Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Imaging Studies
2.4. Intraoperative Assessment
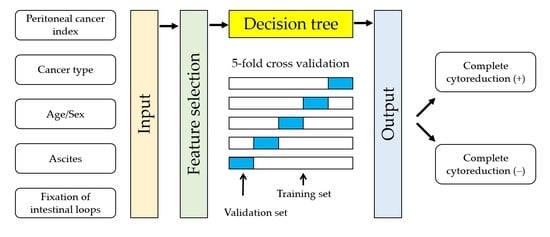
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Imaging and Intraoperative PCI
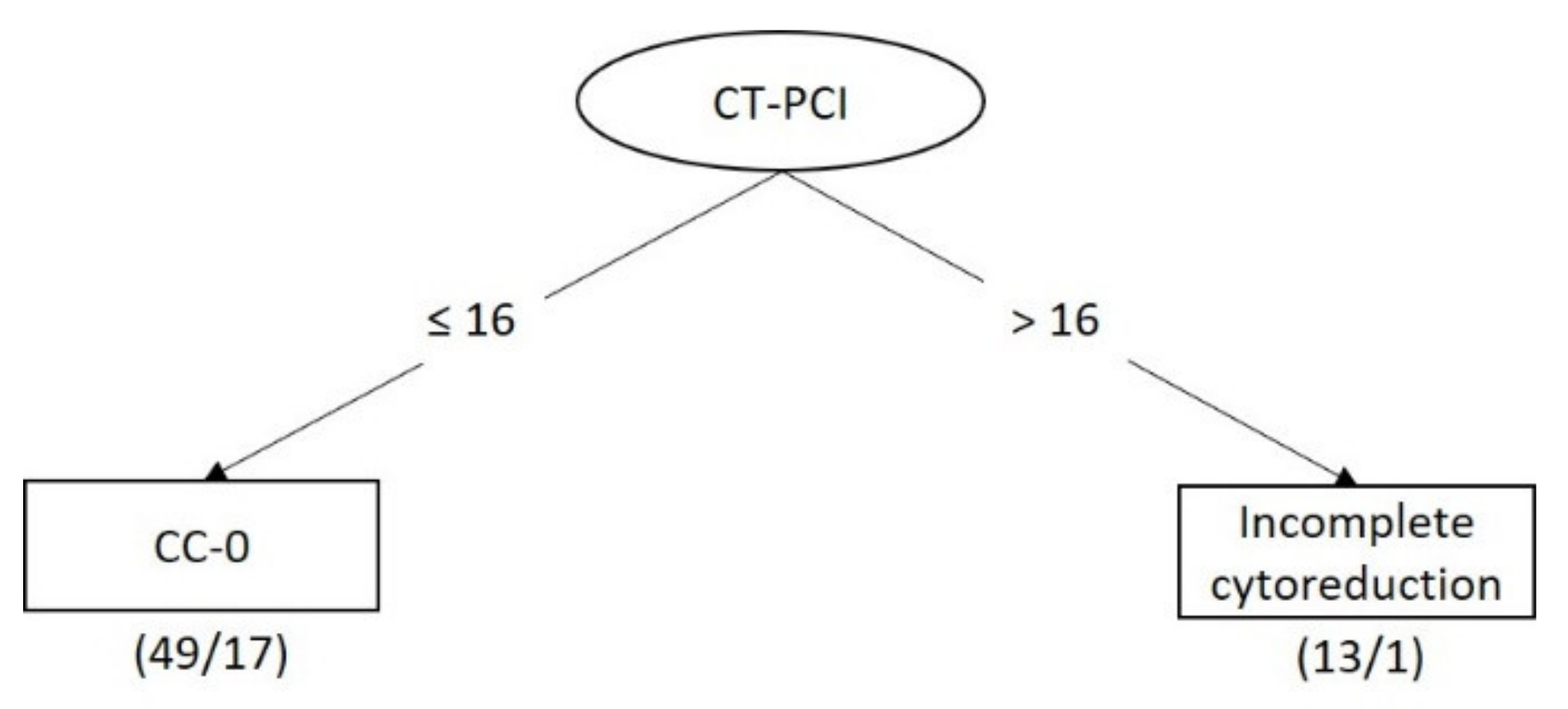
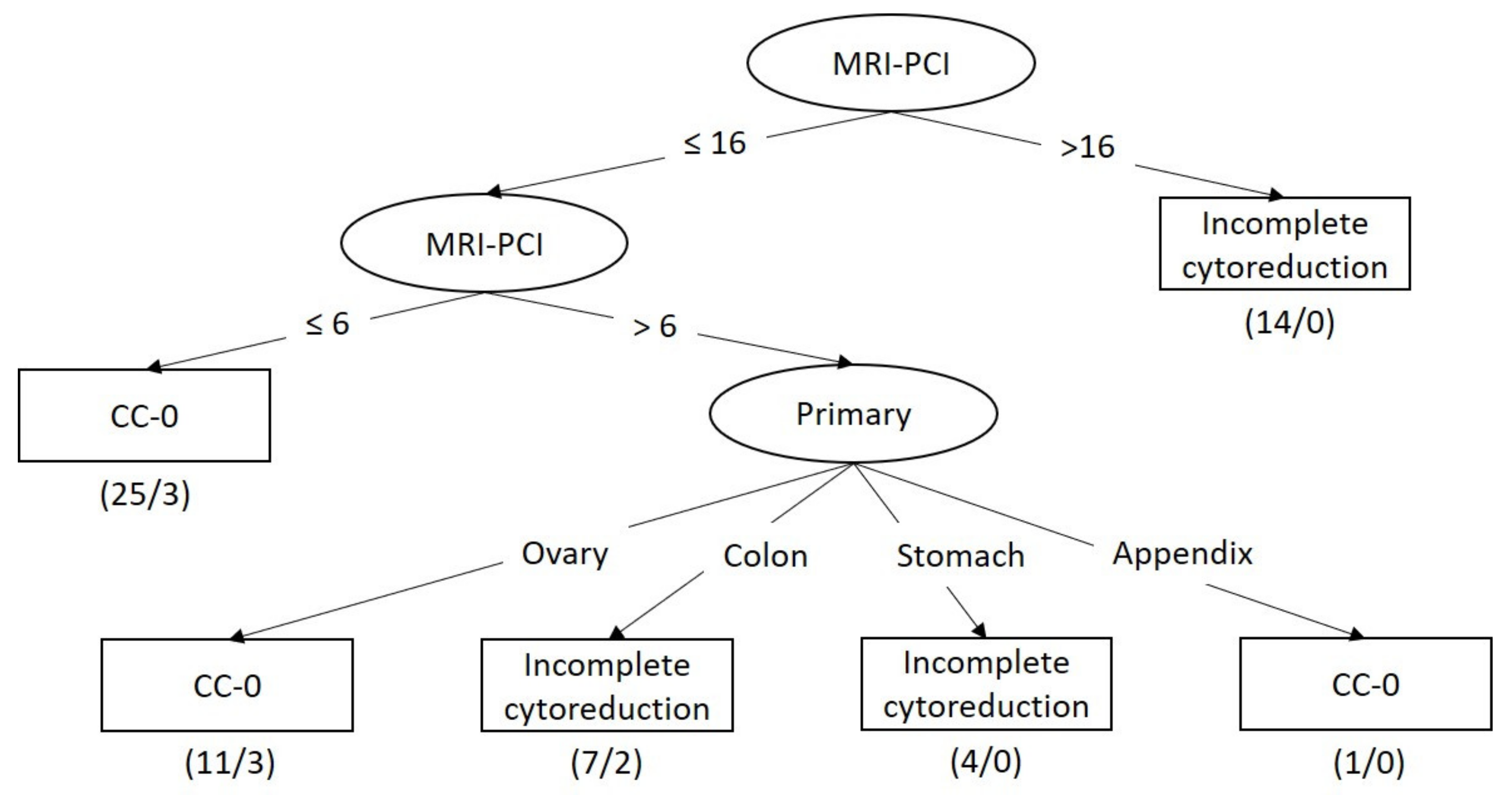
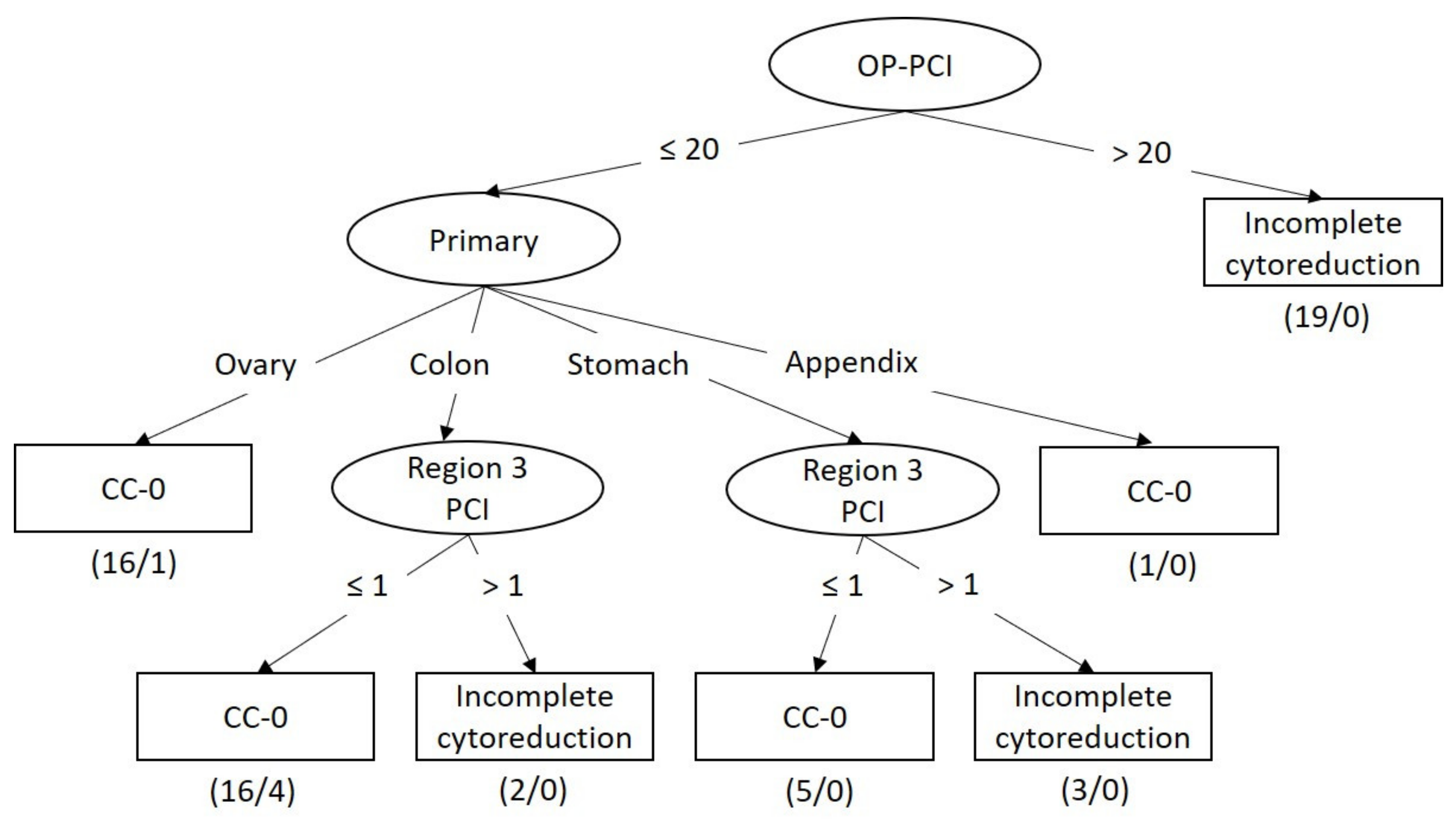
3.3. Model Performance for Predicting Incomplete Cytoreduction
3.4. Classifier Models for Predicting Incomplete Cytoreduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA A Cancer J. Clin. 2015, 65, 283–298. [Google Scholar] [CrossRef]
- Neuwirth, M.G.; Alexander, H.R.; Karakousis, G.C. Then and now: Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J. Gastrointest. Oncol. 2016, 7, 18–28. [Google Scholar]
- Sugarbaker, P.H. Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology 2004, 18, 51–59. [Google Scholar]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations—Part II: Postoperative management and special considerations. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Kim, B.J.; Villeneuve, L.; Vaudoyer, D.; Képénékian, V.; Bakrin, N.; Gilly, F.-N.; Cotte, E.; Glehen, O.; Passot, G. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int. J. Hyperth. 2017, 34, 532–537. [Google Scholar] [CrossRef]
- Aherne, E.A.; Fenlon, H.M.; Shields, C.J.; Mulsow, J.J.; Cronin, C.G. What the Radiologist Should Know About Treatment of Peritoneal Malignancy. Am. J. Roentgenol. 2017, 208, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.; Tyler, R.; Price, M.; Beggs, A.; Youssef, H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open 2019, 3, 585–594. [Google Scholar] [CrossRef]
- Chin, K.M.; Tan, G.H.C.; Chia, C.S.; Ong, J.C.A.; Teo, M.C.C. Novel prognostic score for outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer with metachronous peritoneal carcinomatosis. ANZ J. Surg. 2020, 90, 1958–1964. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for the treatment of advanced primary and recurrent ovarian cancer. Curr. Opin. Obstet. Gynecol. 2009, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of Preoperative Computed Tomography in Estimating Peritoneal Cancer Index in Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2008, 16, 327–333. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Rousset, P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: History, clinical applications, and implementation. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 65–74. [Google Scholar] [CrossRef]
- Diop, A.; Fontarensky, M.; Montoriol, P.-F.; Da Ines, D. CT imaging of peritoneal carcinomatosis and its mimics. Diagn. Interv. Imaging 2014, 95, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Matsusue, E.; Kanasaki, Y.; Kanamori, Y.; Nakanishi, J.; Sugihara, S.; Kigawa, J.; Terakawa, N.; Ogawa, T. Detection of peritoneal dissemination in gynecological malignancy: Evaluation by diffusion-weighted MR imaging. Eur. Radiol. 2007, 18, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Witten, I.H.; Frank, E.; Hall, M.A.; Pal, C.J. Data Mining: Practical Machine Learning Tools and Techniques (Morgan Kaufmann Series in Data Management Systems), 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Low, R.N. Preoperative and surveillance MR imaging of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2016, 7, 58–71. [Google Scholar]
- Lee, R.M.; Zaidi, M.Y.; Gamboa, A.C.; Speegle, S.; Kimbrough, C.W.; Cloyd, J.M.; Leiting, J.L.; Grotz, T.E.; Lee, A.J.; Fournier, K.F.; et al. What is the Optimal Preoperative Imaging Modality for Assessing Peritoneal Cancer Index? An Analysis from the United States HIPEC Collaborative. Clin. Color. Cancer 2020, 19, e1–e7. [Google Scholar] [CrossRef]
- Laghi, A.; Bellini, D.; Rengo, M.; Accarpio, F.; Caruso, D.; Biacchi, D.; Di Giorgio, A.; Sammartino, P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: Systematic review and meta-analysis. La Radiol. Med. 2017, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sant, I.V.T.; Van Eden, W.J.; Engbersen, M.P.; Kok, N.F.M.; Woensdregt, K.; Lambregts, D.M.J.; Shanmuganathan, S.; Beets-Tan, R.G.H.; Aalbers, A.G.J.; Lahaye, M.J. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. BJS 2019, 106, 491–498. [Google Scholar] [CrossRef]
- Dresen, R.C.; De Vuysere, S.; De Keyzer, F.; Van Cutsem, E.; Prenen, H.; Vanslembrouck, R.; De Hertogh, G.; Wolthuis, A.; D’Hoore, A.; Vandecaveye, V. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Abou-Taleb, H.; Yehia, A.; El Malek, N.A.A.; Siefeldein, G.S.; Badary, D.M.; Jabir, M.A. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad. Radiol. 2019, 26, 1650–1658. [Google Scholar] [CrossRef]
- Michielsen, K.; Vergote, I.; De Beeck, K.O.; Amant, F.; Leunen, K.; Moerman, P.; Deroose, C.; Souverijns, G.; Dymarkowski, S.; De Keyzer, F.; et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to CT and FDG-PET/CT. Eur. Radiol. 2013, 24, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Gryparis, A.; DeJong, D.; Hutson, R.; Theophilou, G.; Leach, C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J. Ovarian Res. 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.-Y. To predict or not to predict? The dilemma of predicting the risk of suboptimal cytoreduction in ovarian cancer. Ann. Oncol. 2011, 22, viii23–viii28. [Google Scholar] [CrossRef] [PubMed]
| All | Appendix | Colon | Ovary | Stomach | ANOVA | |
|---|---|---|---|---|---|---|
| No | 62 | 6 | 25 | 20 | 11 | |
| Sex (M/F) | 20/42 | 3/3 | 11/14 | 0/20 | 6/5 | |
| Age (ys) | 56 ± 11 | 57 ± 13 | 56 ± 11 | 55 ± 10 | 54 ± 13 | 0.910 |
| Peritoneal Cancer Index (PCI) | ||||||
| Intraoperative | 15 ± 11 | 29 ± 10 | 13 ± 12 | 14 ± 10 | 12 ± 9 | 0.011 |
| CT | 9 ± 10 *** | 26 ± 10 | 8 ± 9 ** | 9 ± 8 ** | 4 ± 2 ** | <0.001 |
| MRI | 11 ± 9 *** | 25 ± 9 | 10 ± 8 ** | 10 ± 9 ** | 7 ± 8 | 0.001 |
| Cytoreduction Score (CC) | ||||||
| CC-0 | 33 | 1 | 12 | 15 | 5 | |
| CC-1 | 8 | 2 | 3 | 2 | 1 | |
| CC-2 | 4 | 1 | 1 | 2 | 0 | |
| CC-3 | 17 | 2 | 9 | 1 | 5 | |
| Linear Regression | Bland-Altman Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | R | b | 95% CI | Constant | 95% CI | SEE | Bias | LOA | ICC (r) |
| All | |||||||||
| CT-PCI | 0.775 | 0.9 | 0.7, 1.1 | 6.2 | 3.7, 8.8 | 7.1 | −5.3 | −19.3, 8.6 | 0.680 |
| MRI-PCI | 0.856 | 1.0 | 0.9, 1.2 | 3.6 | 1.3, 5.9 | 5.8 | −3.8 | −15.1, 7.5 | 0.792 |
| Appendix | |||||||||
| CT-PCI | 0.851 | 0.8 | 0.1, 1.5 | 7.7 | −11.4, 26.8 | 5.7 | −3.0 | −13.7, 7.7 | 0.833 |
| MRI-PCI | 0.879 | 0.9 | 0.2, 1.7 | 4.8 | −14.2, 23.7 | 5.2 | −3.5 | −12.7, 5.7 | 0.835 |
| Colon | |||||||||
| CT-PCI | 0.745 | 1.0 | 0.6, 1.4 | 4.8 | 0.2, 9.4 | 7.9 | −4.9 | −20.0, 10.2 | 0.646 |
| MRI-PCI | 0.929 | 1.3 | 1.1, 1.6 | −0.1 | −3.0, 2.8 | 4.4 | −3.3 | −13.2, 6.6 | 0.830 |
| Ovary | |||||||||
| CT-PCI | 0.781 | 0.9 | 0.5, 1.3 | 5.7 | 1.4, 10.1 | 6.2 | −4.9 | −16.8, 7.0 | 0.679 |
| MRI-PCI | 0.858 | 1.0 | 0.7, 1.2 | 4.5 | 0.8, 8.1 | 5.1 | −4.1 | −13.8, 5.6 | 0.782 |
| Stomach | |||||||||
| CT-PCI | 0.522 | 2.1 | −0.5, 4.7 | 4.0 | −7.4, 15.5 | 8.3 | −8.3 | −24.4, 7.9 | 0.144 |
| MRI-PCI | 0.450 | 0.5 | −0.3, 1.3 | 8.2 | 0, 16.5 | 8.7 | −4.6 | −22.4, 13.2 | 0.407 |
| Tree Size | Accuracy | Sensitivity | Specificity | Precision | Recall | F-Measure | ROC Area | |
|---|---|---|---|---|---|---|---|---|
| CT Model | ||||||||
| J48 | 3 | 69.4% | 0.414 | 0.939 | 0.857 | 0.414 | 0.558 | 0.635 |
| REPTree | 3 | 69.4% | 0.414 | 0.939 | 0.857 | 0.414 | 0.558 | 0.635 |
| MRI Model | ||||||||
| J48 | 11 | 72.6% | 0.690 | 0.758 | 0.714 | 0.690 | 0.702 | 0.749 |
| REPTree | 9 | 79.0% | 0.690 | 0.879 | 0.833 | 0.690 | 0.755 | 0.786 |
| OP Model | ||||||||
| J48 | 11 | 87.1% | 0.793 | 0.939 | 0.920 | 0.793 | 0.852 | 0.868 |
| REPTree | 3 | 82.3% | 0.655 | 0.970 | 0.950 | 0.655 | 0.776 | 0.783 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-N.; Huang, W.-S.; Huang, T.-H.; Chen, C.-Y.; Huang, C.-Y.; Wang, T.-Y.; Liao, Y.-S.; Lee, L.-W. Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction. Diagnostics 2021, 11, 674. https://doi.org/10.3390/diagnostics11040674
Lin C-N, Huang W-S, Huang T-H, Chen C-Y, Huang C-Y, Wang T-Y, Liao Y-S, Lee L-W. Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction. Diagnostics. 2021; 11(4):674. https://doi.org/10.3390/diagnostics11040674
Chicago/Turabian StyleLin, Chia-Ni, Weh-Shih Huang, Tzu-Hao Huang, Chao-Yu Chen, Cheng-Yi Huang, Ting-Yao Wang, Yu-San Liao, and Li-Wen Lee. 2021. "Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction" Diagnostics 11, no. 4: 674. https://doi.org/10.3390/diagnostics11040674
APA StyleLin, C.-N., Huang, W.-S., Huang, T.-H., Chen, C.-Y., Huang, C.-Y., Wang, T.-Y., Liao, Y.-S., & Lee, L.-W. (2021). Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction. Diagnostics, 11(4), 674. https://doi.org/10.3390/diagnostics11040674