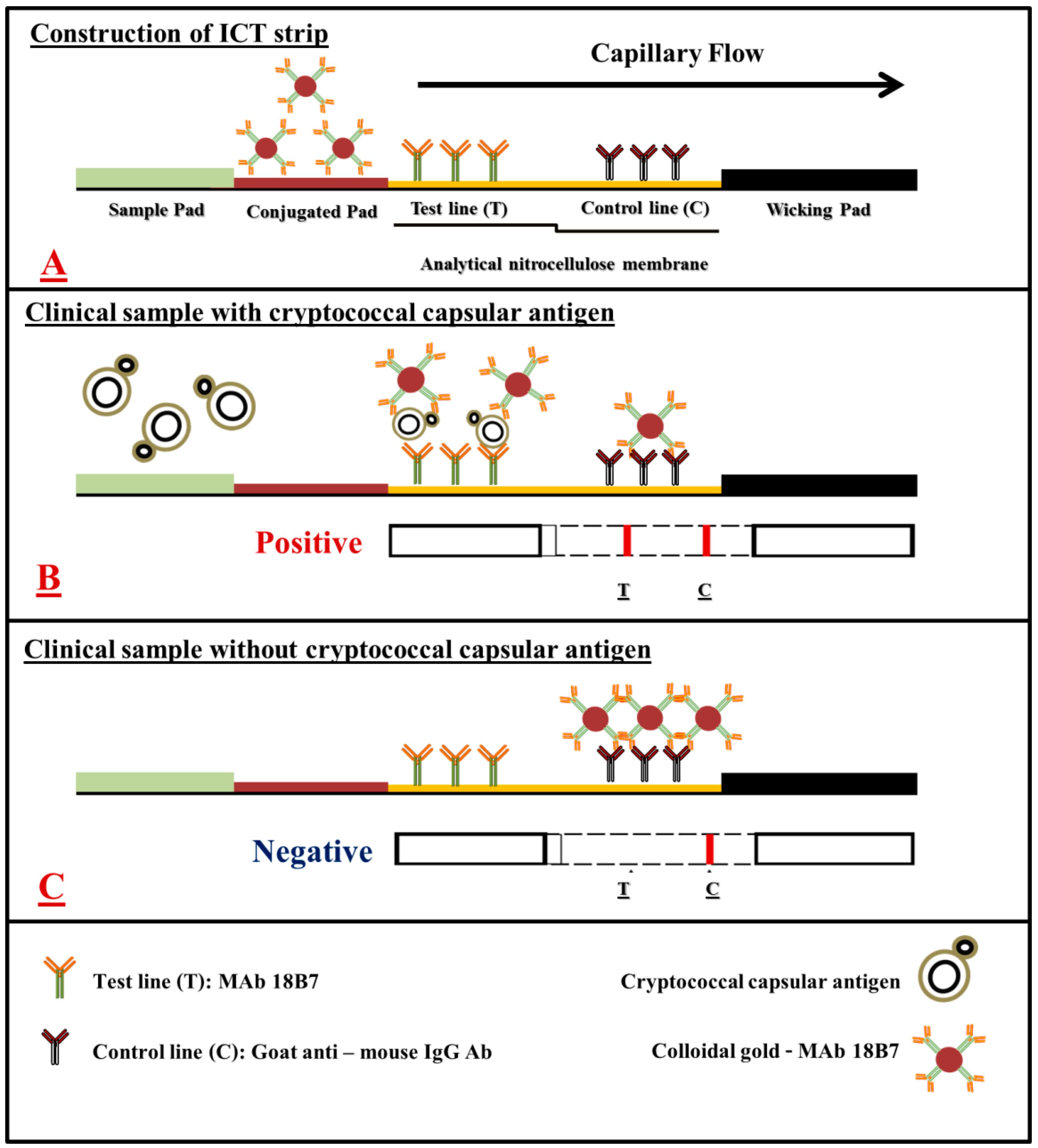
2.4. Development of mAb 18B7 Based Lateral Flow Immunochromatographic Test (mAb 18B7 ICT Strip)
2.4.1. Principle of Assay Format
2.4.2. Preparation of Colloidal Gold Nanoparticle—mAb 18B7 Signal Reporter
2.4.3. Establishment of Test Line and Control Line onto Analytical Nitrocellulose Membrane
2.4.4. Preparation of the Sample Application Pad
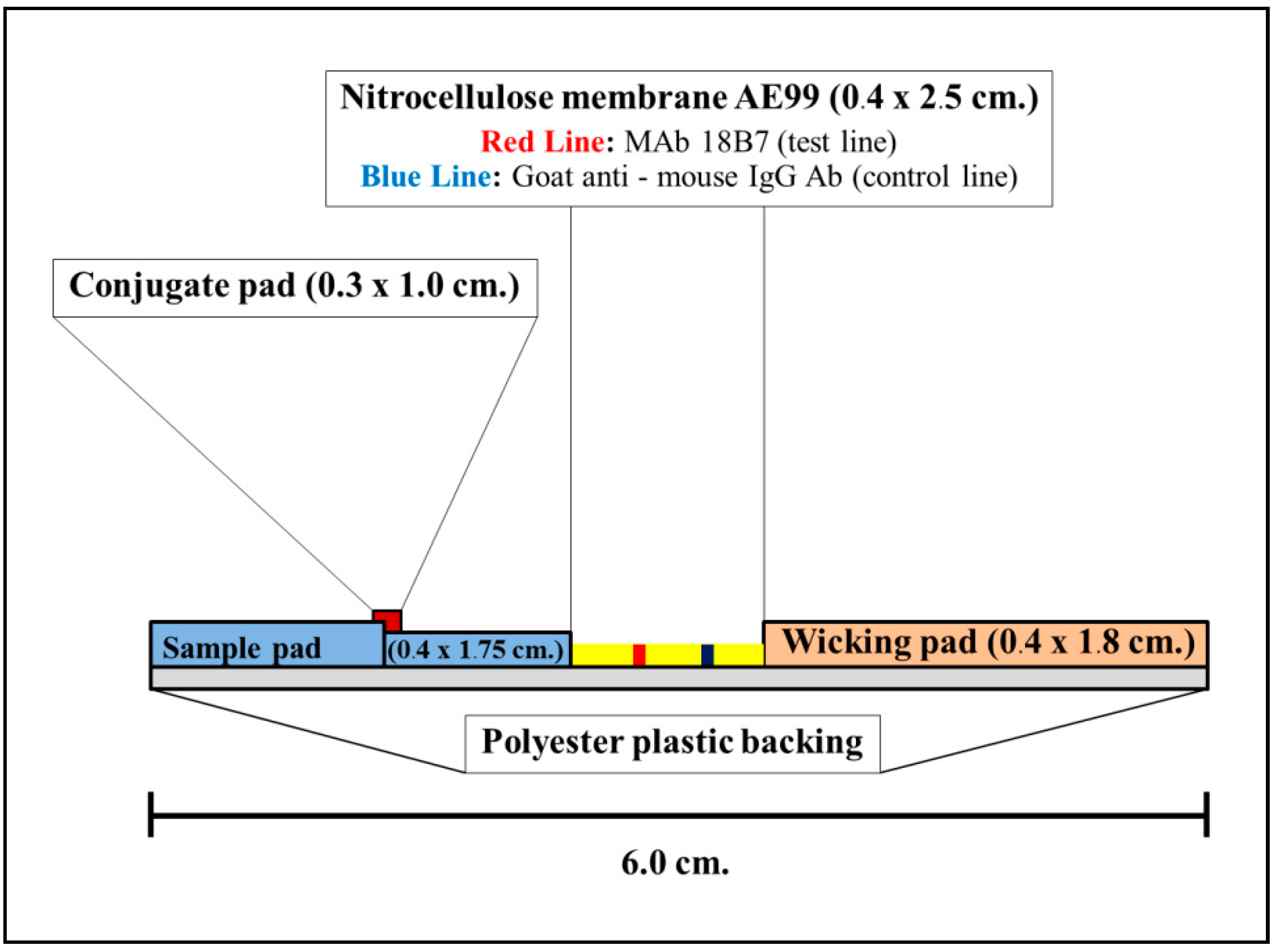
2.4.5. Construction and Material Composition of ICT Strip
- Analytical membrane (Cellulose nitrate membrane AE99; Schleicher & Schuell; size 0.4 × 2.5 cm2)
- The mAb18B7–CG absorbed conjugation releasing pad (Ahlstrom 8964; Glass fiber, Ahlstrom, Finland; size 0.3 × 1.0 cm2)
- Sample application pad (Fushion 5; Whatman Schleicher & Schuell, Germany; size 0.4 × 1.75 cm2)
- Adsorbing (wicking) pad (Whatman; Schleicher & Schuell, Germany size 0.4 × 1.8 cm2).
2.5. Determination of the Lower Limit of Detection (LOD), Cross-Reactivity and Pan Serotype Specific of the mAb 18B7 ICT Strip
2.6. Evaluation of the Diagnostic Kit
2.6.1. Clinical Samples
2.6.2. Validation of Diagnostic Performance of the mAb 18B7 ICT Strip and Statistical Analysis
3. Results
3.1. The Immunoreactivity and Cross-Reactivity of mAb 18B7 against Cryptococcal Capsular Antigen and Other Fungal Pathogenic Antigens by Indirect ELISA
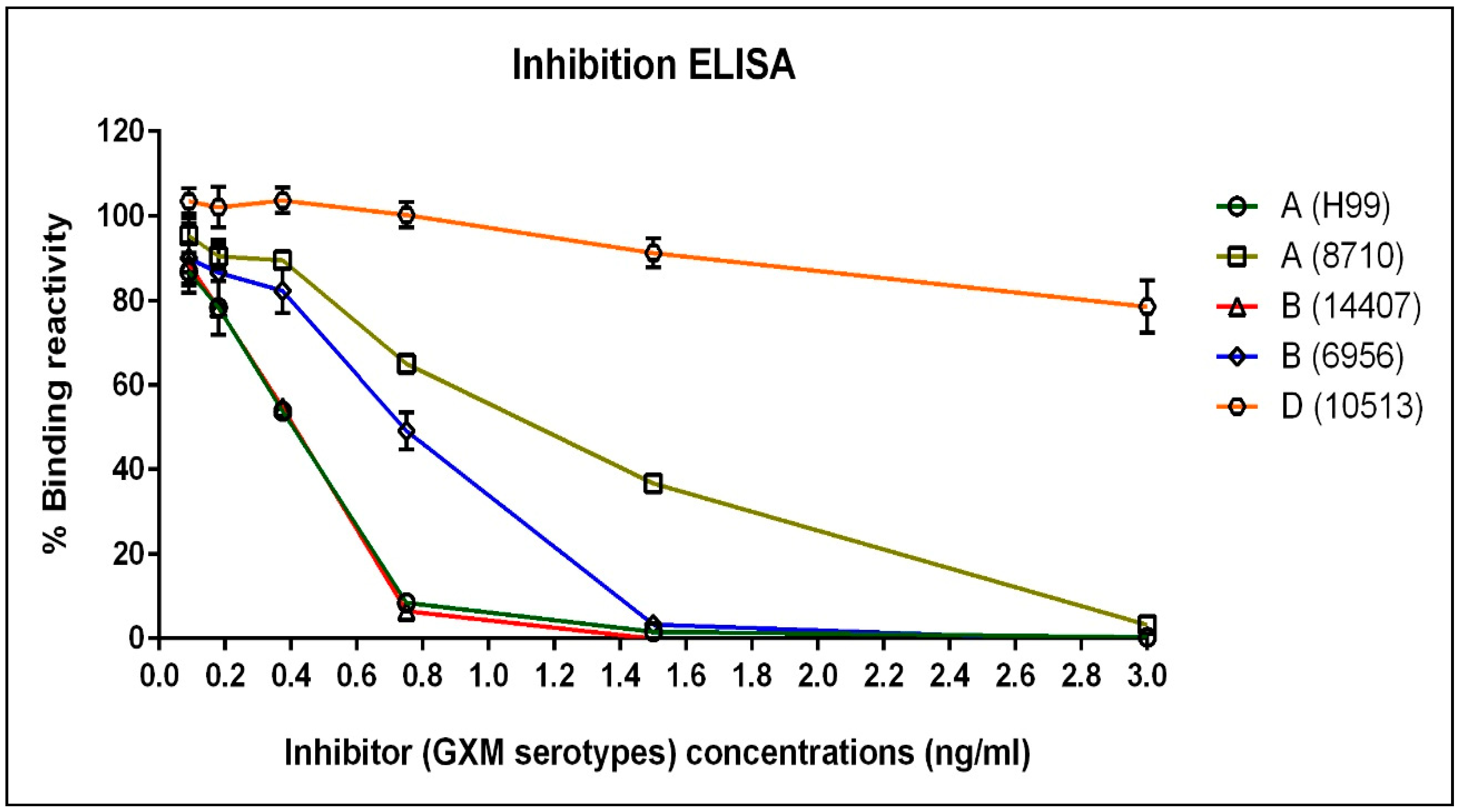
3.2. The Immunoreactivity of mAb 18B7 against Other Serotypes of Cryptococcal Capsular Antigen as Studied by Inhibition ELISA
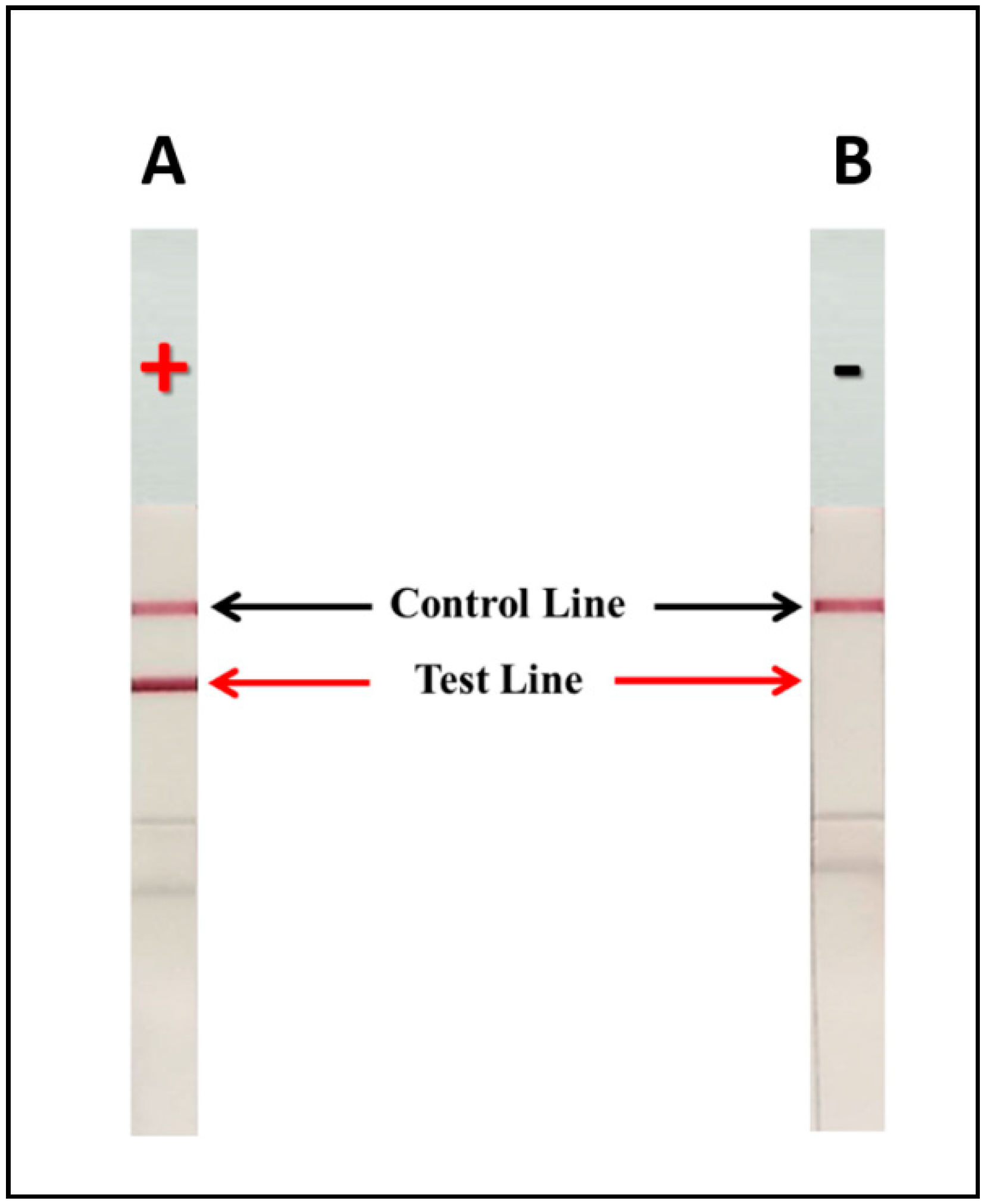
3.3. Development of the mAb 18B7 ICT Strip for the Detection of Cryptococcal Capsular Antigen
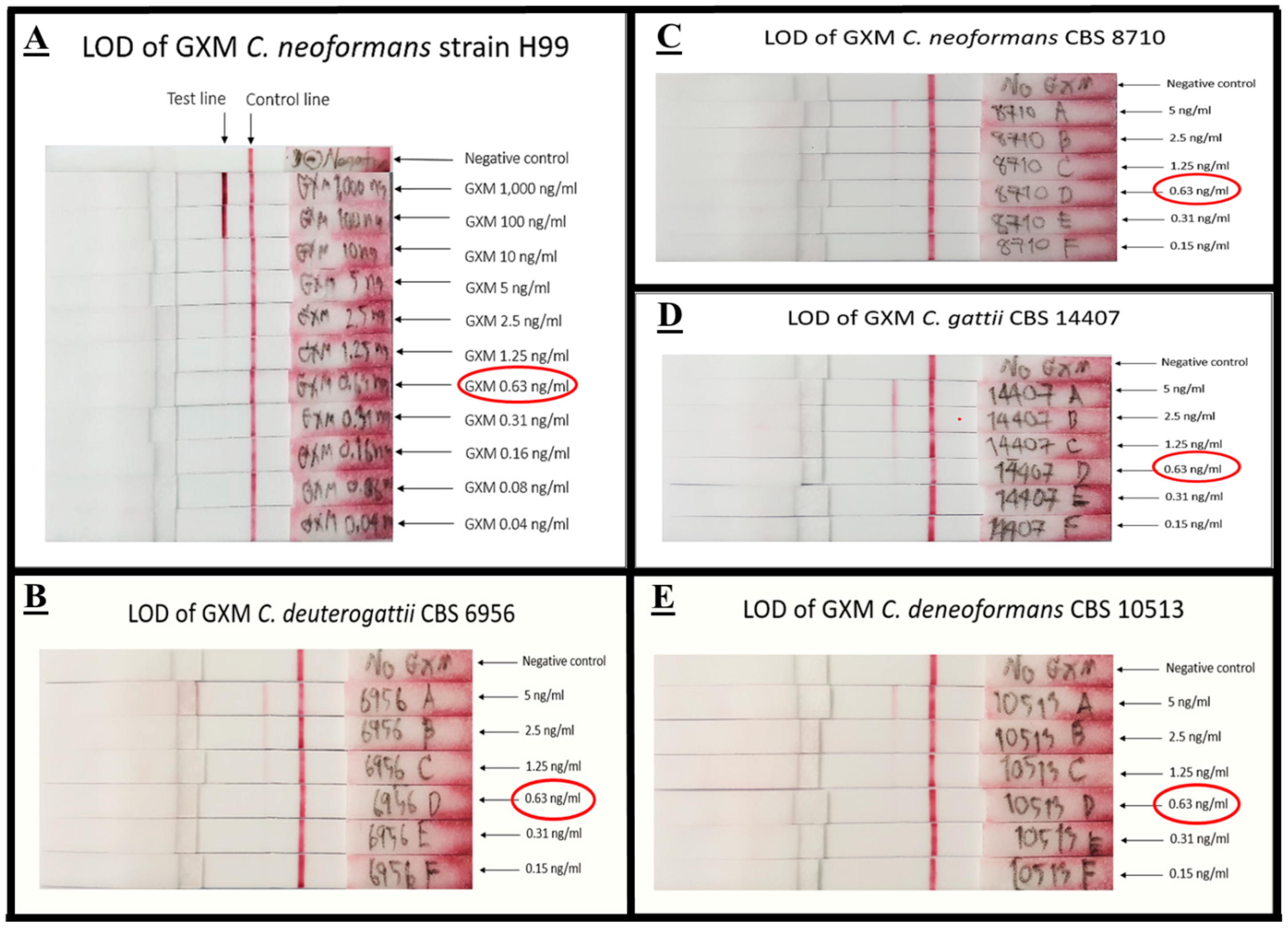
3.3.1. Limit of Detection (LOD) of mAb 18B7 ICT Strip
3.3.2. Cross-Reactivity of mAb 18B7 ICT Strip
3.4. Evaluation of mAb 18B7 ICT Strip with Clinical Sample and Diagnostic Performance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuger, A.; Louie, E.; Holzman, R.S.; Simberkoff, M.S.; Rahal, J.J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann. Intern. Med. 1986, 104, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.A.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.C.; Xess, I.; Jain, N. Evaluation of conventional & serological methods for rapid diagnosis of cryptococcosis. Indian J. Med. Res. 2008, 127, 483–488. [Google Scholar] [PubMed]
- Antinori, S. New Insights into HIV/AIDS-associated cryptococcosis. ISRN AIDS 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Vanselow, M.; Brandt, M.E.; Park, B.J. Diagnosis and management of cryptococcal disease in resource-limited settings. Curr. Fungal Infect. Rep. 2012, 6, 35–40. [Google Scholar] [CrossRef]
- Diamond, R.D.; Bennett, J.E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann. Intern. Med. 1974, 80, 176–181. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Percival, A.; Bauman, S.; Pelfrey, J.; Meintjes, G.; Williams, G.N.; Longley, N.; Harrison, T.S.; Kozel, T.R. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2011, 53, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Trovero, A.C.; Mazza, M.; Rogé, A.; Rivas, M.C.; Bordagorría, X.; Bruno, S.; Davel, G. Production of a latex agglutination reagent for the rapid diagnosis of cryptococcal meningitis. Rev. Argent. Microbiol. 2020, 52, 169–175. [Google Scholar] [CrossRef]
- Rivet-Dañon, D.; Guitard, J.; Grenouillet, F.; Gay, F.; Ait-Ammar, N.; Angoulvant, A.; Marinach, C.; Hennequin, C. Rapid diagnosis of cryptococcosis using an antigen detection immunochromatographic test. J. Infect. 2015, 70, 499–503. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Kumar, P.; Yang, M.; Haynes, B.C.; Skowyra, M.L.; Doering, T.L. Emerging themes in cryptococcal capsule synthesis. Curr. Opin. Struct. Biol. 2011, 21, 597–602. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.; Zaragoza, O.; Casadevall, A. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 2006, 14, 497–505. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.C.; de Jesus, M.; Casadevall, A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J. Biol. Chem. 2006, 281, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Mukherjee, J.; Devi, S.J.N.; Schneerson, R.; Robbins, J.B.; Scharff, M.D. Antibodies elicited by a Cryptococcus neoformans-Tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 1992, 165, 1086–1093. [Google Scholar] [CrossRef]
- Devi, S.J.; Schneerson, R.; Egan, W.; Ulrich, T.J.; Bryla, D.; Robbins, J.B.; Bennett, J.E. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: Synthesis, characterization, and immunogenicity. Infect. Immun. 1991, 59, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.A.; Pappas, P.G.; Perfect, J.; Aberg, J.A.; Casadevall, A.; Cloud, G.A.; James, R.; Filler, S.; Dismukes, W.E. Phase I Evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 2005, 49, 952–958. [Google Scholar] [CrossRef]
- Nimrichter, L.; Frases, S.; Cinelli, L.P.; Viana, N.B.; Nakouzi, A.; Travassos, L.R.; Casadevall, A.; Rodrigues, M.L. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot. Cell 2007, 6, 1400–1410. [Google Scholar] [CrossRef]
- Prakit, K.; Nosanchuk, J.D.; Pruksaphon, K.; Vanittanakom, N.; Youngchim, S. A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 647–656. [Google Scholar] [CrossRef]
- Jeavons, L.; Hamilton, A.J.; Vanittanakom, N.; Ungpakorn, R.; Evans, E.G.V.; Sirisanthana, T.; Hay, R.J. Identification and purification of fpecific Penicillium marneffei antigens and their recognition by human immune sera. J. Clin. Microbiol. 1998, 36, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Pruksaphon, K.; Intaramat, A.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Development and characterization of an immunochromatographic test for the rapid diagnosis of Talaromyces (Penicillium) marneffei. PLoS ONE 2018, 13, e0195596. [Google Scholar] [CrossRef]
- Johnstone, A.; Thorpe, R. “Immunoassays” in Immunochemistry in Practice, 2nd ed.; Johnstone, A., Thorpe, R., Eds.; Blackwell Scientific Pub: London, UK, 1988. [Google Scholar]
- Liu, L.; Yang, D.; Liu, G. Signal amplification strategies for paper-based analytical devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Lyman, C.A.; Devi, S.J.; Nathanson, J.; Frasch, C.E.; Pizzo, P.A.; Walsh, T.J. Detection and quantitation of the glucuronoxylomannan-like polysaccharide antigen from clinical and nonclinical isolates of Trichosporon beigelii and implications for pathogenicity. J. Clin. Microbiol. 1995, 33, 126–130. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Frases, S.; Casadevall, A.; Fischman-Gompertz, O.; Nimrichter, L.; Rodrigues, M.L. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet. Biol. 2009, 46, 496–505. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.C.; Fries, B.C.; Wang, F.; Casadevall, A. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Widiyanti, D.; Koizumi, N.; Fukui, T.; Muslich, L.T.; Segawa, T.; Villanueva, S.Y.A.M.; Saito, M.; Masuzawa, T.; Gloriani, N.G.; Yoshida, S.-I. Development of immunochromatography-based methods for detection of Leptospiral lipopolysaccharide antigen in urine. Clin. Vaccine Immunol. 2013, 20, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kawatsu, K.; Kumeda, Y.; Taguchi, M.; Yamazaki-Matsune, W.; Kanki, M.; Inoue, K. Development and evaluation of immunochromatographic assay for simple and rapid detection of Campylobacter jejuni and Campylobacter coli in human stool specimens. J. Clin. Microbiol. 2008, 46, 1226–1231. [Google Scholar] [CrossRef][Green Version]
- Hansen, J.; Slechta, E.S.; Gates-Hollingsworth, M.A.; Neary, B.; Barker, A.P.; Bauman, S.; Kozel, T.R.; Hanson, K.E. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin. Vaccine Immunol. 2013, 20, 52–55. [Google Scholar] [CrossRef]
- Percival, A.; Thorkildson, P.; Kozel, T.R. Monoclonal antibodies specific for immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, reduce serotype bias in an immunoassay for cryptococcal antigen. Clin. Vaccine Immunol. 2011, 18, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.-A.; Rivera, J.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [CrossRef]
- Gates-Hollingsworth, M.A.; Kozel, T.R. Serotype sensitivity of a lateral flow immunoassay for cryptococcal antigen. Clin. Vaccine Immunol. 2013, 20, 634–635. [Google Scholar] [CrossRef][Green Version]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Meis, J.F.; Gould, I.M.; Fu, W.; Colombo, A.L.; Rodriguez-Noriega, E. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997–2005: An 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 2007, 45, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Wake, R.M.; Beyene, T.; Katende, A.; Letang, E.; Boulware, D.R. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J. Clin. Microbiol. 2018, 57, e01238-18. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.E.; Boulware, D.R. Lateral flow assay for cryptococcal antigen: An improtant advance to improve the continuum of HIV care and reduce cryptococcal meningitis-related mortality. Rev. Inst. Med. Trop. Sao. Paulo 2015, 57, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hristov, D.R.; Rodriguez-Quijada, C.; Gomez-Marquez, J.; Hamad-Schifferli, K. Designing paper-based immunoassays for biomedical applications. Sensors 2019, 19, 554. [Google Scholar] [CrossRef] [PubMed]


| Fungal Species | Isolate Number |
|---|---|
| Talaromyces (Penicillium) marneffei | ATCC 200051 δ |
| Sporothrix schenckii | 52-S1 † |
| Candida albicans | ATCC 900028 δ |
| Candida krusei | CI |
| Candida glabrata | CI |
| Aspergillus fumigatus | 55-A1 † |
| Pseudallescheria boydii | MMC60S211 Ϩ |
| Trichosporon sp. | CI |
| Geotrichum sp. | CI |
| Type of Clinical Samples | Case/Control Samples | Total |
|---|---|---|
| Cerebrospinal fluid (CSF) | Cryptococcosis -Microscopy (India ink) confirmed: 4 cases -Culture proven confirmed: 3 cases | 28 |
| Non-Cryptococcosis # | 25 | |
| Total | 53 | |
| Serum | Cryptococcosis | 52 |
| Non-Cryptococcosis # | 24 | |
| Normal Healthy | 21 | |
| Total | 97 | |
| Total | 150 | |
| (A) | |||
| mAb 18B7 ICT Strip | Rapid Test of CrAg® IMMY | Total | |
| Cryptococcosis (Positive) | Non-Cryptococcosis (Negative) | ||
| Positive | 26 (TP) * | 0 (FP) * | 26 |
| Negative | (FN) * | 25 (TN) * | 27 |
| Total | 28 | 25 | 53 |
| (B) | |||
| Diagnostic Performance Criteria for CSF | Percentages (95% C.I.) | ||
| Sensitivity | 92.86% (76.27–99.10) | ||
| Specificity | 100% (84.24–100) | ||
| Positive predictive value | 100% (84.76–100) | ||
| Negative predictive value | 92.59% (75.53–99.04) | ||
| Accuracy | 96.23% (86.51–99.69) | ||
| Cohen’s kappa coefficient (κ) | 0.925 (0.822–1.000) | ||
| (A) | |||
| mAb 18B7 ICT Strip | Rapid Test of CrAg® IMMY | Total | |
| Cryptococcosis (Positive) | Non-Cryptococcosis (Negative) | ||
| Positive | 50 (TP) * | 1 (FP) * | 51 |
| Negative | 2 (FN) * | 44 (TN) * | 46 |
| Total | 52 | 45 | 97 |
| (B) | |||
| Diagnostic Performance Criteria for Serum | Percentages (95% C.I.) | ||
| Sensitivity | 96.15% (86.28–99.68) | ||
| Specificity | 97.78% (87.37–99.94) | ||
| Positive predictive value | 98.04% (88.73–99.95) | ||
| Negative predictive value | 96.65% (85.16–99.47) | ||
| Accuracy | 96.91% (91.23–99.36) | ||
| Cohen’s kappa coefficient (κ) | 0.938 (0.869–1.000) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathongdejwisit, P.; Pruksaphon, K.; Intaramat, A.; Aiumurai, P.; Sookrung, N.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Youngchim, S. A Novel, Inexpensive In-House Immunochromatographic Strip Test for Cryptococcosis Based on the Cryptococcal Glucuronoxylomannan Specific Monoclonal Antibody 18B7. Diagnostics 2021, 11, 758. https://doi.org/10.3390/diagnostics11050758
Sathongdejwisit P, Pruksaphon K, Intaramat A, Aiumurai P, Sookrung N, Ratanabanangkoon K, Nosanchuk JD, Youngchim S. A Novel, Inexpensive In-House Immunochromatographic Strip Test for Cryptococcosis Based on the Cryptococcal Glucuronoxylomannan Specific Monoclonal Antibody 18B7. Diagnostics. 2021; 11(5):758. https://doi.org/10.3390/diagnostics11050758
Chicago/Turabian StyleSathongdejwisit, Pakornswit, Kritsada Pruksaphon, Akarin Intaramat, Pisinee Aiumurai, Nitat Sookrung, Kavi Ratanabanangkoon, Joshua D. Nosanchuk, and Sirida Youngchim. 2021. "A Novel, Inexpensive In-House Immunochromatographic Strip Test for Cryptococcosis Based on the Cryptococcal Glucuronoxylomannan Specific Monoclonal Antibody 18B7" Diagnostics 11, no. 5: 758. https://doi.org/10.3390/diagnostics11050758
APA StyleSathongdejwisit, P., Pruksaphon, K., Intaramat, A., Aiumurai, P., Sookrung, N., Ratanabanangkoon, K., Nosanchuk, J. D., & Youngchim, S. (2021). A Novel, Inexpensive In-House Immunochromatographic Strip Test for Cryptococcosis Based on the Cryptococcal Glucuronoxylomannan Specific Monoclonal Antibody 18B7. Diagnostics, 11(5), 758. https://doi.org/10.3390/diagnostics11050758








