Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Collection and Handling
2.3. Processing of the Samples
2.4. Statistical Analyses
3. Results
3.1. Study Population
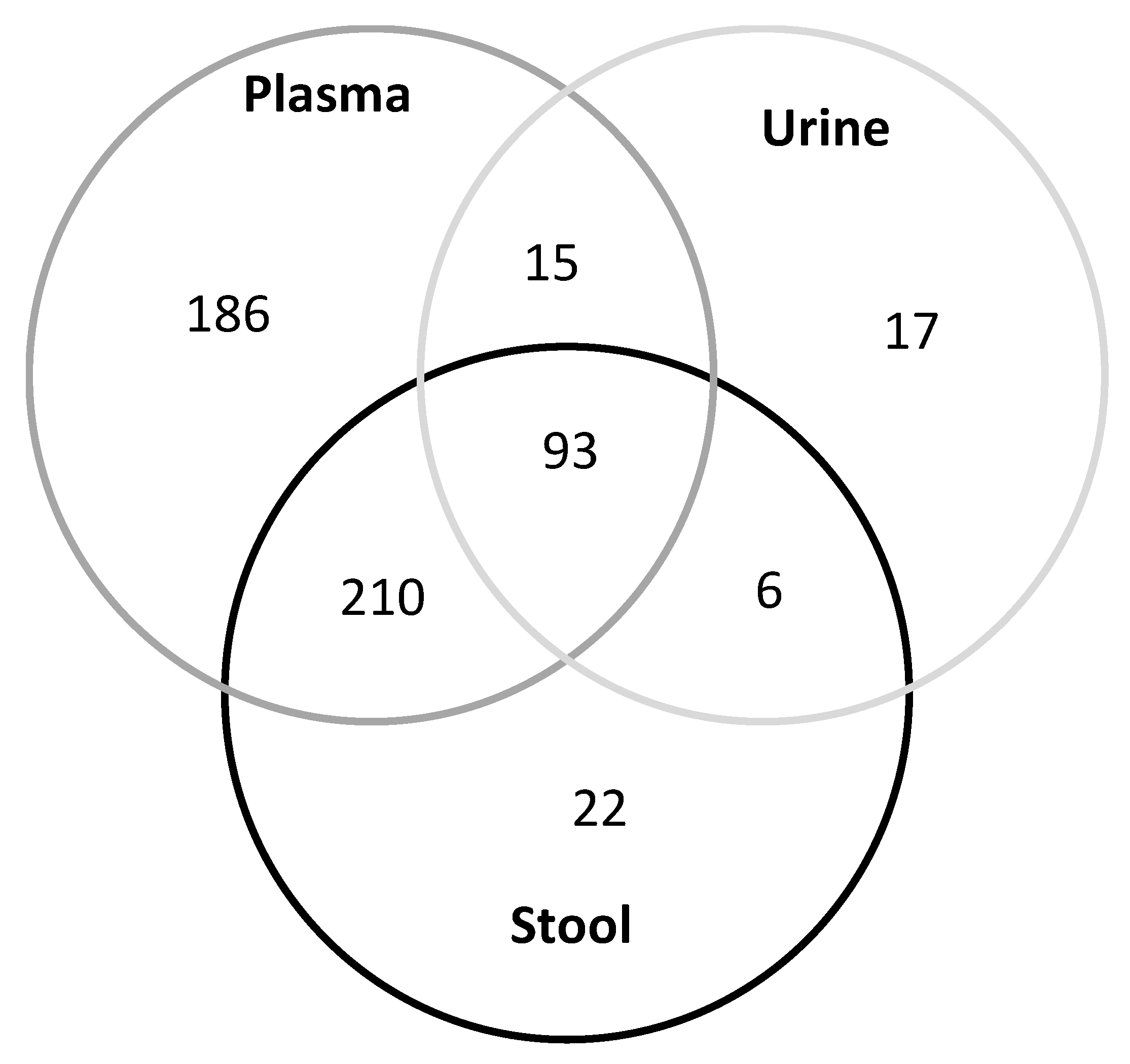
3.2. Metabolite Profiles in Various Human Bio-Samples
3.3. Correlation of Metabolites in Liquid Biopsies
3.4. Differences in Metabolite Concentrations between Participants with and without Advanced Colorectal Neoplasms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senyilmaz, D.; Teleman, A.A. Chicken or the egg: Warburg effect and mitochondrial dysfunction. F1000prime Rep. 2015, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboud, O.A.; Weiss, R.H. New opportunities from the cancer metabolome. Clin. Chem. 2013, 59, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, V.; Bhardwaj, M.; Schrotz-King, P.; Brenner, H. Metabolomics biomarkers for detection of colorectal neoplasms: A systematic review. Cancers 2018, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: A systematic review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisner, R.; Greiner, R.; Tso, V.; Wang, H.; Fedorak, R.N. A machine-learned predictor of colonic polyps based on urinary metabolomics. BioMed Res. Int. 2013, 2013, 303982. [Google Scholar] [CrossRef]
- Amiot, A.; Dona, A.C.; Wijeyesekera, A.; Tournigand, C.; Baumgaertner, I.; Lebaleur, Y.; Sobhani, I.; Holmes, E. 1H NMR spectroscopy of fecal extracts enables detection of advanced colorectal neoplasia. J. Proteome Res. 2015, 14, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chang, D.; Foshaug, R.R.; Eisner, R.; Tso, V.K.; Wishart, D.S.; Fedorak, R.N. Development and validation of a high-throughput mass spectrometry based urine metabolomic test for the detection of colonic adenomatous polyps. Metabolites 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Fang, H.; Tso, V.K.; Sun, Y.; Foshaug, R.R.; Krahn, S.C.; Zhang, F.; Yan, Y.; Xu, H.; Chang, D.; et al. Clinical validation of a novel urine-based metabolomic test for the detection of colonic polyps on chinese population. Int. J. Colorectal Dis. 2017, 32, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Hoffmeister, M.; Arndt, V.; Stegmaier, C.; Altenhofen, L.; Haug, U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: Population-based study. J. Natl. Cancer Inst. 2010, 102, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Carr, P.R.; Weigl, K.; Jansen, L.; Walter, V.; Erben, V.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Healthy lifestyle factors associated with lower risk of colorectal cancer irrespective of genetic risk. Gastroenterology 2018, 155, 1805–1815.e1805. [Google Scholar] [CrossRef] [PubMed]
- Erben, V.; Carr, P.R.; Holleczek, B.; Stegmaier, C.; Hoffmeister, M.; Brenner, H. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: Results from a large cohort of participants of screening colonoscopy. Int. J. Cancer 2019, 144, 2135–2143. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Bezabeh, T.; Wang, Z.; Liang, J.; Huang, Y.; Zhao, J.; Liu, X.; Ye, W.; Tang, W.; et al. 1H NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int. J. Cancer 2019, 145, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Rao, S.; Weir, T.L.; O’Malia, J.; Bazan, M.; Brown, R.J.; Ryan, E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giskeødegård, G.F.; Madssen, T.S.; Euceda, L.R.; Tessem, M.B.; Moestue, S.A.; Bathen, T.F. Nmr-based metabolomics of biofluids in cancer. NMR Biomed. 2018, e3927. [Google Scholar] [CrossRef]
- Liesenfeld, D.B.; Grapov, D.; Fahrmann, J.F.; Salou, M.; Scherer, D.; Toth, R.; Habermann, N.; Böhm, J.; Schrotz-King, P.; Gigic, B.; et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: The colocare study. Am. J. Clin. Nutr. 2015, 102, 433–443. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urinology Think Tank Writing Group. Urine: Waste product or biologically active tissue? Neurourol. Urodyn. 2018, 37, 1162–1168. [Google Scholar] [CrossRef]
- Phua, L.C.; Chue, X.P.; Koh, P.K.; Cheah, P.Y.; Ho, H.K.; Chan, E.C. Non-invasive fecal metabonomic detection of colorectal cancer. Cancer Biol. Ther. 2014, 15, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the intersections between metabolism and cancer biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, K.; Yachida, S.; Sugimoto, M.; Oshima, M.; Nakagawa, T.; Akamoto, S.; Tabata, S.; Saitoh, K.; Kato, K.; Sato, S.; et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by myc. Proc. Natl. Acad. Sci. USA 2017, 114, E7697–e7706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagland, H.R.; Berg, M.; Jolma, I.W.; Carlsen, A.; Søreide, K. Molecular pathways and cellular metabolism in colorectal cancer. Dig. Surg. 2013, 30, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.A.; Brennan, L.; Broadhurst, D.; Fiehn, O.; Cascante, M.; Dunn, W.B.; Schmidt, M.A.; Velagapudi, V. Preanalytical processing and biobanking procedures of biological samples for metabolomics research: A white paper, community perspective (for “precision medicine and pharmacometabolomics task group”-the metabolomics society initiative). Clin. Chem. 2018, 64, 1158–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobard, E.; Trédan, O.; Postoly, D.; André, F.; Martin, A.L.; Elena-Herrmann, B.; Boyault, S. A systematic evaluation of blood serum and plasma pre-analytics for metabolomics cohort studies. Int. J. Mol. Sci. 2016, 17, 2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carayol, M.; Licaj, I.; Achaintre, D.; Sacerdote, C.; Vineis, P.; Key, T.J.; Onland Moret, N.C.; Scalbert, A.; Rinaldi, S.; Ferrari, P. Reliability of serum metabolites over a two-year period: A targeted metabolomic approach in fasting and non-fasting samples from epic. PLoS ONE 2015, 10, e0135437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotter, M.; Brandmaier, S.; Prehn, C.; Adam, J.; Rabstein, S.; Gawrych, K.; Brüning, T.; Illig, T.; Lickert, H.; Adamski, J.; et al. Stability of targeted metabolite profiles of urine samples under different storage conditions. Metabolomics 2017, 13, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breier, M.; Wahl, S.; Prehn, C.; Fugmann, M.; Ferrari, U.; Weise, M.; Banning, F.; Seissler, J.; Grallert, H.; Adamski, J.; et al. Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples. PLoS ONE 2014, 9, e89728. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, M.M. Preanalytics in urinalysis. Clin. Biochem. 2016, 49, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Erben, V.; Poschet, G.; Schrotz-King, P.; Brenner, H. Evaluation of different stool extraction methods for metabolomics measurements in human fecal samples. medRxiv 2020. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, T.; Chen, M.; He, L.; Wang, T.; Liu, X.; Chang, H.; Mao, J.H.; Hang, B.; Snijders, A.M.; et al. Systematic analysis of impact of sampling regions and storage methods on fecal gut microbiome and metabolome profiles. mSphere 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Verla-Tebit, E.; Lilla, C.; Hoffmeister, M.; Brenner, H.; Chang-Claude, J. Cigarette smoking and colorectal cancer risk in germany: A population-based case-control study. Int. J. Cancer 2006, 119, 630–635. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Jette, M.; Sidney, K.; Blumchen, G. Metabolic equivalents (mets) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Tandon, K.; Imam, M.; Ismail, B.E.; Castro, F. Body mass index and colon cancer screening: The road ahead. World J. Gastroenterol. 2015, 21, 1371–1376. [Google Scholar] [CrossRef]
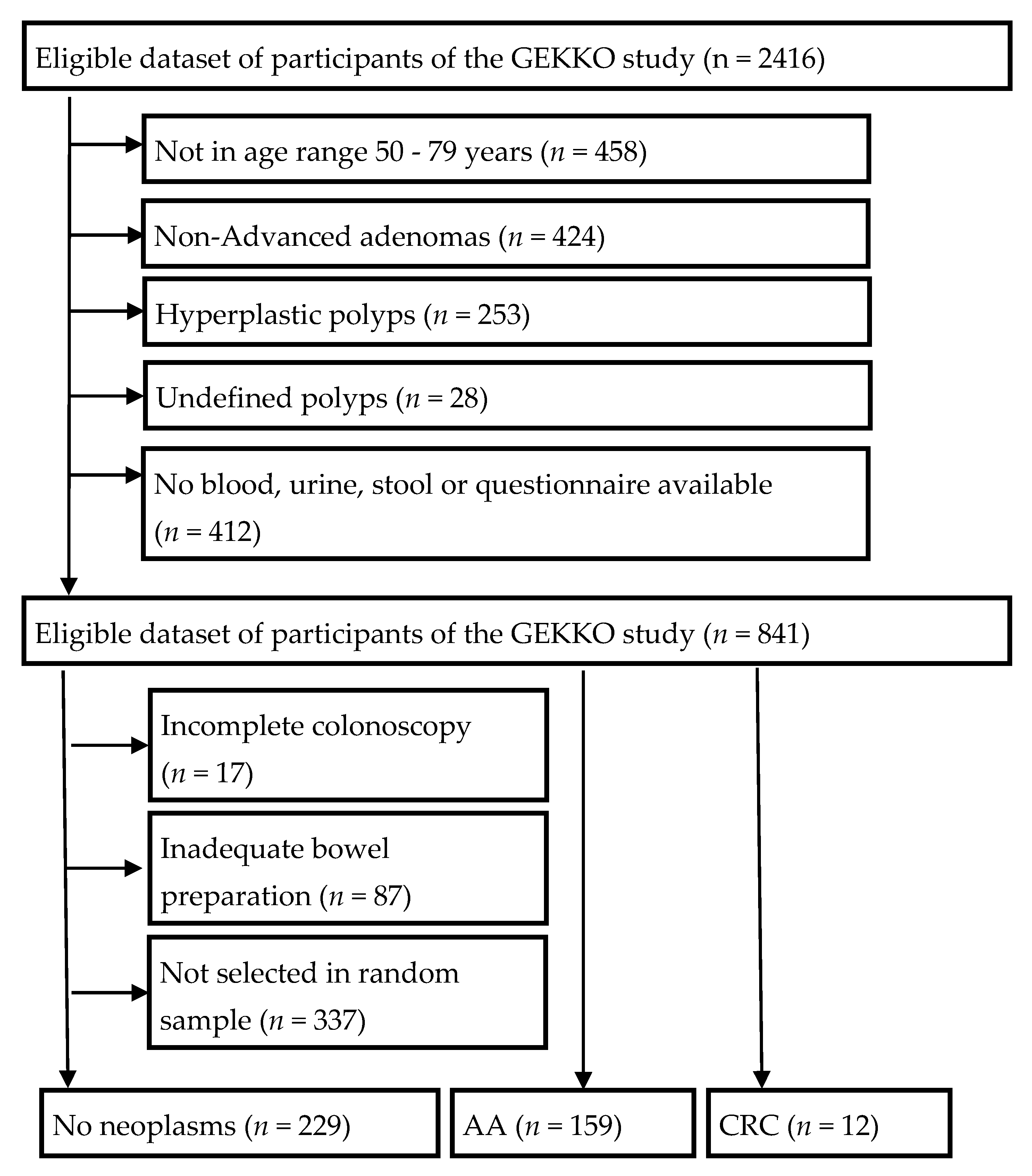

| Characteristics | No Neoplasms | AA/CRC | p Value 1 |
|---|---|---|---|
| n = 229 | n = 159/12 | ||
| Sex, n (%) | |||
| Female | 106 (46%) | 68 (40%) | 0.19 |
| Male | 123 (54%) | 103 (60%) | |
| Age, n (%) | |||
| 50–59 years | 122 (53%) | 61 (36%) | 0.0006 |
| 60–69 years | 65 (28%) | 55 (32%) | |
| 70–79 years | 42 (18%) | 55 (32%) | |
| Mean, (SD) | 60.9 (±8.0) | 64.1 (±8.6) | 0.0002 |
| Smoking status, n (%) | |||
| Current | 23 (10%) | 32 (19%) | 0.0031 |
| Former | 79 (34%) | 71 (42%) | |
| Never | 127 (55%) | 68 (40%) | |
| BMI (kg/m2), mean | 26.1 (±4.2) | 26.9 (±4.6) | 0.06 |
| Alcohol consumption (g/day), mean | |||
| Women | 6.1 (±10.2) | 8.8 (±34.7) | 0.17 |
| Men | 9.0 (±12.1) | 13.9 (±14.5) | 0.007 |
| Leisure time physical activity MET-h/week, mean (SD) | 42.7 (±57.6) | 37.3 (±41.4) | 0.08 |
| Dietary quality score, mean 2 | 31.0 (±6.7) | 28.7 (±6.7) | 0.0005 |
| Healthy Lifestyle score 2 | |||
| 4 or 5 points | 99 (43%) | 50 (29%) | 0.0005 |
| 3 points | 96 (41%) | 66 (39%) | |
| 0 or 1 or 2 points | 34 (15%) | 55 (32%) |
| Total | Blood vs. Stool | Blood vs. Urine | Stool vs. Urine | ||||
|---|---|---|---|---|---|---|---|
| Pos. n (%) | Neg. n (%) | Pos. n (%) | Neg. n (%) | Pos. n (%) | Neg. n (%) | ||
| Correlation −0.5 to ≤−0.4 | 1 (0.16) | 0 | 0 | ||||
| Correlation −0.4 to ≤−0.3 | 1 (0.16) | 2 (0.32) | 1 (0.16) | ||||
| Correlation −0.3 to ≤−0.2 | 8 (1.27) | 11 (1.77) | 11 (1.77) | ||||
| Correlation −0.2 to ≤−0.1 | 38 (6.04) | 52 (8.36) | 88 (14.13) | ||||
| Correlation −0.1 to ≤0.0 | 201 (31.96) | 183 (29.42) | 266 (42.70) | ||||
| Correlation 0.0 to ≤0.1 | 268 (42.61) | 233 (37.46) | 188 (30.18) | ||||
| Correlation 0.1 to ≤0.2 | 80 (12.72) | 82 (13.18) | 59 (9.47) | ||||
| Correlation 0.2 to ≤0.3 | 21 (3.34) | 20 (3.22) | 9 (1.44) | ||||
| Correlation 0.3 to ≤0.4 | 2 (0.32) | 10 (1.61) | 0 | ||||
| Correlation 0.4 to ≤0.5 | 3 (0.48) | 10 (1.61) | 1 (0.16) | ||||
| Correlation 0.5 to ≤0.6 | 4 (0.64) | 5 (0.80) | 0 | ||||
| Correlation 0.6 to ≤0.7 | 1 (0.16) | 4 (0.64) | 0 | ||||
| Correlation 0.7 to ≤0.8 | 1 (0.16) | 4 (0.64) | 0 | ||||
| Correlation 0.8 to ≤0.9 | 0 | 5 (0.80) | 0 | ||||
| Correlation 0.9 to ≤1.00 | 0 | 1 (0.16) | 0 | ||||
| Significant correlations | |||||||
| Total study population | 630 | 68 | 25 | 126 | 28 | 39 | 63 |
| Participants without neoplasms | 630 | 59 | 25 | 114 | 28 | 20 | 49 |
| Participants with advanced colorectal neoplasms | 630 | 54 | 20 | 88 | 34 | 27 | 42 |
| Total study population, significant correlations | |||||||
| Alkaloids | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Amine Oxides | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Amino Acids | 20 | 1 | 0 | 17 | 0 | 5 | 0 |
| Amino acid related | 30 | 11 | 1 | 26 | 1 | 10 | 3 |
| Bile Acids | 14 | 3 | 1 | 13 | 0 | 2 | 1 |
| Biogenic Amines | 9 | 1 | 0 | 3 | 0 | 1 | 1 |
| Carbohydrates and related | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Carboxylic Acids | 7 | 1 | 0 | 3 | 0 | 0 | 0 |
| Cresols | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fatty Acids | 12 | 6 | 2 | 1 | 5 | 0 | 2 |
| Hormones and related | 4 | 2 | 0 | 4 | 0 | 0 | 0 |
| Indoles and Derivatives | 4 | 2 | 0 | 3 | 0 | 1 | 0 |
| Nucleobases and related | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| Vitamins and Cofactors | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Acylcarnitines | 40 | 17 | 6 | 15 | 8 | 4 | 19 |
| Glycerophospholipids (Lysophosphatidylcholines and Phosphatidylcholines) | 90 | 5 | 3 | 18 | 3 | 1 | 2 |
| Sphingomyelins | 15 | 0 | 1 | 0 | 1 | 0 | 0 |
| Cholesteryl Esters | 22 | 3 | 1 | 2 | 2 | 0 | 11 |
| Ceramides | 28 | 5 | 1 | 2 | 1 | 1 | 0 |
| Dihydroceramides | 8 | 0 | 0 | 1 | 1 | 0 | 1 |
| Glycosylceramides (Mono-, Di-, and Trihexosylceramides) | 34 | 0 | 0 | 0 | 0 | 1 | 0 |
| Diglycerides | 44 | 3 | 5 | 7 | 2 | 0 | 9 |
| Triglycerides | 242 | 7 | 4 | 5 | 4 | 11 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erben, V.; Poschet, G.; Schrotz-King, P.; Brenner, H. Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms. Diagnostics 2021, 11, 561. https://doi.org/10.3390/diagnostics11030561
Erben V, Poschet G, Schrotz-King P, Brenner H. Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms. Diagnostics. 2021; 11(3):561. https://doi.org/10.3390/diagnostics11030561
Chicago/Turabian StyleErben, Vanessa, Gernot Poschet, Petra Schrotz-King, and Hermann Brenner. 2021. "Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms" Diagnostics 11, no. 3: 561. https://doi.org/10.3390/diagnostics11030561
APA StyleErben, V., Poschet, G., Schrotz-King, P., & Brenner, H. (2021). Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms. Diagnostics, 11(3), 561. https://doi.org/10.3390/diagnostics11030561






