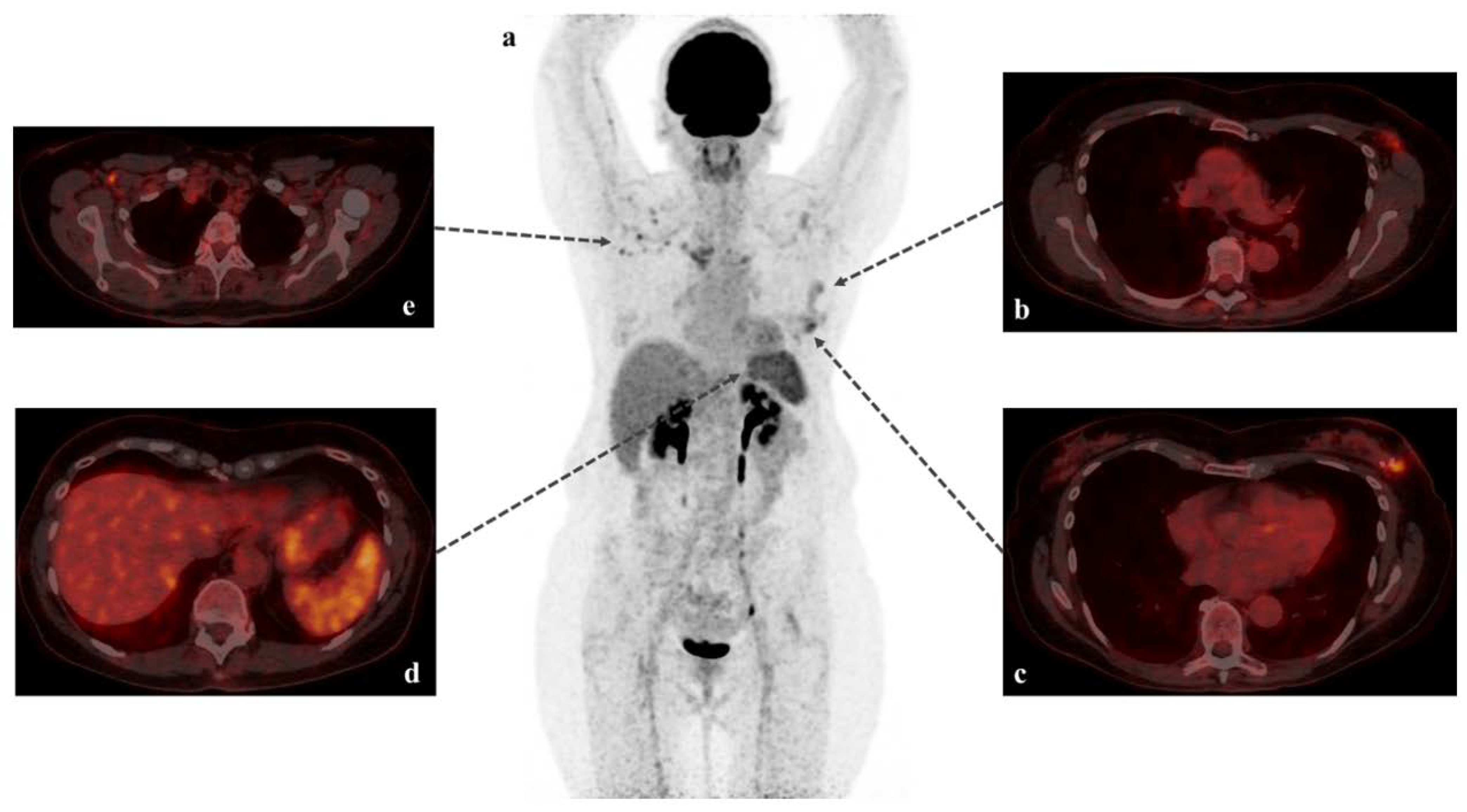
Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sugawara, Y.; Zasadny, K.R.; Kison, P.V.; Baker, L.H.; Wahl, R.L. Splenic Fluorodeoxyglucose Uptake Increased by Granulocyte Colony-Stimulating Factor Therapy: PET Imaging Results. J. Nucl. Med. 1999, 40, 1456–1462. [Google Scholar] [PubMed]
- Emami, H.; Singh, P.; MacNabb, M.; Vucic, E.; Lavender, Z.; Rudd, J.H.F.; Fayad, Z.A.; Lehrer-Graiwer, J.; Korsgren, M.; Figueroa, A.L.; et al. Splenic Metabolic Activity Predicts Risk of Future Cardiovascular Events: Demonstration of a Cardiosplenic Axis in Humans. JACC Cardiovasc. Imaging 2015, 8, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Salaun, P.Y.; Gastinne, T.; Bodet-Milin, C.; Campion, L.; Cambefort, P.; Moreau, A.; Le Gouill, S.; Berthou, C.; Moreau, P.; Kraeber-Bodéré, F. Analysis of 18F-FDG PET Diffuse Bone Marrow Uptake and Splenic Uptake in Staging of Hodgkin’s Lymphoma: A Reflection of Disease Infiltration or Just Inflammation? Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Pijl, J.P.; Kwee, T.C.; Slart, R.H.J.A.; Yakar, D.; Wouthuyzen-Bakker, M.; Glaudemans, A.W.J.M. Clinical Implications of Increased Uptake in Bone Marrow and Spleen on FDG-PET in Patients with Bacteremia. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Avner, M.; Orevi, M.; Caplan, N.; Popovtzer, A.; Lotem, M.; Cohen, J.E. COVID-19 Vaccine as a Cause for Unilateral Lymphadenopathy Detected by 18F-FDG PET/CT in a Patient Affected by Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 1–2. [Google Scholar] [CrossRef]
- Nawwar, A.A.; Searle, J.; Hagan, I.; Lyburn, I.D. COVID-19 Vaccination Induced Axillary Nodal Uptake on [18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.; Thomas, A.; Iravani, A. 18F-Fluorodeoxyglucose PET/CT Findings in a Systemic Inflammatory Response Syndrome after COVID-19 Vaccine. Lancet 2021, 397, e9. [Google Scholar] [CrossRef]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal Neoantigen Vaccines Induce Persistent Memory T Cell Responses and Epitope Spreading in Patients with Melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seban, R.-D.; Champion, L.; Deleval, N.; Richard, C.; Provost, C. Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics 2021, 11, 676. https://doi.org/10.3390/diagnostics11040676
Seban R-D, Champion L, Deleval N, Richard C, Provost C. Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics. 2021; 11(4):676. https://doi.org/10.3390/diagnostics11040676
Chicago/Turabian StyleSeban, Romain-David, Laurence Champion, Nicolas Deleval, Capucine Richard, and Claire Provost. 2021. "Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine" Diagnostics 11, no. 4: 676. https://doi.org/10.3390/diagnostics11040676
APA StyleSeban, R.-D., Champion, L., Deleval, N., Richard, C., & Provost, C. (2021). Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics, 11(4), 676. https://doi.org/10.3390/diagnostics11040676





