Thermostable Heptaplex PCR Assay for the Detection of Six Respiratory Bacterial Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Clinical Isolates
2.2. Primers
2.3. Preparation of PAC and IAC Templates
2.4. Preparation of DNA Templates from Clinical Isolates
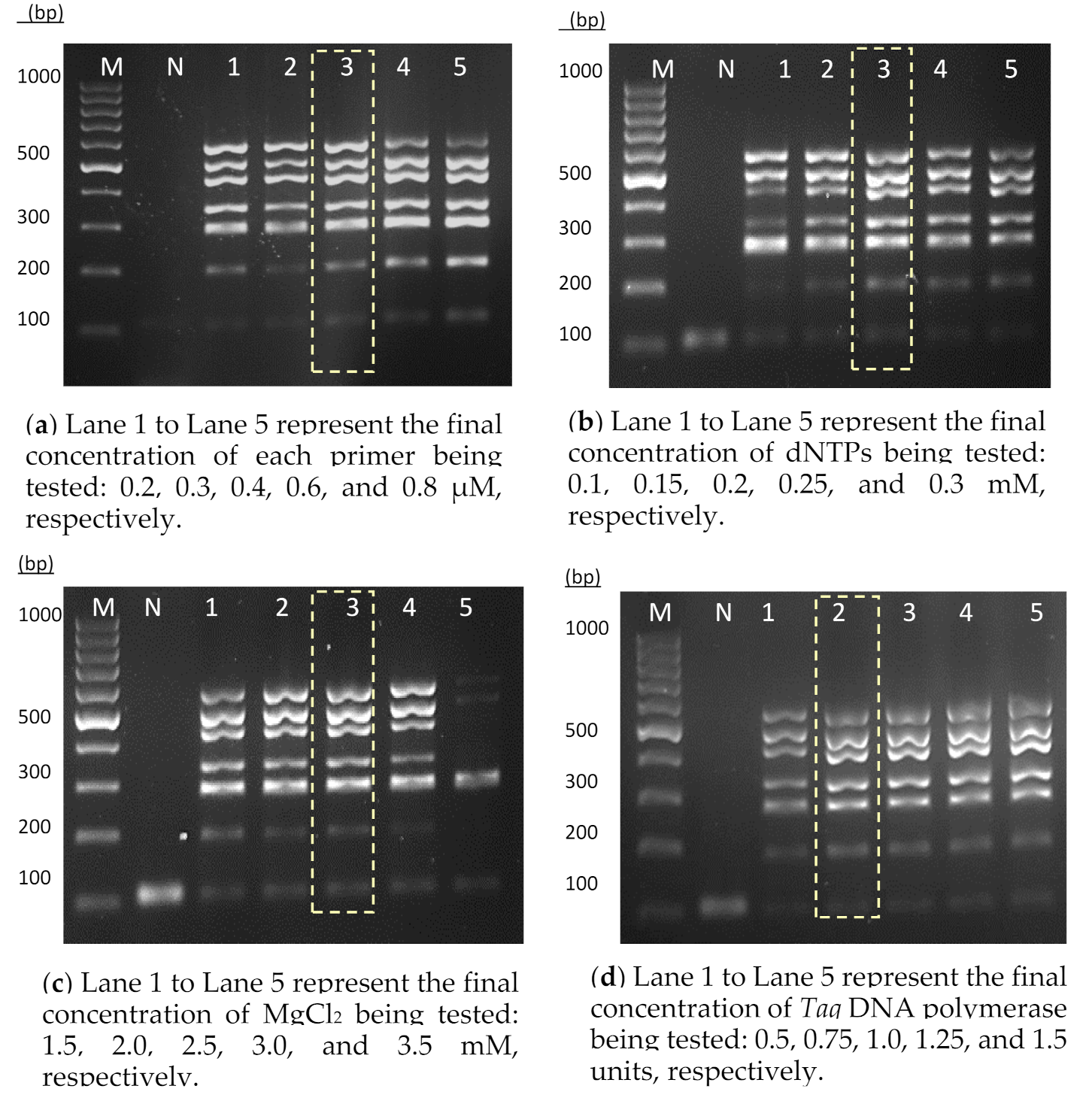
2.5. Development of Heptaplex PCR Assay
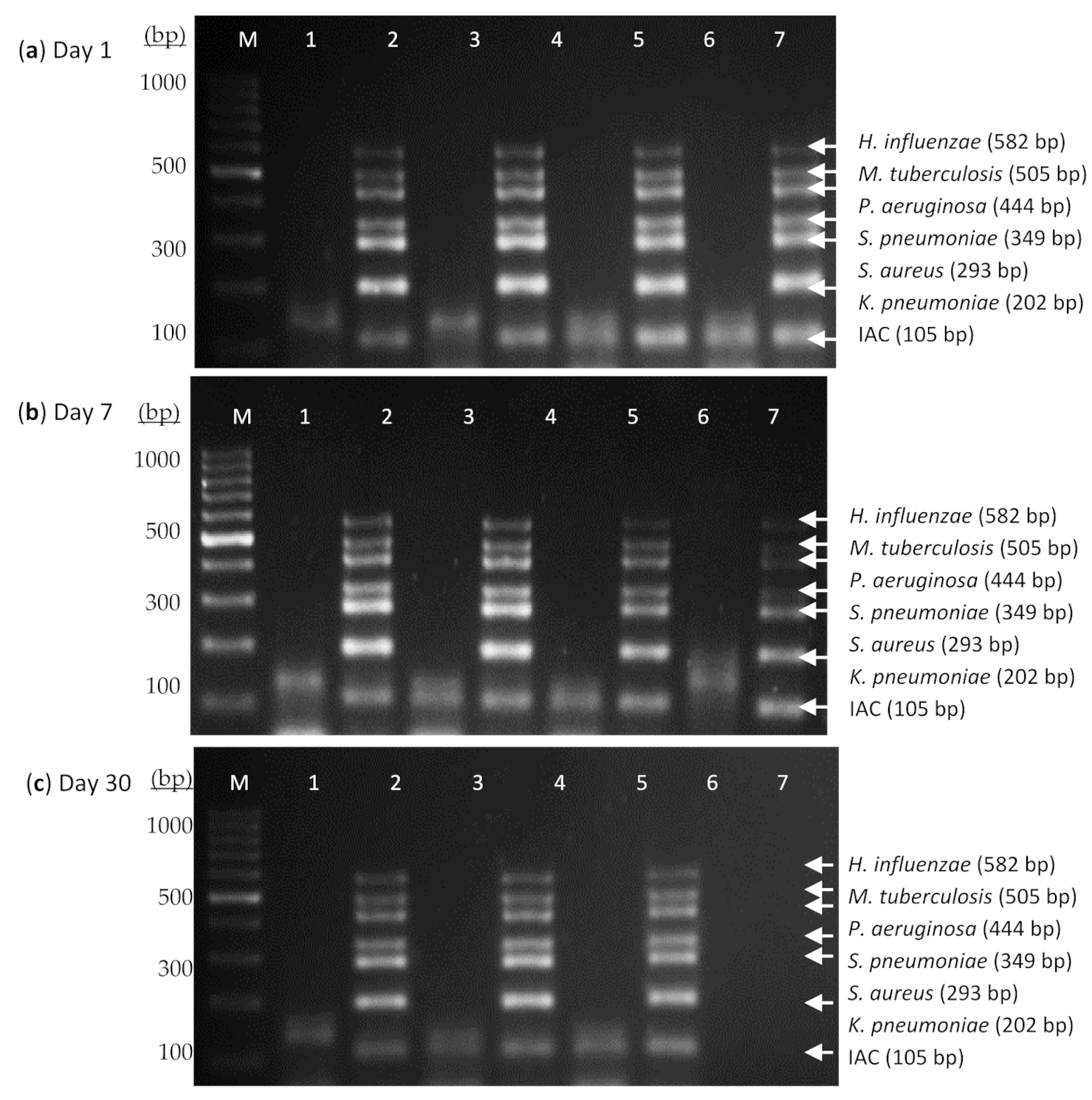
2.6. Thermostabilization and Stability Evaluation of the Heptaplex PCR Assay
2.7. Analytical Sensitivity and Specificity Evaluation of the Heptaplex PCR Assay
3. Results
3.1. Development of the Heptaplex PCR Assay
3.2. Thermostabilization and Stability Evaluation of the Heptaplex PCR Assay
3.3. Sensitivity and Specificity Evaluation of the Heptaplex PCR on the Reference Strains and Clinical Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, C.M.A.V.D.; Hulscher, M.E.J.L.; Akkermans, R.P.; Wille, J.; Geerlings, S.E.; Prins, J.M. Appropriate antibiotic use reduces length of hospital stay. J. Antimicrob. Chemother. 2016, 72, 923–932. [Google Scholar] [CrossRef]
- Steele, L.; Orefuwa, E.; Dickmann, P. Drivers of earlier infectious disease outbreak detection: A systematic literature review. Int. J. Infect. Dis. 2016, 53, 15–20. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 DALYs and HALE Collaborators; Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Kon, K.; Rai, M. The Microbiology of Respiratory System Infections; Kon, K., Rai, M., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Khan, S.; Priti, S.; Ankit, S. Bacteria Etiological Agents Causing Lower Respiratory Tract Infections and Their Resistance Patterns. Iran. Biomed. J. 2015, 19, 240–246. [Google Scholar]
- Robinson, J. Colonization and infection of the respiratory tract: What do we know? Paediatr. Child Heal. 2004, 9, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Eun, B.W.; Nahm, M.H. Diagnosis of Pneumococcal Pneumonia: Current Pitfalls and the Way Forward. Infect. Chemother. 2013, 45, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.L.; Elina, H.T.; Lim, B.H.; Yean, C.Y.; Ravichandran, M.; Lalitha, P.; Peuchant, O.; Duvert, J.P.; Clerc, M.; Raherison, S.; et al. Development of a dry reagent-based triplex PCR for the detection of toxigenic and non-toxigenic Vibrio cholerae. J. Med. Microbiol. 2011, 60, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Klatser, P.R.; Kuijper, S.; van Ingen, C.W.; Kolk, A.H. Stabilized, freeze-dried PCR mix for detection of mycobacteria. J. Clin. Microbiol. 1998, 36, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.S. Shelf Life of Medical Devices, Guidance Document. In Division of Small Manufacturers Assistance; Center for Devices and Radiological Health, Food and Drug Administration: Silver Spring, MD, USA, 1991; p. 24. [Google Scholar]
- Simões, E.A.F.; Patel, C.; Sung, W.-K.; Lee, C.W.H.; Loh, K.H.; Lucero, M.; Nohynek, H.; Nai, G.; Thien, P.L.; Koh, C.W.; et al. Pathogen Chip for Respiratory Tract Infections. J. Clin. Microbiol. 2013, 51, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Wunderink, R.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Balk, R.A.; Fakhran, S.S.; Chappell, J.D.; Casimir, G.; Courtney, D.M.; et al. Staphylococcus aureusCommunity-acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin. Infect. Dis. 2016, 63, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, M.G.C.; Vazquez, R.G.; Mendez, I.M.; Vargas, C.R.; Cerezo, S.G. Detection of the glmM Gene in Helicobacter pylori Isolates with a Novel Primer by PCR. J. Clin. Microbiol. 2011, 49, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Herrera, E.; Sperhacke, R.D.; Becker, D.; Kritski, A.; Zaha, A.; Rossetti, M.L.R. Internal control in PCR for Mycobacterium tuberculosis: Usefulness and improvement of the diagnosis. Braz. Arch. Biol. Technol. 2008, 51, 485–491. [Google Scholar] [CrossRef]
- Foo, P.C.; Chan, Y.Y.; Too, W.C.S.; Tan, Z.N.; Wong, W.K.; Lalitha, P.; Lim, B.H. Development of a thermostabilized, one-step, nested, tetraplex PCR assay for simultaneous identification and differentiation of Entamoeba species, Entamoeba histolytica and Entamoeba dispar from stool samples. J. Med. Microbiol. 2012, 61 Pt 9, 1219–1225. [Google Scholar] [CrossRef]
- Fluorogenics. Technology and Processes. Lyophilisation. 2012. Available online: http://www.fluorogenics.co.uk/technology-and-processes/ (accessed on 5 October 2018).
- Lin, F.-R.; Shen, Y.-H.; Fang, C.-W.; Shie, S.-S.; Huang, C.-G.; Yang, S.; Yang, S.-L.; Tsao, K.-C.; Huang, Y.-C.; Lai, M.-W.; et al. Incidence of and Factors Associated with False Positives in Laboratory Diagnosis of Norovirus Infection by Amplification of the RNA-Dependent RNA Polymerase Gene. PLoS ONE 2014, 9, e109876. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Panning, M.; Guenther, S.; Schmitz, H. False-Negative Results of PCR Assay with Plasma of Patients with Severe Viral Hemorrhagic Fever. J. Clin. Microbiol. 2002, 40, 4394–4395. [Google Scholar] [CrossRef] [PubMed]
- Molloy, P.J.; Persing, D.H.; Berardi, V.P. False-Positive Results of PCR Testing for Lyme Disease. Clin. Infect. Dis. 2001, 33, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Avashia, N. Research Techniques Made Simple: Polymerase Chain Reaction (PCR). J. Investig. Dermatol. 2013, 133, e6. [Google Scholar] [CrossRef] [PubMed]
- Welinder-Olsson, C.; Dotevall, L.; Hogevik, H.; Jungnelius, R.; Trollfors, B.; Wahl, M.; Larsson, P. Comparison of broad-range bacterial PCR and culture of cerebrospinal fluid for diagnosis of community-acquired bacterial meningitis. Clin. Microbiol. Infect. 2007, 13, 879–886. [Google Scholar] [CrossRef] [PubMed]



| Primers | Sequence (5′ → 3′) | Target Amplicon | Size (bp) |
|---|---|---|---|
| IAC_F 1AC_R | F: AAC TTA TCC CCA ATC GCG CA R: GCC CTT TCT TCT CAA GCG GT | H. pylori (IAC) | 105 |
| 1_F 2_R | F: ATT TCT CCG GCG TCA AGT GT R: CTC AAC ATC GTC GCA AAG GC | K. pneumoniae | 202 |
| 3_F 4_R | F: CGC AAA CTG TTG GCC ACT AT R: CTC GCC ATC ATG ATT CAA GT | S. aureus | 293 |
| 5_F 6_R | F: TTG ACC CAT CAG GGA GAA AG R: CTT GAT GCC ACT TAG CCA AC | S. pneumoniae | 349 |
| 7_F 8_R | F: GAT GGA AAT GCT GAA ATT CG R: GGA CGC TCT TTA CCA TAG GA | P. aeruginosa | 444 |
| 9_F 10_R | F: TGG AGG ATC CGT ACG AGA AG R: TTG ACA GTG GAC ACC TTG GA | M. tuberculosis complex | 505 |
| 11_F 12_R | F: GCG AAA GTC CAA GCC TCT CT R: TCA CCG TAA GAT ACT GTG CCT | H. influenzae | 582 |
| Organism | Reference Strains, n | Clinical Isolates, n | Total Test, n | Positive Test, n (%) | Negative Test, n (%) | |
|---|---|---|---|---|---|---|
| 1. | K. pneumoniae | 2 | 19 | 21 | 21 (100) | 0 (0) |
| 2. | S. aureus | 2 | 16 | 18 | 18 (100) | 0 (0) |
| 3. | S. pneumoniae | 3 | 17 | 20 | 20 (100) | 0 (0) |
| 4. | P. aeruginosa | 2 | 21 | 23 | 23 (100) | 0 (0) |
| 5. | M. tuberculosis | 2 | 16 | 18 | 18 (100) | 0 (0) |
| 6. | H. influenzae | 2 | 17 | 19 | 19 (100) | 0 (0) |
| 7. | A. hydrophila | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 8. | A. baumannii | 1 | 2 | 3 | 0 (0) | 3 (100) |
| 9. | Acinetobacter spp. | 0 | 3 | 3 | 0 (0) | 3 (100) |
| 10. | A. xylosoxidans | 0 | 2 | 2 | 0 (0) | 2 (100) |
| 11. | B. cereus | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 12 | B. subtilis | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 13. | B. pseudomallei | 0 | 1 | 1 | 0 (0) | 1 (100) |
| 14. | C. freundii | 0 | 2 | 2 | 0 (0) | 2 (100) |
| 15. | Enterobacter species | 0 | 2 | 2 | 0 (0) | 2 (100) |
| 16. | E. aerogenes | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 17. | E. cloacae | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 18. | E. coli | 1 | 6 | 7 | 0 (0) | 7 (100) |
| 19. | E. coli O157 | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 20. | Klebsiella spp. | 0 | 1 | 1 | 0 (0) | 1 (100) |
| 21. | L. monocytogenes | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 22. | M. catarrhalis | 0 | 2 | 2 | 0 (0) | 2 (100) |
| 23. | N. gonorrhoeae | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 24. | N. meningitidis | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 25. | P. mirabilis | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 26. | R. gilardii | 0 | 1 | 1 | 0 (0) | 1 (100) |
| 27. | S. epidermis | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 28. | S. marcescens | 0 | 1 | 1 | 0 (0) | 1 (100) |
| 29. | S. mutans | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 30. | S. pyogenes | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 31. | S. sanguinis | 1 | 0 | 1 | 0 (0) | 1 (100) |
| 32. | S. viridians | 1 | 2 | 3 | 0 (0) | 3 (100) |
| 33. | Serratia marcescens | 0 | 1 | 1 | 0 (0) | 1 (100) |
| 34. | Streptococcus group A | 0 | 5 | 5 | 0 (0) | 5 (100) |
| 35. | Streptococcus group B | 0 | 4 | 4 | 0 (0) | 4 (100) |
| 36. | Streptococcus group C | 0 | 2 | 2 | 0 (0) | 2 (100) |
| 37. | Streptococcus group G | 0 | 2 | 2 | 0 (0) | 2 (100) |
| Evaluation of the developed multiplex PCR assay for RTIs: | ||||||
| Total test, N | : | 175 | ||||
| Total positive | : | 119/119 | (100%) | |||
| Total negative | : | 57/57 | (100%) | |||
| Test accuracy | : | 175/175 | (100%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nik Zuraina, N.M.N.; Goni, M.D.; Amalina, K.N.; Hasan, H.; Mohamad, S.; Suraiya, S. Thermostable Heptaplex PCR Assay for the Detection of Six Respiratory Bacterial Pathogens. Diagnostics 2021, 11, 753. https://doi.org/10.3390/diagnostics11050753
Nik Zuraina NMN, Goni MD, Amalina KN, Hasan H, Mohamad S, Suraiya S. Thermostable Heptaplex PCR Assay for the Detection of Six Respiratory Bacterial Pathogens. Diagnostics. 2021; 11(5):753. https://doi.org/10.3390/diagnostics11050753
Chicago/Turabian StyleNik Zuraina, Nik Mohd Noor, Mohammed Dauda Goni, Khazani Nur Amalina, Habsah Hasan, Suharni Mohamad, and Siti Suraiya. 2021. "Thermostable Heptaplex PCR Assay for the Detection of Six Respiratory Bacterial Pathogens" Diagnostics 11, no. 5: 753. https://doi.org/10.3390/diagnostics11050753
APA StyleNik Zuraina, N. M. N., Goni, M. D., Amalina, K. N., Hasan, H., Mohamad, S., & Suraiya, S. (2021). Thermostable Heptaplex PCR Assay for the Detection of Six Respiratory Bacterial Pathogens. Diagnostics, 11(5), 753. https://doi.org/10.3390/diagnostics11050753







