Contribution of Gray Matter Atrophy and White Matter Damage to Cognitive Impairment in Mildly Disabled Relapsing-Remitting Multiple Sclerosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Magnetic Resonance Imaging
2.3. Brain Segmentation and Volumetry
2.4. Voxel-Based Morphometry Analysis (VBM)
2.5. DTI Analysis
2.6. Thalamic Segmentation and Vertex Analysis
2.7. Statistical Analysis of Quantitative Variables
3. Results
3.1. Demographic Characteristics and Cognitive Performance
3.2. Neuroimaging Analyses
3.2.1. Segmentation of Brain and Subcortical Structures
3.2.2. VBM
3.2.3. Diffusion Tensor Imaging
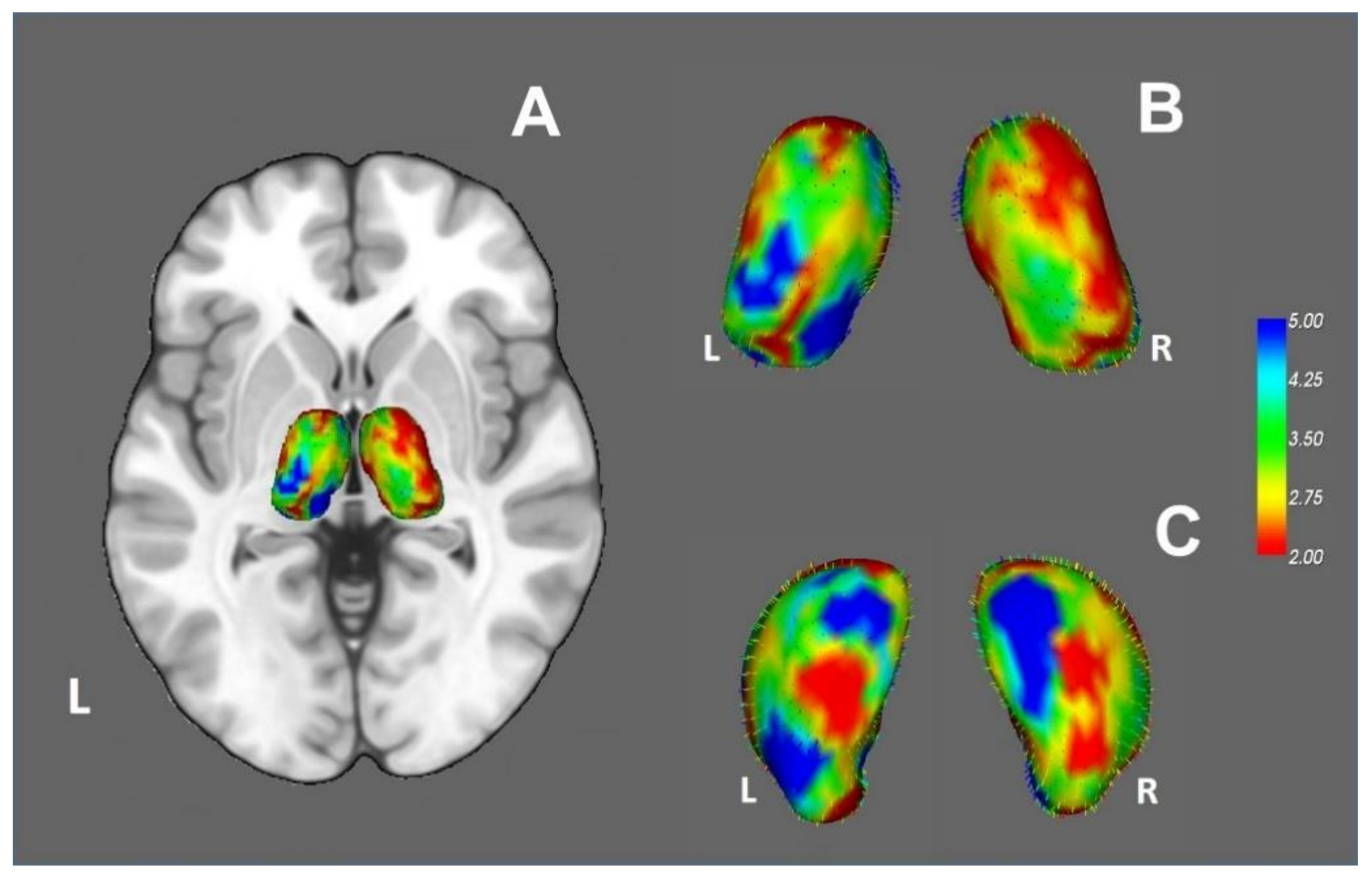
3.2.4. Vertex Analysis and Thalamic Parcellation
3.2.5. Correlations between MRI Measurements, Disease Severity, and Cognitive Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Krishnamurthi, R.V.; Theadom, A.M.; Abajobir, A.A.; Mishra, S.R.; Ahmed, M.B.; Abate, K.H.; Mengistie, M.A.; Wakayo, T.; Abd-Allah, F.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Julian, L.J. Cognitive Functioning in Multiple Sclerosis. Neurol. Clin. 2011, 29, 507–525. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Prestipino, E.; Bellinvia, A.; Niccolai, C.; Razzolini, L.; Pastò, L.; Fratangelo, R.; Tudisco, L.; Fonderico, M.; Mattiolo, P.L.; et al. Cognitive impairment in multiple sclerosis: An exploratory analysis of environmental and lifestyle risk factors. PLoS ONE 2019, 14, e0222929. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.; Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Patti, F.; Chisari, C.; Gallo, P.; Grossi, P.; Ghezzi, A.; et al. Aging with multiple sclerosis: Prevalence and profile of cognitive impairment. Neurol. Sci. 2019, 40, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Grzegorski, T.; Losy, J. Cognitive impairment in multiple sclerosis – a review of current knowledge and recent research. Rev. Neurosci. 2017, 28, 1–16. [Google Scholar] [CrossRef]
- Azevedo, C.J.; Cen, S.Y.; Khadka, S.; Liu, S.; Kornak, J.; Shi, Y.; Zheng, L.; Hauser, S.L.; Pelletier, D. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann. Neurol. 2018, 83, 223–234. [Google Scholar] [CrossRef]
- Minagar, A.; Barnett, M.H.; Benedict, R.H.B.; Pelletier, D.; Pirko, I.; Sahraian, M.A.; Frohman, E.; Zivadinov, R. The thalamus and multiple sclerosis Modern views on pathologic, imaging, and clinical aspects. Neurology 2013, 80, 210–219. [Google Scholar] [CrossRef]
- Pinter, D.; Khalil, M.; Pirpamer, L.; Damulina, A.; Pichler, A.; Fruhwirth, V.; Ropele, S.; Schmidt, R.; Fuchs, S.; Enzinger, C. Long-term course and morphological MRI correlates of cognitive function in multiple sclerosis. Mult. Scler. J. 2020, 135245852094147. [Google Scholar] [CrossRef] [PubMed]
- Burggraaff, J.; Liu, Y.; Prieto, J.C.; Simoes, J.; de Sitter, A.; Ruggieri, S.; Brouwer, I.; Lissenberg-Witte, B.I.; Rocca, M.A.; Valsasina, P.; et al. Manual and automated tissue segmentation confirm the impact of thalamus atrophy on cognition in multiple sclerosis: A multicenter study. NeuroImage Clin. 2021, 29, 102549. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Ciccarelli, O. The value of including thalamic atrophy as a clinical trial endpoint in multiple sclerosis. Neurology 2018, 90, 677–678. [Google Scholar] [CrossRef]
- Bethune, A.; Tipu, V.; Sled, J.G.; Narayanan, S.; Arnold, D.L.; Mabbott, D.; Rockel, C.; Ghassemi, R.; Till, C.; Banwell, B. Diffusion tensor imaging and cognitive speed in children with multiple sclerosis. J. Neurol. Sci. 2011, 309, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, S.; Rocca, M.A.; Riccitelli, G.; Pagani, E.; Rovaris, M.; Caputo, D.; Ghezzi, A.; Capra, R.; Bertolotto, A.; Comi, G.; et al. Corpus callosum damage and cognitive dysfunction in benign MS. Hum. Brain Mapp. 2009, 30, 2656–2666. [Google Scholar] [CrossRef]
- Pokryszko-Dragan, A.; Banaszek, A.; Nowakowska-Kotas, M.; Jeżowska-Jurczyk, K.; Dziadkowiak, E.; Gruszka, E.; Zagrajek, M.; Bilińska, M.; Budrewicz, S.; Sąsiadek, M.; et al. Diffusion tensor imaging findings in the multiple sclerosis patients and their relationships to various aspects of disability. J. Neurol. Sci. 2018, 391, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dineen, R.A.; Vilisaar, J.; Hlinka, J.; Bradshaw, C.M.; Morgan, P.S.; Constantinescu, C.S.; Auer, D.P. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009, 132, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Masera, S.; Lorenzatti, M.; Condello, C.; Merola, A.; Mattioda, A.; Tribolo, A.; Capello, E.; Mancardi, G.L.; Mutani, R.; et al. Demyelination, Inflammation, and Neurodegeneration in Multiple Sclerosis Deep Gray Matter. J. Neuropathol. Exp. Neurol. 2009, 68, 489–502. [Google Scholar] [CrossRef]
- Henry, R.G.; Shieh, M.; Amirbekian, B.; Chung, S.; Okuda, D.T.; Pelletier, D. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J. Neurol. Sci. 2009, 282, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Weeda, M.M.; Pruis, I.J.; Westerveld, A.S.R.; Brouwer, I.; Bellenberg, B.; Barkhof, F.; Vrenken, H.; Lukas, C.; Schneider, R.; Pouwels, P.J.W. Damage in the Thalamocortical Tracts is Associated With Subsequent Thalamus Atrophy in Early Multiple Sclerosis. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Conradsson, D.; Ytterberg, C.; von Koch, L.; Johansson, S. Changes in disability in people with multiple sclerosis: A 10-year prospective study. J. Neurol. 2018, 265, 119–126. [Google Scholar] [CrossRef]
- Parmenter, B.A.; Weinstock-Guttman, B.; Garg, N.; Munschauer, F.; Benedict, R.H.B. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult. Scler. 2007, 13, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ozer, S.; Noonan, K.; Burke, M.; Young, J.; Barber, S.; Forster, A.; Jones, R. The validity of the Memory Alteration Test and the Test Your Memory test for community-based identification of amnestic mild cognitive impairment. Alzheimer’s Dement. 2016, 12, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Molinuevo, J.L.; Sanchez-Valle, R.; Bosch, B.; Villar, A. Screening for amnestic mild cognitive impairment and early Alzheimer’s disease with M@T (Memory Alteration Test) in the primary care population. Int. J. Geriatr. Psychiatry 2007, 22, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Ricchiuti, L.; De Caro, M.F.; Patti, F.; Vecchio, R.; Sorbi, S.; Trojano, M. The Rao’s Brief Repeatable Battery and Stroop Test: Normative values with age, education and gender corrections in an Italian population. Mult. Scler. 2006, 12, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.; Bernabeu-Sanz, A.; López-Mir, F.; González, P.; Luna, L.; Naranjo, V. BRAIM: A computer-aided diagnosis system for neurodegenerative diseases and brain lesion monitoring from volumetric analyses. Comput. Methods Programs Biomed. 2017, 145, 167–179. [Google Scholar] [CrossRef]
- Oishi, K.; Zilles, K.; Amunts, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Hua, K.; Woods, R.; Toga, A.W.; et al. Human brain white matter atlas: Identification and assignment of common anatomical structures in superficial white matter. Neuroimage 2008, 43, 447–457. [Google Scholar] [CrossRef]
- Leemans, A.; Jeurissen, B.; Sijbers, J.; Jones, D. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. 17th Sci. Meet. Int. Soc. Magn. Reson. Med. 2009, 17, 3537. [Google Scholar]
- Jones, D.K.; Basser, P.J. “Squashing peanuts and smashing pumpkins”: How noise distorts diffusion-weighted MR data. Magn. Reson. Med. 2004, 52, 979–993. [Google Scholar] [CrossRef]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000, 44, 625–632. [Google Scholar] [CrossRef]
- Wang, R.; Benner, T.; Sorensen, A.G.; Wedeen, V.J. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc. Int. Soc. Magn. Reson. Med. 2007, 15, 3720. [Google Scholar]
- Catani, M.; Thiebaut de Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008, 44, 1105–1132. [Google Scholar] [CrossRef]
- Wakana, S.; Jiang, H.; Nagae-Poetscher, L.M.; van Zijl, P.C.M.; Mori, S. Fiber tract-based atlas of human white matter anatomy. Radiology 2004, 230, 77–87. [Google Scholar] [CrossRef]
- Witelson, S.F. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 1989, 112 Pt 3, 799–835. [Google Scholar] [CrossRef]
- Lebel, C.; Caverhill-Godkewitsch, S.; Beaulieu, C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 2010, 52, 20–31. [Google Scholar] [CrossRef]
- Preti, M.G.; Baglio, F.; Laganà, M.M.; Griffanti, L.; Nemni, R.; Clerici, M.; Bozzali, M.; Baselli, G. Assessing corpus callosum changes in Alzheimer’s disease: Comparison between tract-based spatial statistics and atlas-based tractography. PLoS ONE 2012, 7, e35856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catani, M.; Dell’Acqua, F.; Budisavljevic, S.; Howells, H.; Thiebaut de Schotten, M.; Froudist-Walsh, S.; D’Anna, L.; Thompson, A.; Sandrone, S.; Bullmore, E.T.; et al. Frontal networks in adults with autism spectrum disorder. Brain 2016, 139, 616–630. [Google Scholar] [CrossRef]
- Behrens, T.E.J.; Johansen-Berg, H.; Woolrich, M.W.; Smith, S.M.; Wheeler-Kingshott, C.A.M.; Boulby, P.A.; Barker, G.J.; Sillery, E.L.; Sheehan, K.; Ciccarelli, O.; et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003, 6, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Traynor, C.; Heckemann, R.A.; Hammers, A.; O’Muircheartaigh, J.; Crum, W.R.; Barker, G.J.; Richardson, M.P. Reproducibility of thalamic segmentation based on probabilistic tractography. Neuroimage 2010, 52, 69–85. [Google Scholar] [CrossRef]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Deluca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017, 23, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Corfield Freya, L.D. A Systematic Review and Meta-Analysis of the Brief Cognitive Assessment for Multiple Sclerosis (BICAMS). Neurol. Ther. 2018, 7, 287–306. [Google Scholar] [CrossRef]
- Schoonheim, M.M.; Hulst, H.E.; Brandt, R.B.; Strik, M.; Wink, A.M.; Uitdehaag, B.M.J.; Barkhof, F.; Geurts, J.J.G. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 2015, 84, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Deppe, M.; Krämer, J.; Tenberge, J.G.; Marinell, J.; Schwindt, W.; Deppe, K.; Groppa, S.; Wiendl, H.; Meuth, S.G. Early silent microstructural degeneration and atrophy of the thalamocortical network in multiple sclerosis. Hum. Brain Mapp. 2016, 37, 1866–1879. [Google Scholar] [CrossRef]
- Tovar-Moll, F.; Evangelou, I.E.; Chiu, A.W.; Richert, N.D.; Ostuni, J.L.; Ohayon, J.M.; Auh, S.; Ehrmantraut, M.; Talagala, S.L.; McFarland, H.F.; et al. Thalamic Involvement and Its Impact on Clinical Disability in Patients with Multiple Sclerosis: A Diffusion Tensor Imaging Study at 3T. Am. J. Neuroradiol. 2009, 30, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.; Ostwaldt, A.C.; Opfer, R.; Suppa, P.; Spies, L.; Winkler, G. MRI-based brain volumetry at a single time point complements clinical evaluation of patients with multiple sclerosis in an outpatient setting. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Magon, S.; Tsagkas, C.; Gaetano, L.; Patel, R.; Naegelin, Y.; Amann, M.; Parmar, K.; Papadopoulou, A.; Wuerfel, J.; Stippich, C.; et al. Volume loss in the deep gray matter and thalamic subnuclei: A longitudinal study on disability progression in multiple sclerosis. J. Neurol. 2020, 267, 1536–1546. [Google Scholar] [CrossRef]
- Rosselli, M.; Ardila, A.; Bernal, B. Angular gyrus connectivity model for language: A functional neuroimaging meta-analysis [Modelo de conectividad de la circunvolución angular en el lenguaje: Metaanálisis de neuroimágenes funcionales]. Rev. Neurol. 2015, 60, 495–503. [Google Scholar] [PubMed]
- Scolari, M.; Seidl-Rathkopf, K.N.; Kastner, S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr. Opin. Behav. Sci. 2015, 1, 32–39. [Google Scholar] [CrossRef]
- Martino, J.; Brogna, C.; Robles, S.G.; Vergani, F.; Duffau, H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 2010, 46, 691–699. [Google Scholar] [CrossRef]
- Duffau, H.; Moritz-Gasser, S.; Mandonnet, E. A re-examination of neural basis of language processing: Proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Sarubbo, S.; De Benedictis, A.; Maldonado, I.L.; Basso, G.; Duffau, H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct. Funct. 2013, 218, 21–37. [Google Scholar] [CrossRef]
- Chen, H.F.; Huang, L.L.; Li, H.Y.; Qian, Y.; Yang, D.; Qing, Z.; Luo, C.M.; Li, M.C.; Zhang, B.; Xu, Y. Microstructural disruption of the right inferior fronto-occipital and inferior longitudinal fasciculus contributes to WMH-related cognitive impairment. CNS Neurosci. Ther. 2020, 26, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Fan, Q.; Machado, N.; Eloyan, A.; Bireley, J.D.; Russo, A.W.; Tobyne, S.M.; Patel, K.R.; Brewer, K.; Rapaport, S.F.; et al. Corpus callosum axon diameter relates to cognitive impairment in multiple sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Granberg, T.; Fan, Q.; Treaba, C.A.; Ouellette, R.; Herranz, E.; Mangeat, G.; Louapre, C.; Cohen-Adad, J.; Klawiter, E.C.; Sloane, J.A.; et al. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 2017, 140, 2912–2926. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Portaccio, E.; Mancini, A.; Calabresi, P. Multiple sclerosis and cognition: Synaptic failure and network dysfunction. Nat. Rev. Neurosci. 2018, 19, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Zemmoura, I.; Duffau, H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front. Neuroanat. 2018, 12, 77. [Google Scholar] [CrossRef]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef]
- Olson, I.R.; Der Heide, R.J.V.; Alm, K.H.; Vyas, G. Development of the Uncinate Fasciculus: Implications for Theory and Developmental Disorders; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 14, pp. 50–61. [Google Scholar]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Vann, S.D.; Aggleton, J.P.; Maguire, E.A. What Does the Retrosplenial Cortex Do?—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19812579/ (accessed on 6 August 2020).
- Delano-Wood, L.; Stricker, N.H.; Sorg, S.F.; Nation, D.A.; Jak, A.J.; Woods, S.P.; Libon, D.J.; Delis, D.C.; Frank, L.R.; Bondi, M.W. Posterior Cingulum White Matter Disruption and Its Associations with Verbal Memory and Stroke Risk in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2012, 29, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, S.H.; Jung, H.Y. Characteristics of the uncinate fasciculus and cingulum in patients with mild cognitive impairment: Diffusion tensor tractography study. Brain Sci. 2019, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Ly, M.; Adluru, N.; Destiche, D.J.; Lu, S.Y.; Oh, J.M.; Hoscheidt, S.M.; Alexander, A.L.; Okonkwo, O.C.; Rowley, H.A.; Sager, M.A.; et al. Fornix Microstructure and Memory Performance is Associated with Altered Neural Connectivity during Episodic Recognition; Cambridge University Press: Cambridg, UK, 2016; Volume 22, pp. 191–204. [Google Scholar]
- Valdés Cabrera, D.; Stobbe, R.; Smyth, P.; Giuliani, F.; Emery, D.; Beaulieu, C. Diffusion tensor imaging tractography reveals altered fornix in all diagnostic subtypes of multiple sclerosis. Brain Behav. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]


| MS (n = 30) | HC (n = 30) | p | |
|---|---|---|---|
| Sex (M/F) (number) | (21/9) | (21/9) | 0.83 |
| † Age years (range) | 41 (24–63) | 40 (22–60) | 0.71 |
| ‖ Disease duration years (SD) | 9.5 ± 6.3 | n.a. | |
| † Education years | 13 (9–17) | 15; 4 (9–17) | 0.04 |
| † EDSS | 2 (0–2.5) | n.a. | |
| ‖ z-SDMT scores (SD) | 41.31 ± 11.25 | 56.56 ± 9.44 | <0.001 |
| ‖ z-M@T global score (SD) | 48.18 ± 5.5 | 48.55 ± 5.3 | 0.799 |
| ‖ z-M@T-encoding | 8.93 ± 1.1 | 9.81 ± 0.4 | <0.001 |
| ‖ z-M@T-orientation | 5.24 ± 1.7 | 4.96 ± 0.19 | 0.97 |
| ‖ z-M@T-semantic | 13.55 ± 3.05 | 14.50 ± 0.58 | 0.29 |
| ‖ z-M@T-free recall | 7.59 ± 1.8 | 9.12 ± 0.86 | 0.001 |
| ‖ z-M@T-cued-recall | 8.76 ± 1.52 | 9.7 ± 0.54 | 0.003 |
| ‖ z-M@T-episodic ** | 16.3 ± 3.15 | 19 ± 0.95 | <0.001 |
| ‖ GMV (mL) | 633.4 ± 104.2 | 717.64 ± 87.2 | 0.002 |
| ‖ WMV (mL) | 399.91 ± 63.52 | 453.82 ± 61.8 | 0.002 |
| ‖ CSFV (mL) | 493.29 ± 111.9 | 469.87 ± 85.34 | 0.374 |
| ‖ Putamen (mL) | 4.06 ± 0.82 | 4.47 ± 0.36 | 0.017 |
| ‖ Thalami (mL) | 9.51 ± 1.72 | 10.32 ± 1.23 | 0.01 |
| ‖ Hippocampus (mL) | 3.89 ± 0.65 | 4.82 ± 0.54 | <0.001 |
| ‖ Caudate (mL) | 4.11 ± 0.7 | 5.03 ± 0.56 | <0.001 |
| ‖ ICV (mL) | 1526.6 ± 159 | 1641.33 ± 164.3 | 0.009 |
| ‖ BPF | 0.67 ± 0.6 | 0.71 ± 0.38 | 0.008 |
| ‖ WM lesion load (mL) | 9.55 ± 4.53 | n.a. |
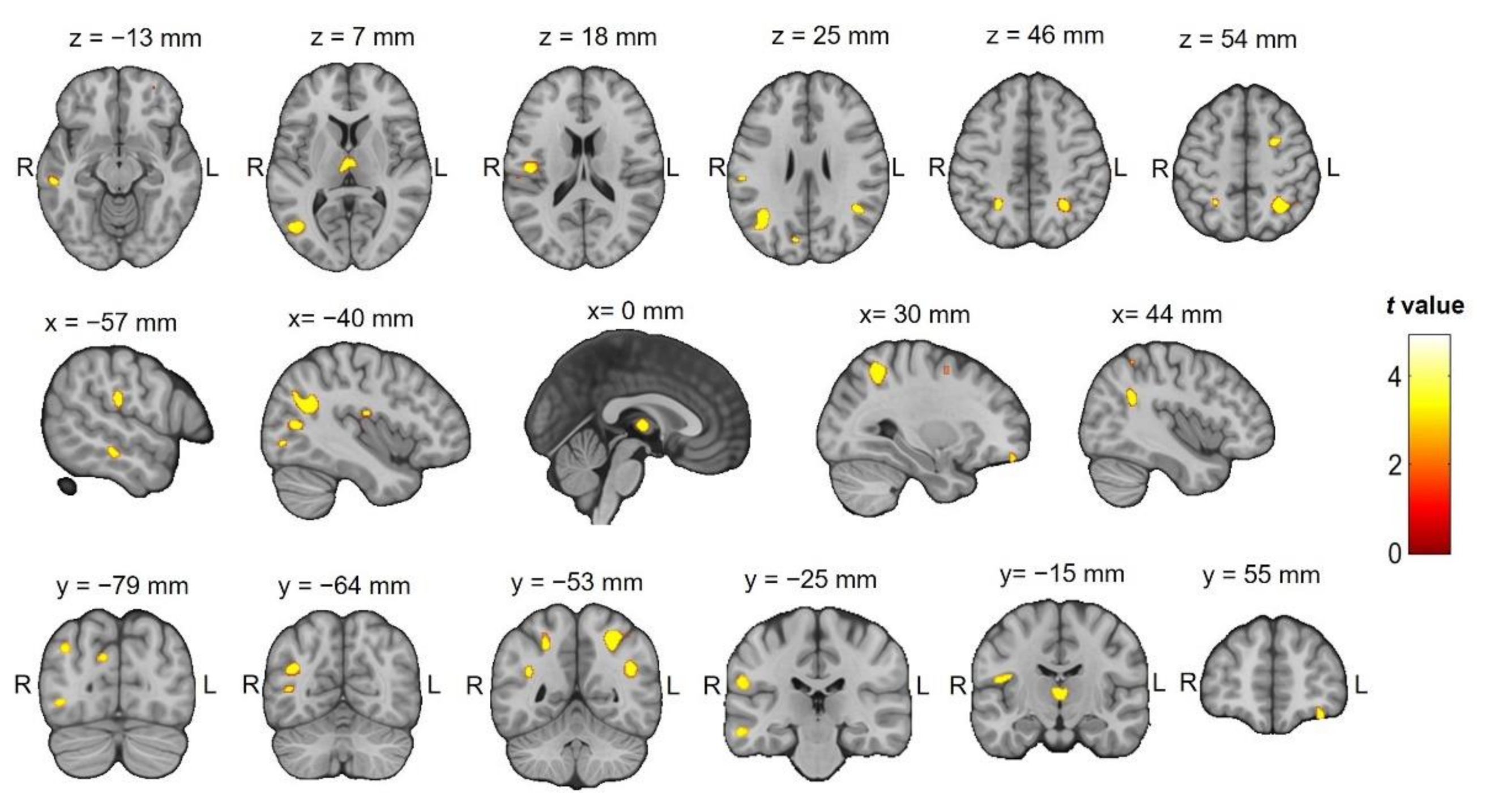
| Brain Area | Hemisphere | BA | x | y | z | Z Value | Cluster |
|---|---|---|---|---|---|---|---|
| Temporal Lobe, Middle Temporal Gyrus | L | 39 | −37 | −63 | 25 | 4.89 | 420 |
| Parietal Lobe, Superior Parietal Lobule | R | 7 | 30 | −55 | 49 | 4.72 | 370 |
| Temporal Lobe, Superior Temporal Gyrus | R | 39 | 43 | −52 | 28 | 4.28 | 137 |
| Temporal Lobe, Middle Temporal Gyrus | L | 21 | −57 | −27 | −13 | 4.17 | 68 |
| Thalamus, Medial Dorsal Nucleus | L | −4 | −13 | 6 | 4.05 | 174 | |
| Thalamus, Medial Dorsal Nucleus | R | 6 | −13 | 6 | 3.94 | ||
| Insula | L | 13 | −46 | −16 | 18 | 3.90 | 106 |
| Temporal Lobe, Middle Temporal Gyrus | L | 39 | −43 | −69 | 10 | 3.9 | 229 |
| Parietal Lobe, Sub-Gyral | L | 7 | −25 | −49 | 52 | 3.89 | 106 |
| Parietal Lobe, Postcentral Gyrus | L | 40 | −58 | −24 | 22 | 3.84 | 87 |
| Frontal Lobe, Sub-Gyral cingulate | R | 6 | 27 | 2 | 52 | 3.78 | 85 |
| Occipital Lobe, Cuneus | L | 18 | −10 | −78 | 27 | 3.74 | 22 |
| Occipital Lobe, Inferior Occipital Gyrus | L | 19 | −41 | −79 | −4 | 3.67 | 57 |
| Frontal Lobe, Superior Frontal Gyrus | R | 10 | −36 | −79 | 33 | 3.41 | 33 |
| Parietal Lobe, Inferior Parietal Lobule | R | 40 | 42 | -49 | 55 | 3.37 | 21 |
| MS | HC | p | |
|---|---|---|---|
| L-Thalamus FA | 0.22 ± 0.012 | 0.25 ± 0.04 | <0.001 |
| L-Thalamus MD | 0.0011 ± 0.00009 | 0.001 ± 0.00005 | <0.001 |
| R-Thalamus FA | 0.21 ± 0.012 | 0.25 ± 0.03 | <0.001 |
| R-Thalamus MD | 0.0014 ± 0.0018 | 0.0017 ± 0.0015 | 0.65 |
| L-Frontal FA | 0.28 ± 0.02 | 0.34 ± 0.016 | <0.001 |
| L-Frontal MD | 0.0015 ± 0.00023 | 0.0013 ± 0.00011 | <0.001 |
| R-Frontal FA | 0.29 ± 0.03 | 0.33 ± 0.02 | <0.001 |
| R-Frontal MD | 0.0013 ± 0.00027 | 0.0013 ± 0.00016 | 0.87 |
| L-Parietal FA | 0.28 ± 0.02 | 0.34 ± 0.016 | <0.001 |
| L- Parietal MD | 0.0014 ± 0.0002 | 0.001 ± 0.0001 | <0.001 |
| R- Parietal FA | 0.29 ± 0.03 | 0.33 ± 0.02 | <0.001 |
| R- Parietal MD | 0.0013 ± 0.0003 | 0.001 ± 0.0001 | <0.001 |
| L-Temporal FA | 0.28 ± 0.02 | 0.34 ± 0.01 | <0.001 |
| L- Temporal MD | 0.0013 ± 0.0002 | 0.001 ± 0.00001 | <0.001 |
| R- Temporal FA | 0.29 ± 0.03 | 0.33 ± 0.02 | <0.001 |
| R- Temporal MD | 0.0013 ± 0.0003 | 0.001 ± 0.0001 | <0.001 |
| L-Occipital FA | 0.28 ± 0.02 | 0.33 ± 0.016 | <0.001 |
| L- Occipital MD | 0.0013 ± 0.0002 | 0.001 ± 0.0001 | <0.001 |
| R- Occipital FA | 0.29 ± 0.03 | 0.33 ± 0.02 | <0.001 |
| R- Occipital MD | 0.0015 ± 0.0013 | 0.001 ± 0.0001 | 0.03 |
| EDSS | z-SDMT | M@T-Encoding | M@T-Free Recall | M@T-Cued Recall | M@T-Episodic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
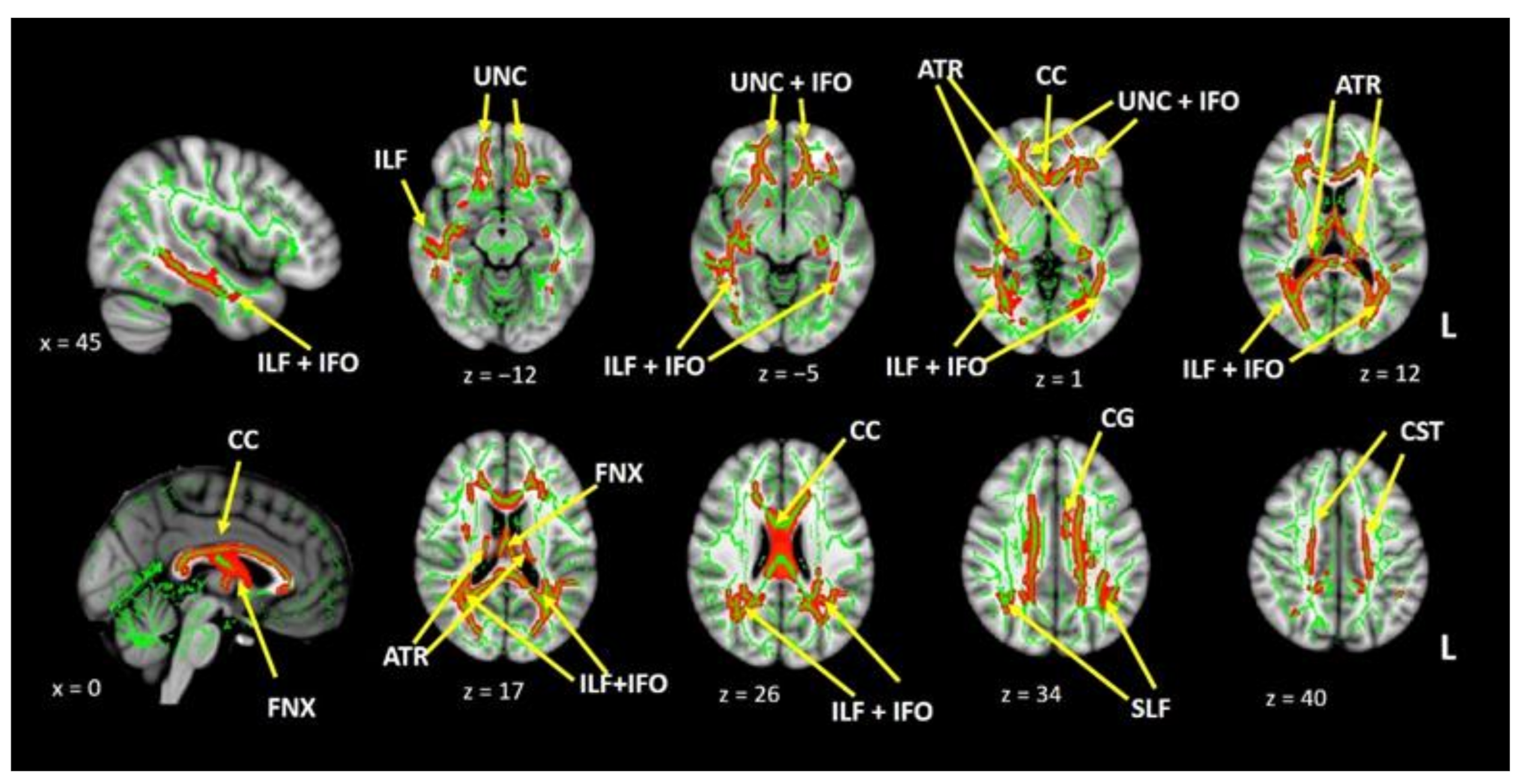
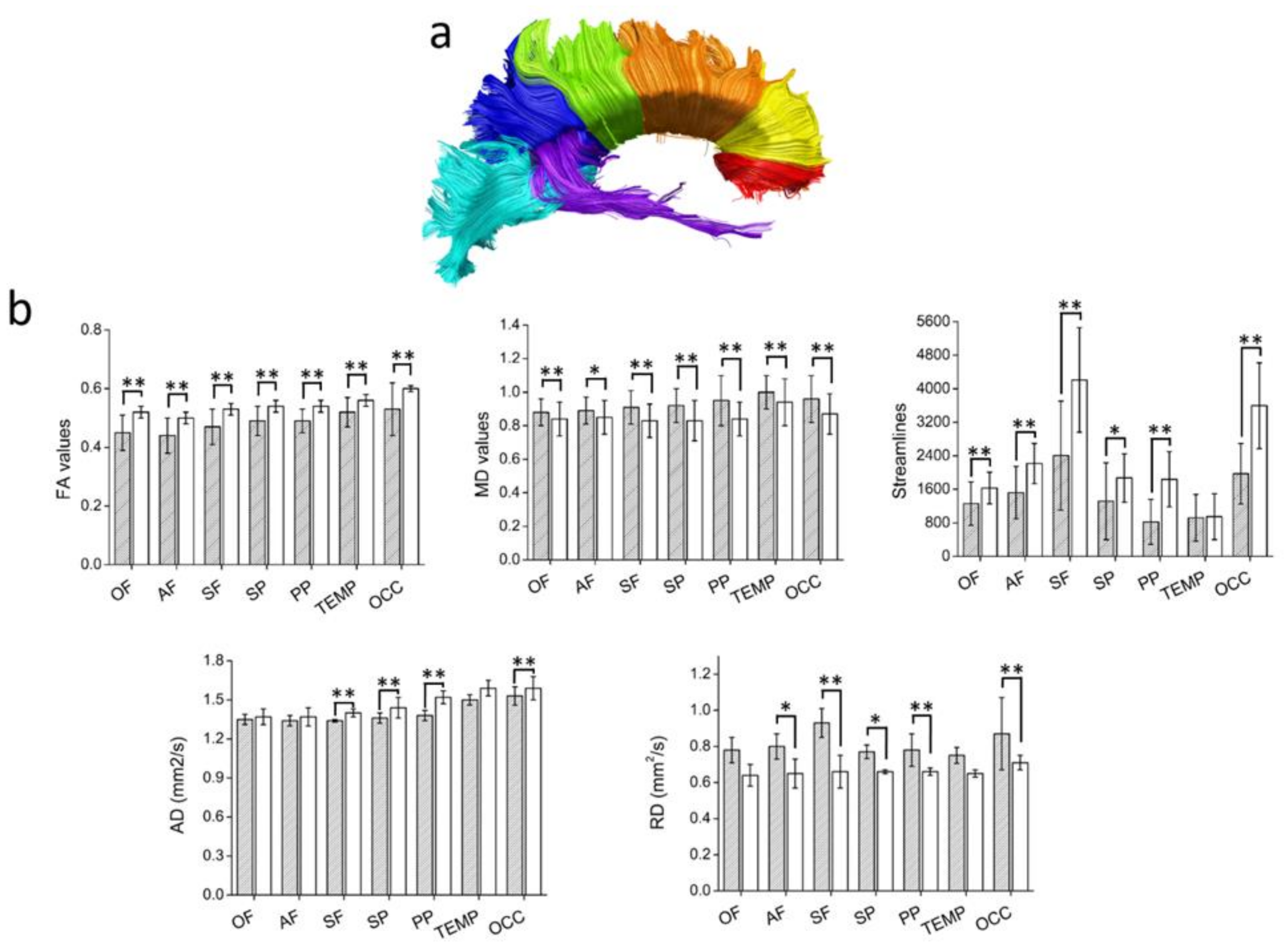
| CC STR | −0.4 | 0.03 | ns | ns | 0.64 | <0.001 | ns | ns | −0.58 | 0.001 | 0.5 | 0.007 |
| CC-Superior parietal STR | −0.48 | 0.009 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CC-Superior parietal AD | 0.62 | <0.001 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CC-Posterior Parietal STR | −0.41 | 0.02 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CC-Posterior Parietal AD | 0.45 | 0.01 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CC-Occipital STR | ns | ns | 0.41 | 0.027 | ns | ns | ns | ns | ns | ns | ns | ns |
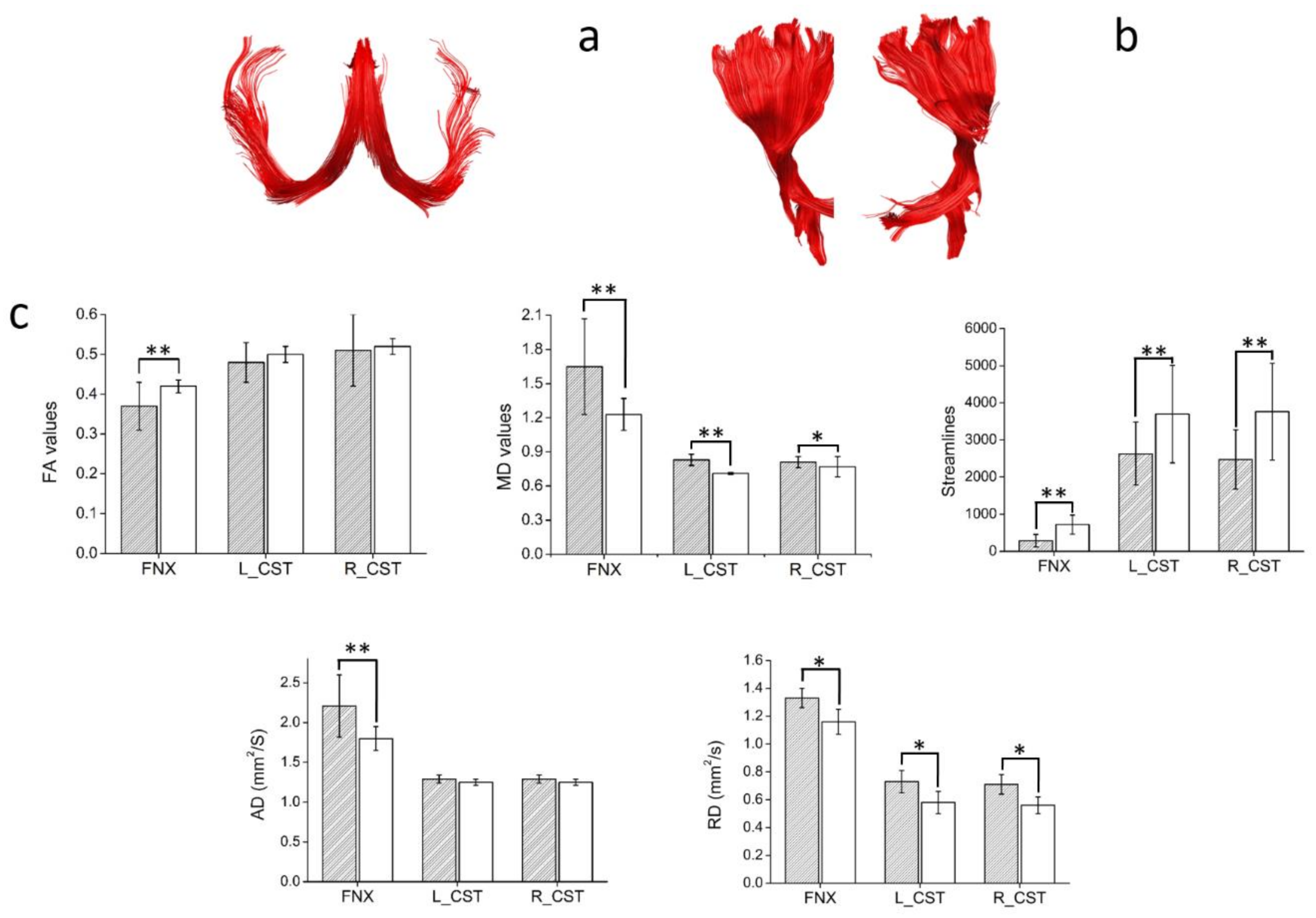
| FNX STR | −0.52 | 0.004 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| FNX FA | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | 0.4 | 0.04 |
| FNX AD | 0.55 | 0.002 | ns | ns | ns | ns | ns | ns | ns | ns | 0.43 | 0.02 |
| L-UNC STR | ns | ns | ns | ns | 0.56 | 0.001 | 0.62 | <0.001 | 0.43 | 0.018 | 0.57 | 0.001 |
| L-UNC AD | ns | ns | ns | ns | 0.61 | <0.001 | ns | ns | ns | ns | ns | ns |
| L-ILF STR | ns | ns | ns | ns | ns | ns | 0.51 | 0.004 | 0.37 | 0.04 | 0.477 | 0.009 |
| R-IFO STR | ns | ns | 0.46 | 0.012 | ns | ns | ns | ns | ns | ns | ns | ns |
| R-IFO AD | ns | ns | −0.47 | 0.01 | ns | ns | ns | ns | ns | ns | ns | ns |
| R-CG STR | ns | ns | ns | ns | 0.54 | 0.002 | ns | ns | ns | ns | 0.38 | 0.04 |
| R-CG AD | ns | ns | ns | ns | 0.51 | 0.005 | ns | ns | ns | ns | ns | ns |
| L-CST AD | 0.39 | 0.03 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| R-CST AD | 0.56 | 0.001 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| EDSS | z-SDMT | |||
|---|---|---|---|---|
| r | p | r | p | |
| L-Thalamus FA | −0.65 | <0.001 | 0.4 | 0.03 |
| L-Thalamus MD | 0.7 | <0.001 | ns | ns |
| R-Thalamus FA | −0.65 | <0.001 | ns | ns |
| R-Thalamus MD | ns | ns | ns | ns |
| L- frontal lobe FA | −0.4 | 0.03 | −0.4 | 0.03 |
| L- frontal lobe MD | 0.55 | 0.002 | 0.55 | 0.002 |
| L- parietal lobe FA | −0.44 | 0.01 | −0.44 | 0.01 |
| L- parietal lobe MD | 0.58 | <0.001 | 0.58 | <0.001 |
| L-Temporal FA | −0.44 | 0.016 | −0.44 | 0.01 |
| L-Temporal Lobe MD | 0.55 | 0.002 | 0.55 | 0.002 |
| L-Occipital lobe FA | −0.41 | 0.02 | −0.41 | 0.02 |
| L-Occipital lobe MD | 0.52 | 0.003 | 0.52 | 0.003 |
| R- frontal lobe FA | −0.53 | 0.003 | −0.53 | 0.001 |
| R- frontal lobe MD | 0.56 | 0.001 | 0.56 | 0.002 |
| R-Parietal Lobe FA | −0.52 | 0.003 | −0.52 | 0.003 |
| R-Parietal Lobe MD | 0.55 | 0.001 | 0.55 | 0.001 |
| R-Temporal lobe FA | −0.52 | 0.003 | −0.53 | 0.003 |
| R-Temporal lobe MD | 0.57 | 0.001 | 0.57 | 0.001 |
| R-occipital lobe FA | −0.52 | 0.003 | −0.52 | 0.003 |
| R-occipital lobe MD | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabéu-Sanz, Á.; Morales, S.; Naranjo, V.; Sempere, Á.P. Contribution of Gray Matter Atrophy and White Matter Damage to Cognitive Impairment in Mildly Disabled Relapsing-Remitting Multiple Sclerosis Patients. Diagnostics 2021, 11, 578. https://doi.org/10.3390/diagnostics11030578
Bernabéu-Sanz Á, Morales S, Naranjo V, Sempere ÁP. Contribution of Gray Matter Atrophy and White Matter Damage to Cognitive Impairment in Mildly Disabled Relapsing-Remitting Multiple Sclerosis Patients. Diagnostics. 2021; 11(3):578. https://doi.org/10.3390/diagnostics11030578
Chicago/Turabian StyleBernabéu-Sanz, Ángela, Sandra Morales, Valery Naranjo, and Ángel P. Sempere. 2021. "Contribution of Gray Matter Atrophy and White Matter Damage to Cognitive Impairment in Mildly Disabled Relapsing-Remitting Multiple Sclerosis Patients" Diagnostics 11, no. 3: 578. https://doi.org/10.3390/diagnostics11030578
APA StyleBernabéu-Sanz, Á., Morales, S., Naranjo, V., & Sempere, Á. P. (2021). Contribution of Gray Matter Atrophy and White Matter Damage to Cognitive Impairment in Mildly Disabled Relapsing-Remitting Multiple Sclerosis Patients. Diagnostics, 11(3), 578. https://doi.org/10.3390/diagnostics11030578







