Individualised Timing of Radio-Guided Parathyroidectomy Using Multi-Phase SPECT/CT Increases In Vivo Sensitivity and Accuracy and Reduces Operating Time: A Randomised Clinical Trial
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Design and Setting
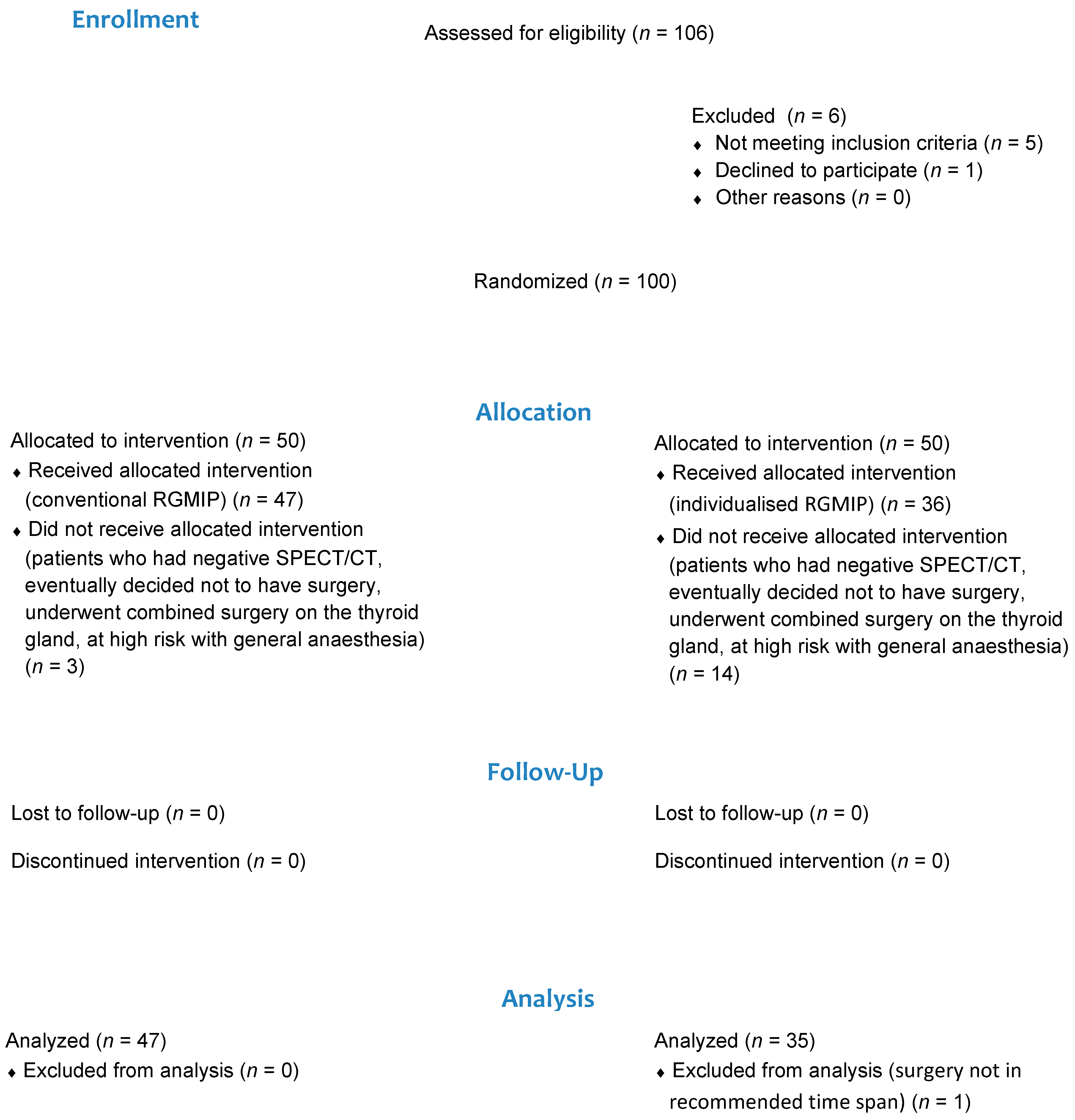
2.3. Participants and Randomisation
2.4. SPECT/CT
2.4.1. Group I
2.4.2. Group II
2.4.3. Surgery
2.4.4. Follow-Up
2.4.5. Statistical Analysis
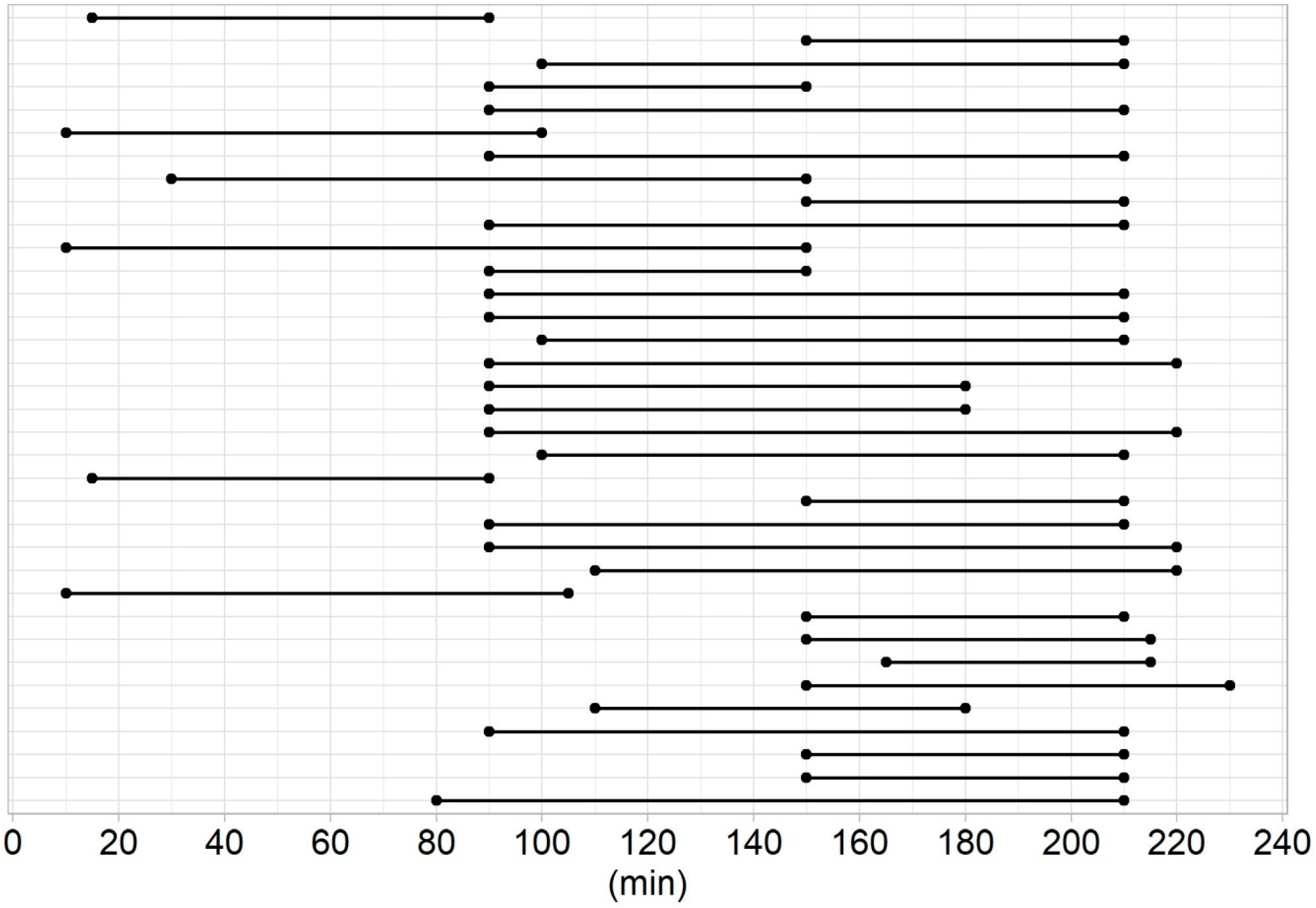
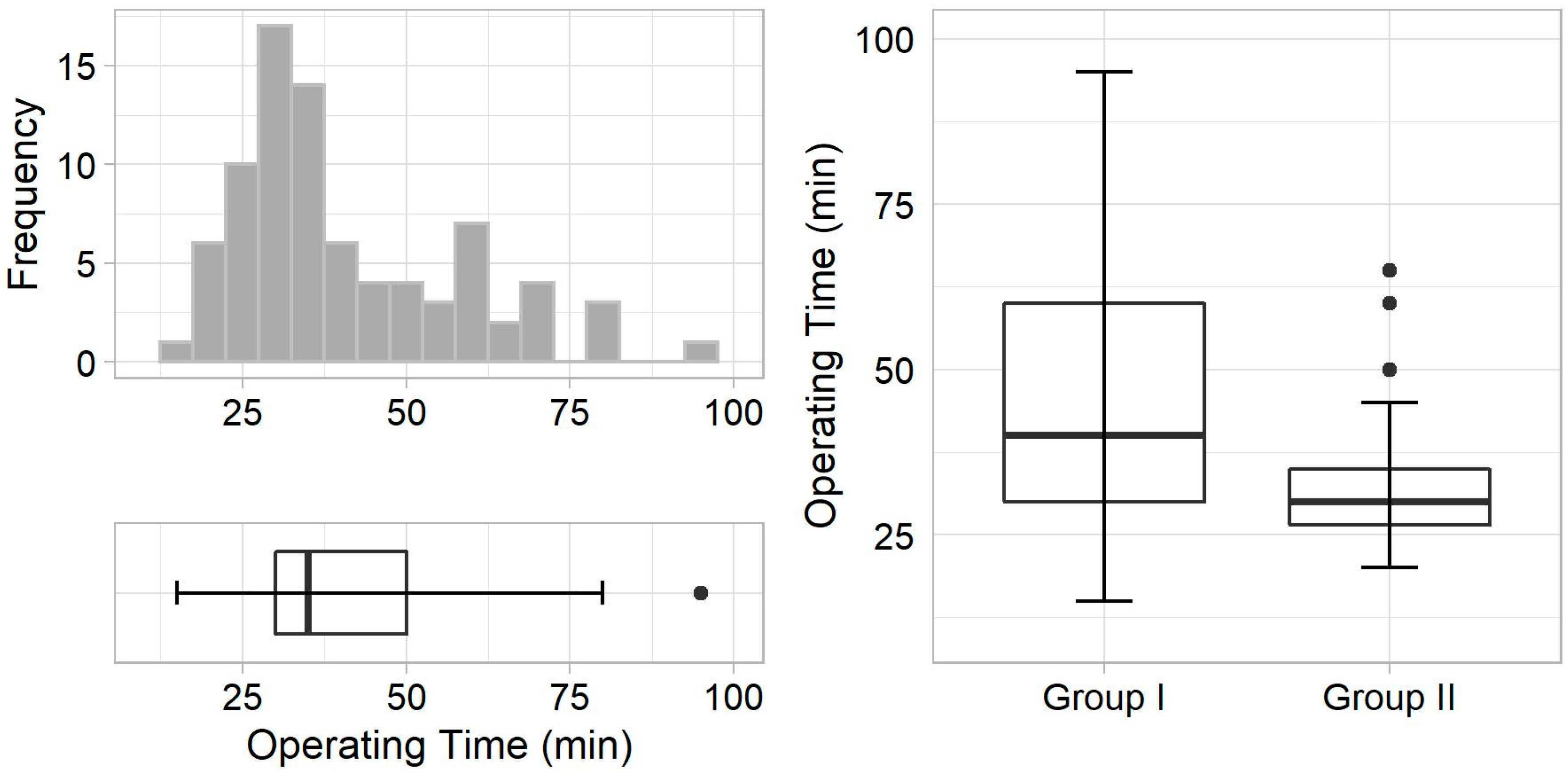
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruda, J.M.; Hollenbeak, C.S.; Stack, B.C., Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol. Head Neck Surg. 2005, 132, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Lebastchi, A.H.; Donovan, P.I.; Udelsman, R. Paradigm shift in the surgical management of multigland parathyroid hyperplasia: An individualized approach. JAMA Surg. 2014, 149, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, S.I.; Gooi, Z.; Tufano, R.P. Minimally invasive parathyroid surgery. Gland Surg. 2015, 4, 410–419. [Google Scholar] [PubMed]
- Slepavicius, A.; Beisa, V.; Janusonis, V.; Strupas, K. Focused versus conventional parathyroidectomy for primary hyperparathyroidism: A prospective, randomized, blinded trial. Langenbecks Arch. Surg. 2008, 393, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R.; Lin, Z.; Donovan, P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann. Surg. 2011, 253, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Schalin-Jäntti, C.; Caldarella, C.; Ceriani, L.; Giovanella, L.; Eisele, D.W. Detection rate of (99m) Tc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: A meta-analysis. Head Neck. 2016, 38 (Suppl. 1), E2159–E2172. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Mazeh, H.; Chen, H.; Sippel, R.S. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J. Surg. 2013, 37, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Oksüz, M.O.; Dittmann, H.; Wicke, C.; Müssig, K.; Bares, R.; Pfannenberg, C.; Eschmann, S.M. Accuracy of parathyroid imaging: A comparison of planar scintigraphy, SPECT, SPECT-CT, and C-11 methionine PET for the detection of parathyroid adenomas and glandular hyperplasia. Diagn. Interv. Radiol. 2011, 17, 297–307. [Google Scholar] [CrossRef]
- Norman, J.; Politz, D. Measuring individual parathyroid gland hormone production in real-time during radioguided parathyroidectomy. Experience in over 8000 operations. Minerva Endocrinol. 2008, 33, 147–157. [Google Scholar]
- García-Talavera, P.; García-Talavera, J.R.; González, C.; Martín, E.; Martín, M.; Gómez, A. Efficacy of in-vivo counting in parathyroid radioguidedsurgery and usefulness of its association withscintigraphy and intraoperative PTHi. Nucl. Med. Commun. 2011, 32, 847–852. [Google Scholar] [CrossRef]
- Murphy, C.; Norman, J. The 20% rule: A simple, instantaneous radioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery 1999, 126, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- McGreal, G.; Winter, D.C.; Sookhai, S.; Evoy, D.; Ryan, M.; O’Sullivan, G.C.; Redmond, H.P. Minimally invasive, radioguided surgery for primary hyperparathyroidism. Ann. Surg. Oncol. 2001, 8, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Hinson, A.M.; Lawson, B.R.; Franco, A.T.; Stack, B.C., Jr. Association of Parathyroid Gland Biopsy Excision Technique with ex Vivo Radiation Counts During Radioguided Parathyroid Surgery. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mack, E.; Starling, J.R. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: Which is most reliable? Ann. Surg. 2005, 242, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Gurpinar, B.; Schalch, P.; Joseph, N.J. Guidelines for radioguided parathyroid surgery. Arch Otolaryngol. Head Neck Surg. 2007, 133, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Quillo, A.R.; Bumpous, J.M.; Goldstein, R.E.; Fleming, M.M.; Flynn, M.B. Minimally invasive parathyroid surgery, the Norman 20% rule: Is it valid? Am Surg. 2011, 77, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.I.; Rivera, M.; Irvin, G.L., 3rd; Solorzano, C.C. Operative failure in the era of focused parathyroidectomy: A contemporary series of 845 patients. Arch Surg. 2010, 145, 628–633. [Google Scholar] [CrossRef]
- Lim, M.S.; Jinih, M.; Ngai, C.H.; Foley, N.M.; Redmond, H.P. The utility of the radionuclide probe in parathyroidectomy for primary hyperparathyroidism. Ann. R. Coll. Surg. Engl. 2017, 99, 369–372. [Google Scholar] [CrossRef]
- O’Doherty, M.J.; Kettle, A.G.; Wells, P.; Collins, R.E.; Coakley, A.J. Parathyroid imaging with technetium-99m-sestamibi: Preoperative localization and tissue uptake studies. J. Nucl. Med. 1992, 33, 313–318. [Google Scholar]
- Bénard, F.; Lefebvre, B.; Beuvon, F.; Langlois, M.F.; Bisson, G. Rapid washout of technetium-99m-MIBI from a large parathyroid adenoma. J. Nucl. Med. 1995, 36, 241–243. [Google Scholar]
- Norman, J.; Politz, D. 5000 parathyroid operations without frozen section or PTH assays: Measuring individual parathyroid gland hormone production in real time. Ann. Surg. Oncol. 2009, 16, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Gulec, S.A.; Rubello, D.; Boni, G.; Puccini, M.; Pelizzo, M.R.; Manca, G.; Casara, D.; Sotti, G.; Erba, P.; et al. Preoperative localization and radioguided parathyroid surgery. J. Nucl. Med. 2003, 44, 1443–1458. [Google Scholar] [PubMed]
- Buicko, J.L.; Kichler, K.M.; Amundson, J.R.; Scurci, S.; Kozol, R.A. The Sestamibi Paradox: Improving Intraoperative Localization of Parathyroid Adenomas. Am. Surg. 2017, 83, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.G.; Ituarte, P.H.; Harari, A.; Wu, J.X.; Yeh, M.W. Trends in the frequency and quality of parathyroid surgery: Analysis of 17,082 cases over 10 years. Ann. Surg. 2015, 261, 746–750. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group I (n = 47) | Group II (n = 35) | p-Value |
|---|---|---|---|
| Age, years | 64.0 (55.0–71.0) | 62.0 (53.0–67.5) | 0.183 * |
| Females | 34 (72) | 27 (77) | 0.799 ** |
| Males | 13 (28) | 8 (23) | |
| Ectopic adenoma | 17 (36) | 12 (34) | >0.999 ** |
| Result | Group I (n = 47) | Group II (n = 35) | p-Value |
|---|---|---|---|
| Successful surgery | 46 (98) | 35 (100) | >0.999 * |
| Histology, hyperplasia | 1 (2) | 1 (3) | >0.999 * |
| Histology, adenoma | 45 (96) | 34 (97) | >0.999 * |
| Multiple adenomas | 1 (2) | 1 (3) | >0.999 * |
| Time delay, min | 160 (125–190) | 190 (140–205) | 0.260 ** |
| Parathyroid gland volume, mL | 1.8 (1.0–3.0) | 1.3 (1.0–2.4) | 0.234 ** |
| Operating time, min | 40.0 (30.0–60.0) | 30.0 (26.5–35.0) | 0.003 ** |
| Adenoma count rate, cpm | 1200 (860–1400) | 1200 (1100–1405) | 0.318 ** |
| Background count rate, cpm | 700 (500–800) | 720 (600–850) | 0.094 ** |
| In vivo index | 2.0 (1.5–2.2) | 1.8 (1.4–2.0) | 0.285 ** |
| Recurrent laryngeal nerve paralysis | 1 (2) | 0 (0) | >0.999 * |
| Hypocalcaemia | 2 (4) | 1 (3) | >0.999 * |
| Parameter | Group I | Group II | p-Value * |
|---|---|---|---|
| In vivo sensitivity | 40/47 (85.1%) | 35/35 (100%) | 0.047 |
| In vivo specificity | 45/47 (95.7%) | 35/35 (100%) | 0.609 |
| In vivo accuracy | 85/94 (90.4%) | 70/70 (100%) | 0.021 |
| Ex vivo sensitivity | 47/47 (100%) | 35/35 (100%) | >0.999 |
| Ex vivo specificity | 47/47 (100%) | 35/35 (100%) | >0.999 |
| Ex vivo accuracy | 94/94 (100%) | 70/70 (100%) | >0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formánek, M.; Dedek, V.; Koláček, M.; Havel, M.; Zeleník, K.; Komínek, P. Individualised Timing of Radio-Guided Parathyroidectomy Using Multi-Phase SPECT/CT Increases In Vivo Sensitivity and Accuracy and Reduces Operating Time: A Randomised Clinical Trial. Diagnostics 2021, 11, 677. https://doi.org/10.3390/diagnostics11040677
Formánek M, Dedek V, Koláček M, Havel M, Zeleník K, Komínek P. Individualised Timing of Radio-Guided Parathyroidectomy Using Multi-Phase SPECT/CT Increases In Vivo Sensitivity and Accuracy and Reduces Operating Time: A Randomised Clinical Trial. Diagnostics. 2021; 11(4):677. https://doi.org/10.3390/diagnostics11040677
Chicago/Turabian StyleFormánek, Martin, Vladimír Dedek, Michal Koláček, Martin Havel, Karol Zeleník, and Pavel Komínek. 2021. "Individualised Timing of Radio-Guided Parathyroidectomy Using Multi-Phase SPECT/CT Increases In Vivo Sensitivity and Accuracy and Reduces Operating Time: A Randomised Clinical Trial" Diagnostics 11, no. 4: 677. https://doi.org/10.3390/diagnostics11040677
APA StyleFormánek, M., Dedek, V., Koláček, M., Havel, M., Zeleník, K., & Komínek, P. (2021). Individualised Timing of Radio-Guided Parathyroidectomy Using Multi-Phase SPECT/CT Increases In Vivo Sensitivity and Accuracy and Reduces Operating Time: A Randomised Clinical Trial. Diagnostics, 11(4), 677. https://doi.org/10.3390/diagnostics11040677






