Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Treatment
2.2. CT Image Acquisition
2.3. CT Texture Analysis
2.4. Radiomic Feature Selection
2.5. Radiomic Model Construction and Evaluation
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Radiomic Feature Selection
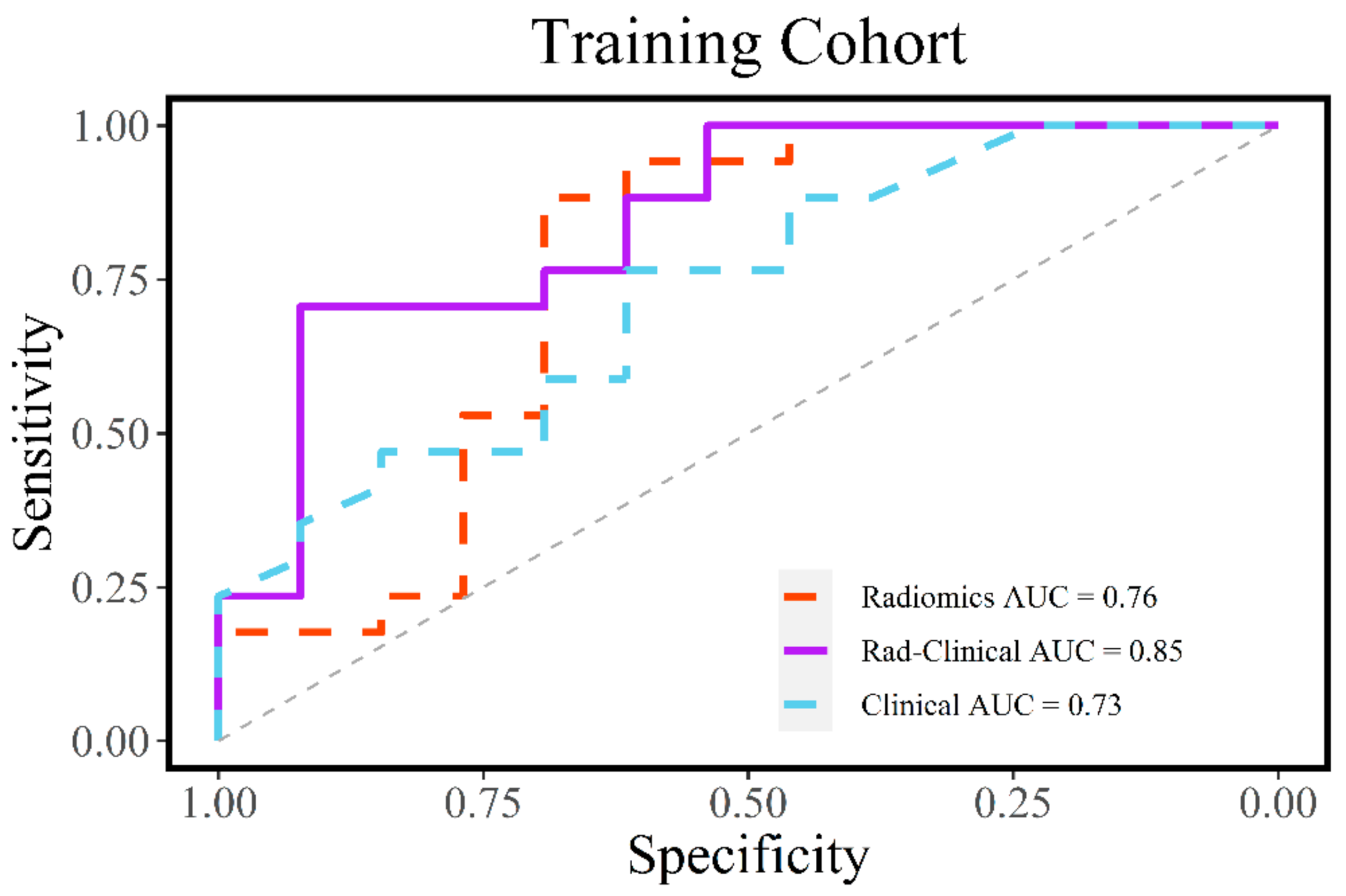
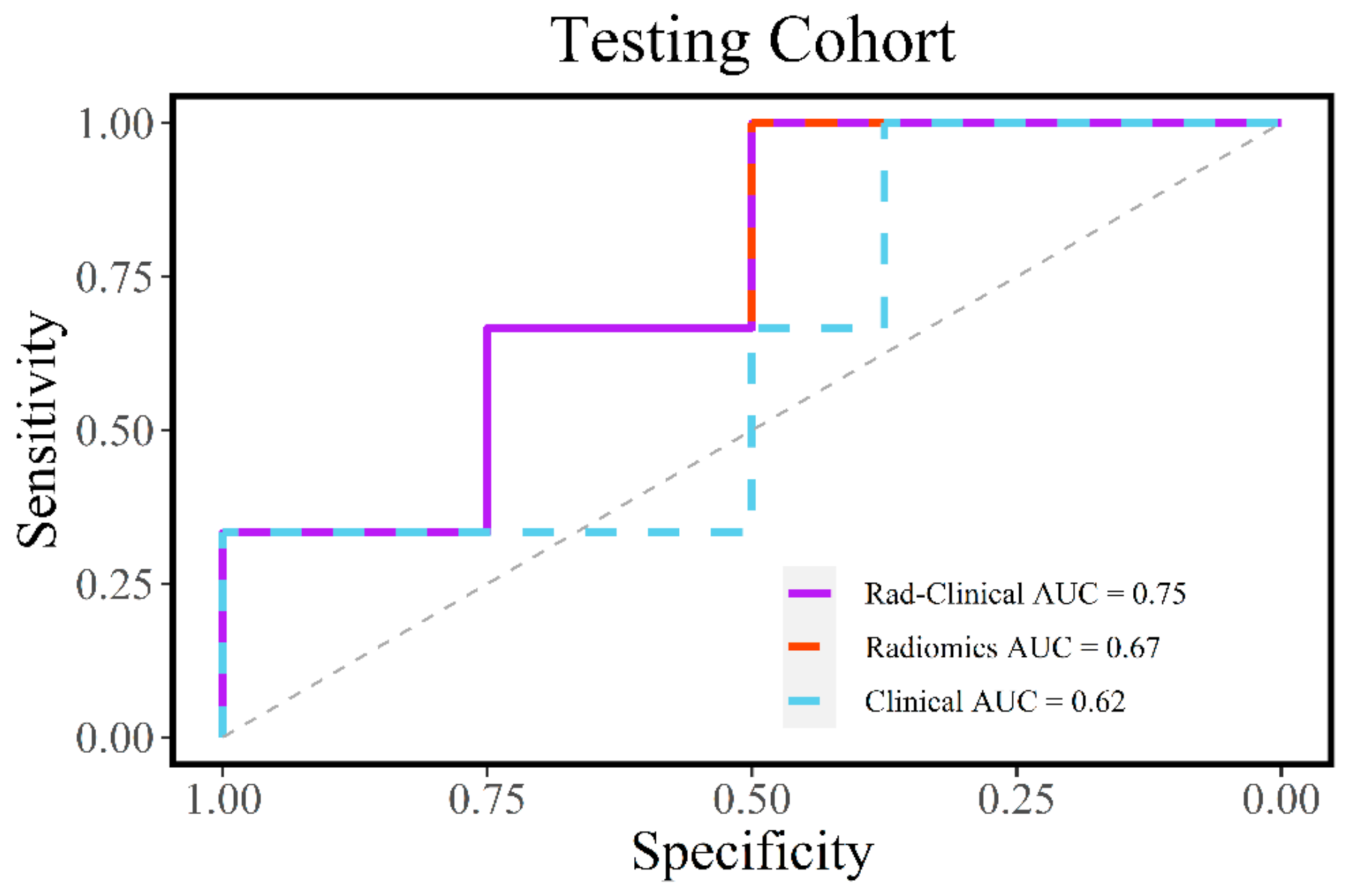
3.3. Evaluation of Radiomic and Clinical Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L. EUROCARE Working Group: Prognoses and Improvement for Head and Neck Cancers Diagnosed in Europe in Early 2000s: The EUROCARE-5 Population-Based Study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef]
- Mermod, M.; Tolstonog, G.; Simon, C.; Monnier, Y. Extracapsular Spread in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2016, 62, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.K.; Fan, C.-Y.; Hanna, E. Significance of Extracapsular Lymph Node Metastases in Patients with Head and Neck Squamous Cell Carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 119–123. [Google Scholar] [CrossRef]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Weinberger, P.M.; Yu, Z.; Haffty, B.G.; Kowalski, D.; Harigopal, M.; Brandsma, J.; Sasaki, C.; Joe, J.; Camp, R.L.; Rimm, D.L.; et al. Molecular Classification Identifies a Subset of Human Papillomavirus--Associated Oropharyngeal Cancers with Favorable Prognosis. J. Clin. Oncol. 2006, 24, 736–747. [Google Scholar] [CrossRef]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic Performance of Post-Treatment FDG PET or FDG PET/CT Imaging in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083–2095. [Google Scholar] [CrossRef]
- Machtay, M.; Natwa, M.; Andrel, J.; Hyslop, T.; Anne, P.R.; Lavarino, J.; Intenzo, C.M.; Keane, W. Pretreatment FDG-PET Standardized Uptake Value as a Prognostic Factor for Outcome in Head and Neck Cancer. Head Neck 2009, 31, 195–201. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ouhmich, F.; Gonzalez-Cabrera, C.; Felli, E.; Saviano, A.; Agnus, V.; Savadjiev, P.; Baumert, T.F.; Pessaux, P.; Marescaux, J.; et al. Radiomics in Hepatocellular Carcinoma: A Quantitative Review. Hepatol. Int. 2019, 13, 546–559. [Google Scholar] [CrossRef]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid Review: Radiomics and Breast Cancer. Breast Cancer Res. Treat 2018, 169, 217–229. [Google Scholar] [CrossRef]
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and Radiogenomics in Lung Cancer: A Review for the Clinician. Lung Cancer 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Sun, Y.; Reynolds, H.M.; Parameswaran, B.; Wraith, D.; Finnegan, M.E.; Williams, S.; Haworth, A. Multiparametric MRI and Radiomics in Prostate Cancer: A Review. Australas Phys. Eng. Sci. Med. 2019, 42, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.V.; Khan, M.; Ramachandra, V.K.; Al-Kadi, O. Texture Analysis of CT Images in the Characterization of Oral Cancers Involving Buccal Mucosa. Dentomaxillofac. Radiol. 2012, 41, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cintra, M.; Brennan, K.; Zhou, M.; Colevas, A.D.; Fischbein, N.; Zhu, S.; Gevaert, O. Development and Validation of Radiomic Signatures of Head and Neck Squamous Cell Carcinoma Molecular Features and Subtypes. EBioMedicine 2019, 45, 70–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Mohamed, A.S.R.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and the Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 1–9. [Google Scholar] [CrossRef]
- Cheng, Z.; Nakatsugawa, M.; Zhou, X.C.; Hu, C.; Greco, S.; Kiess, A.; Page, B.; Alcorn, S.; Haller, J.; Utsunomiya, K.; et al. Utility of a Clinical Decision Support System in Weight Loss Prediction After Head and Neck Cancer Radiotherapy. JCO Clin. Cancer Inform. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Bogowicz, M.; Tanadini-Lang, S.; Veit-Haibach, P.; Pruschy, M.; Bender, S.; Sharma, A.; Hüllner, M.; Studer, G.; Stieb, S.; Hemmatazad, H.; et al. Perfusion CT Radiomics as Potential Prognostic Biomarker in Head and Neck Squamous Cell Carcinoma. Acta Oncol. 2019, 58, 1514–1518. [Google Scholar] [CrossRef]
- Zhai, T.-T.; Langendijk, J.A.; van Dijk, L.V.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; Noordzij, W.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. The Prognostic Value of CT-Based Image-Biomarkers for Head and Neck Cancer Patients Treated with Definitive (Chemo-)Radiation. Oral Oncol. 2019, 95, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, H.; Huang, S.; Chen, X.; Zhou, H.; Chang, H.; Xia, Y.; Wang, G.; Yang, X. Early Prediction of Acute Xerostomia during Radiation Therapy for Nasopharyngeal Cancer Based on Delta Radiomics from CT Images. Quant. Imaging Med. Surg. 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Lin, H.; Gold, K.A.; Sturgis, E.M.; Garden, A.S.; Lee, J.J.; William, W.N. Relationship between Level of Lymph Node Metastasis and Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma. Cancer 2016, 122, 534–545. [Google Scholar] [CrossRef]
- Villaflor, V.M.; Melotek, J.M.; Karrison, T.G.; Brisson, R.J.; Blair, E.A.; Portugal, L.; De Souza, J.A.; Ginat, D.T.; Stenson, K.M.; Langerman, A.; et al. Response-Adapted Volume de-Escalation (RAVD) in Locally Advanced Head and Neck Cancer. Ann. Oncol. 2016, 27, 908–913. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image Biomarker Standardisation Initiative. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Buch, K.; Fujita, A.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT. Am. J. Neuroradiol. 2015, 36, 1343–1348. [Google Scholar] [CrossRef]
- Fujita, A.; Buch, K.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer: Texture Analysis Features on CT. J. Comput. Assist. Tomogr. 2016, 40, 43–47. [Google Scholar] [CrossRef]
- Kann, B.H.; Aneja, S.; Loganadane, G.V.; Kelly, J.R.; Smith, S.M.; Decker, R.H.; Yu, J.B.; Park, H.S.; Yarbrough, W.G.; Malhotra, A.; et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension Using Deep Learning Neural Networks. Sci. Rep. 2018, 8, 14036. [Google Scholar] [CrossRef]
- Zwirner, K.; Hilke, F.J.; Demidov, G.; Socarras Fernandez, J.; Ossowski, S.; Gani, C.; Thorwarth, D.; Riess, O.; Zips, D.; Schroeder, C.; et al. Radiogenomics in Head and Neck Cancer: Correlation of Radiomic Heterogeneity and Somatic Mutations in TP53, FAT1 and KMT2D. Strahlenther. Onkol. 2019, 195, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-26357-9. [Google Scholar]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Lo Muzio, L.; Santarelli, A. American Joint Committee on Cancer Staging System 7th Edition versus 8th Edition: Any Improvement for Patients with Squamous Cell Carcinoma of the Tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 415–423. [Google Scholar] [CrossRef]
- Majercakova, K.; Valero, C.; López, M.; García, J.; Farré, N.; Quer, M.; León, X. Postoperative Staging of the Neck Dissection Using Extracapsular Spread and Lymph Node Ratio as Prognostic Factors in HPV-Negative Head and Neck Squamous Cell Carcinoma Patients. Oral Oncol. 2018, 77, 37–42. [Google Scholar] [CrossRef]
- León, X.; Neumann, E.; Gutiérrez, A.; García, J.; López, M.; Quer, M. Weighted Lymph Node Ratio: New Tool in the Assessment of Postoperative Staging of the Neck Dissection in HPV-Negative Head and Neck Squamous Cell Carcinoma Patients. Head Neck 2020, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Veres, D.S.; Máthé, D.; Hegedűs, N.; Horváth, I.; Kiss, F.J.; Taba, G.; Tóth-Bodrogi, E.; Kovács, T.; Szigeti, K. Radiomic Detection of Microscopic Tumorous Lesions in Small Animal Liver SPECT Imaging. Ejnmmi Res. 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Miles, K.A. Quantifying Tumour Heterogeneity with CT. Cancer Imaging 2013, 13, 140–149. [Google Scholar] [CrossRef]
- Yun, G.; Kim, Y.H.; Lee, Y.J.; Kim, B.; Hwang, J.-H.; Choi, D.J. Tumor Heterogeneity of Pancreas Head Cancer Assessed by CT Texture Analysis: Association with Survival Outcomes after Curative Resection. Sci. Rep. 2018, 8, 7226. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chang, C.-K.; Tu, C.-Y.; Liao, W.-C.; Wu, B.-R.; Chou, K.-T.; Chiou, Y.-R.; Yang, S.-N.; Zhang, G.; Huang, T.-C. Radiomic Features Analysis in Computed Tomography Images of Lung Nodule Classification. PLoS ONE 2018, 13, e0192002. [Google Scholar] [CrossRef]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant Metastasis Occurs Late during the Genetic Evolution of Pancreatic Cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Zhai, T.-T.; Langendijk, J.A.; van Dijk, L.V.; van der Schaaf, A.; Sommers, L.; Vemer-van den Hoek, J.G.M.; Bijl, H.P.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; et al. Pre-Treatment Radiomic Features Predict Individual Lymph Node Failure for Head and Neck Cancer Patients. Radiother. Oncol. 2020, 146, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.-T.; Wesseling, F.; Langendijk, J.A.; Shi, Z.; Kalendralis, P.; van Dijk, L.V.; Hoebers, F.; Steenbakkers, R.J.H.M.; Dekker, A.; Wee, L.; et al. External Validation of Nodal Failure Prediction Models Including Radiomics in Head and Neck Cancer. Oral Oncol. 2021, 112, 105083. [Google Scholar] [CrossRef] [PubMed]

| All Subjects | Training Cohort | Testing Cohort | p-Value | |
|---|---|---|---|---|
| n | 41 | 30 | 11 | |
| Age (years) | 57 ± 6 | 58 ± 7 | 56 ± 3 | 0.57 |
| Sex | ||||
| Female | 3 | 3 | 0 | 0.68 |
| Male | 38 | 27 | 11 | |
| Time Interval (days) | 32 ± 3 | 32 ± 3 | 33 ± 3 | 0.48 |
| Lymph Node Reduction (%) | 66% [53–82%] | 77% [53–82%] | 62% [58–67%] | 0.34 |
| Lymph Node Response | 0.19 | |||
| Good Response (> 66%) | 20 | 17 | 3 | |
| Poor Response (≤ 66%) | 21 | 13 | 8 | |
| Overall Stage | 0.95 | |||
| IVa | 39 | 28 | 11 | |
| IVb | 2 | 2 | 0 | |
| T Stage | 0.04 | |||
| 1 | 6 | 5 | 1 | |
| 2 | 15 | 7 | 8 | |
| 3 | 11 | 10 | 1 | |
| 4 | 9 | 8 | 1 | |
| N Stage | 0.75 | |||
| 2a | 2 | 1 | 1 | |
| 2b | 20 | 14 | 6 | |
| 2c | 18 | 14 | 4 | |
| 3 | 1 | 1 | 0 |
| Correlation Coefficient | Beta Coefficient ± SE | p Value | |
|---|---|---|---|
| (Intercept) | −1.26 ± 1.09 | 0.25 | |
| Minimum | 0.0045 | 0.014 ± 0.015 | 0.35 |
| Skewness | −0.083 | −0.49 ± 0.39 | 0.21 |
| Low Gray Level Run Emphasis | 1.20 | 9.89 ± 7.11 | 0.16 |
| Predicted: Good Response | Predicted: Poor Response | ||
|---|---|---|---|
| Observed: good response | 12 | 5 | 17 |
| Observed: poor response | 1 | 12 | 13 |
| 13 | 17 |
| Predicted: Good Response | Predicted: Poor Response | ||
|---|---|---|---|
| Observed: good response | 3 | 0 | 3 |
| Observed: poor response | 4 | 4 | 8 |
| 7 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.H.; Cao, D.; Ginat, D.T. Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer. Diagnostics 2021, 11, 588. https://doi.org/10.3390/diagnostics11040588
Zhang MH, Cao D, Ginat DT. Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer. Diagnostics. 2021; 11(4):588. https://doi.org/10.3390/diagnostics11040588
Chicago/Turabian StyleZhang, Michael H., David Cao, and Daniel T. Ginat. 2021. "Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer" Diagnostics 11, no. 4: 588. https://doi.org/10.3390/diagnostics11040588
APA StyleZhang, M. H., Cao, D., & Ginat, D. T. (2021). Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer. Diagnostics, 11(4), 588. https://doi.org/10.3390/diagnostics11040588





