Characteristic Features of Infrared Thermographic Imaging in Primary Raynaud’s Phenomenon
Abstract
1. Introduction
2. Materials and Methods
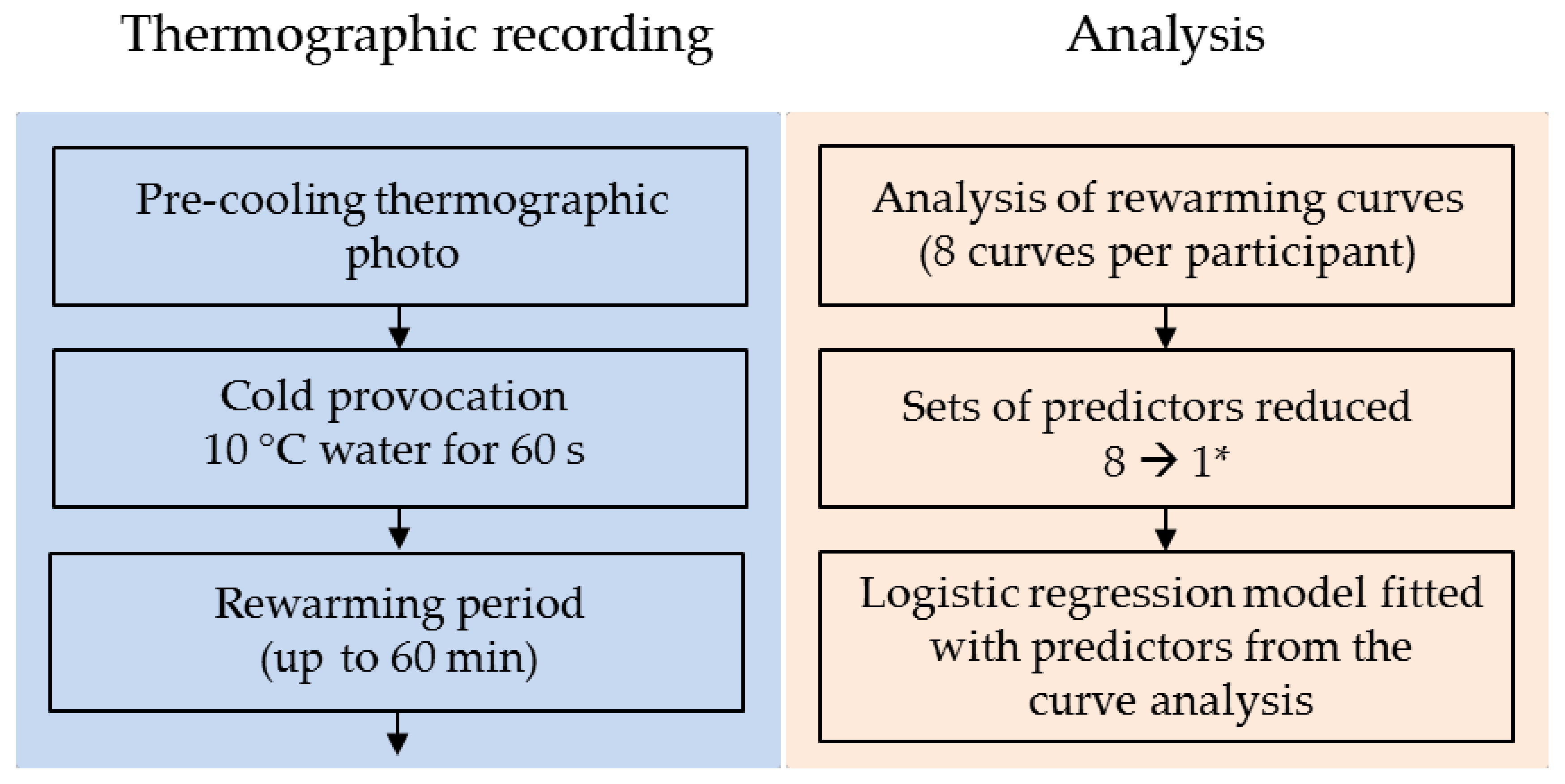
2.1. Thermographic Imaging Procedure
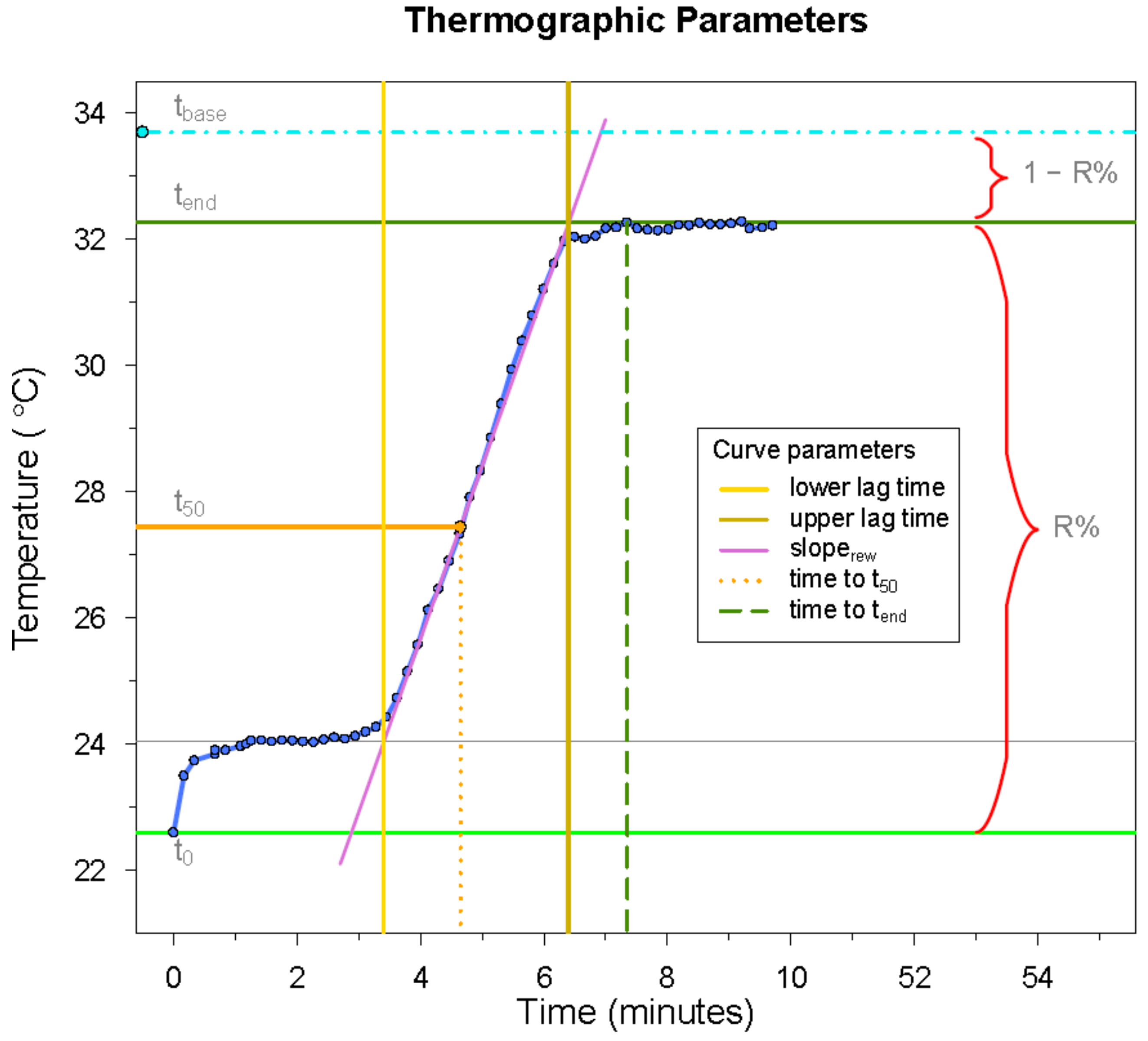
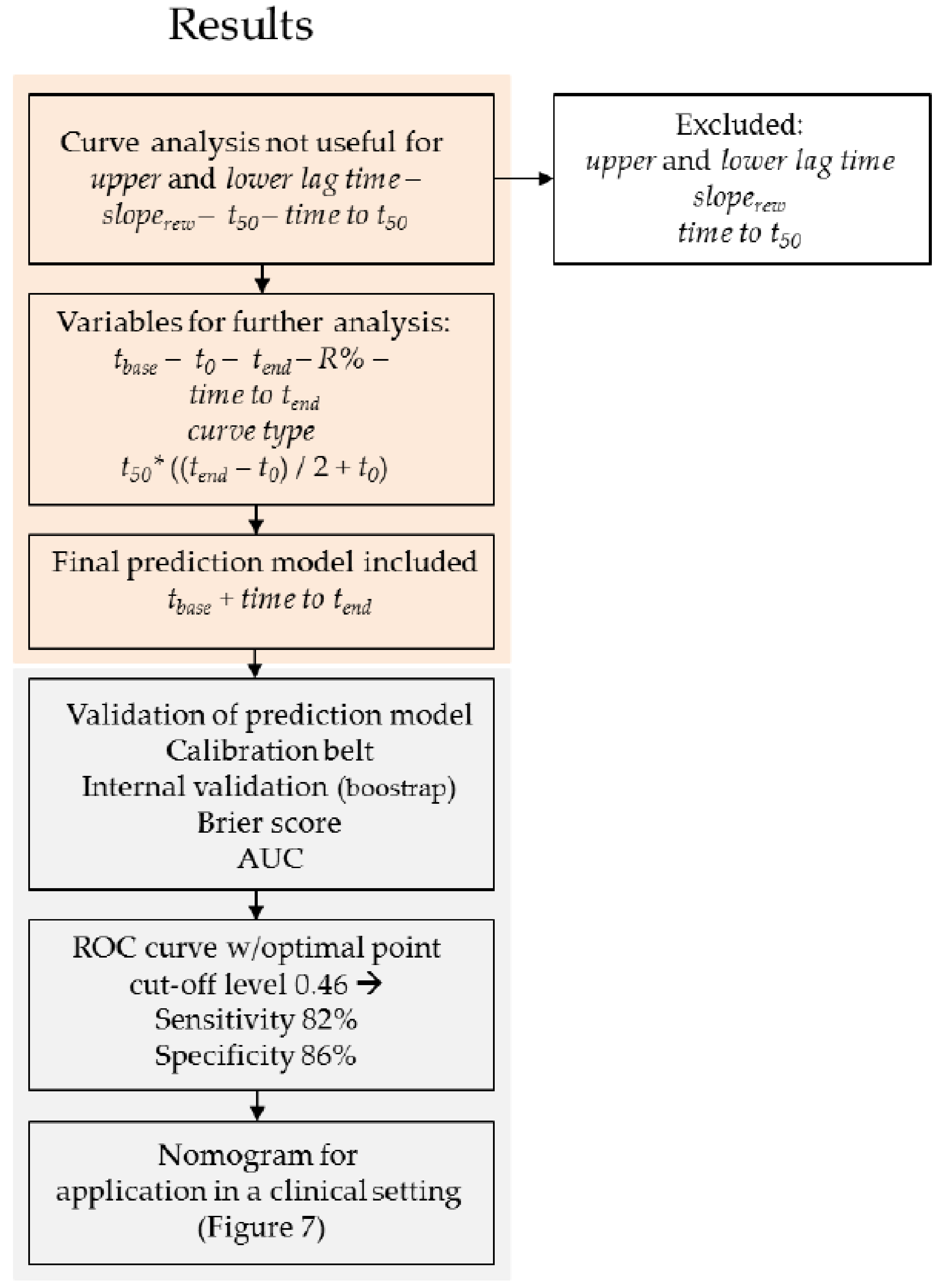
2.2. Data Preparation and Curve Analysis
2.3. Statistics
3. Results
3.1. Clinical Characteristics
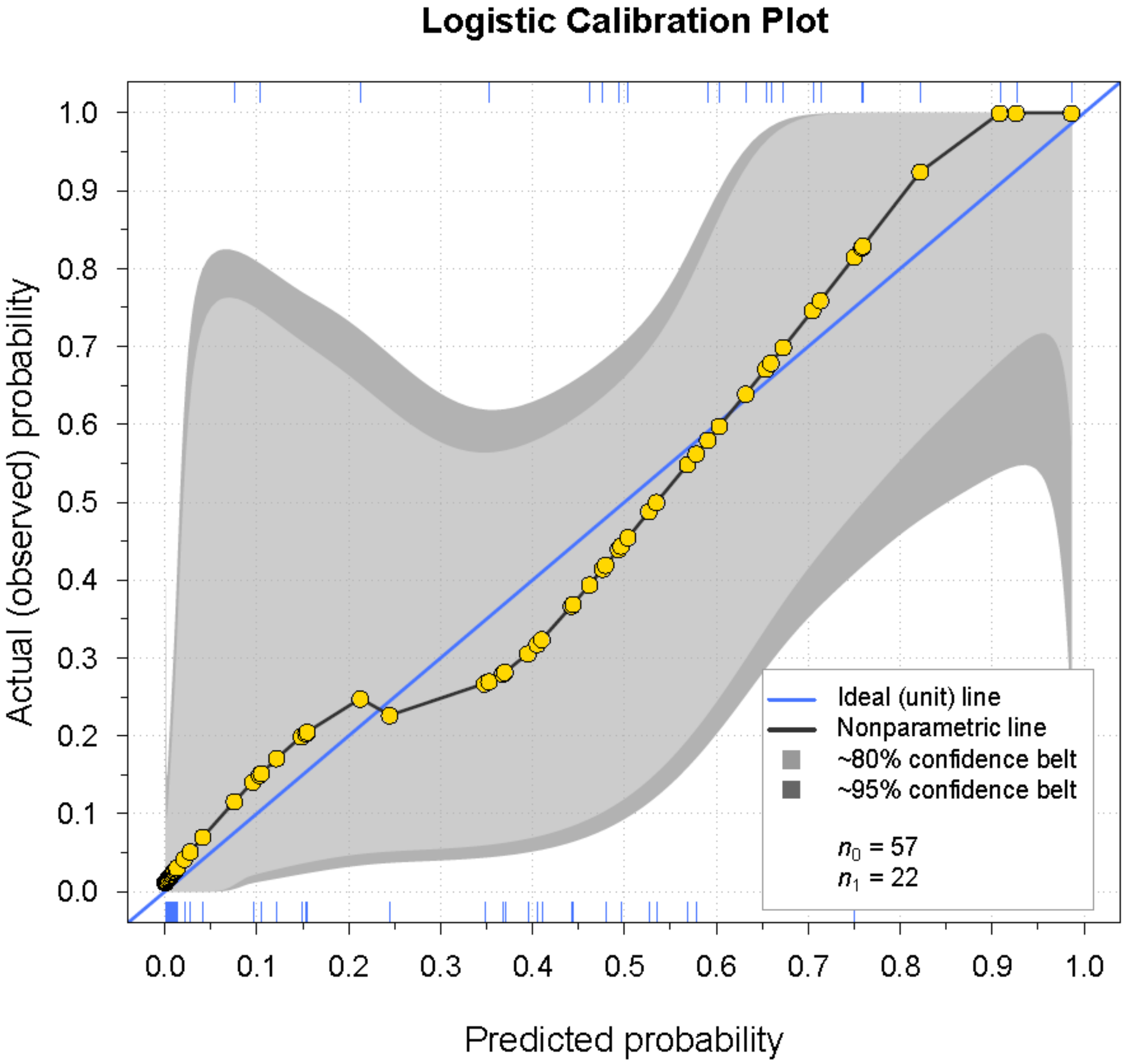
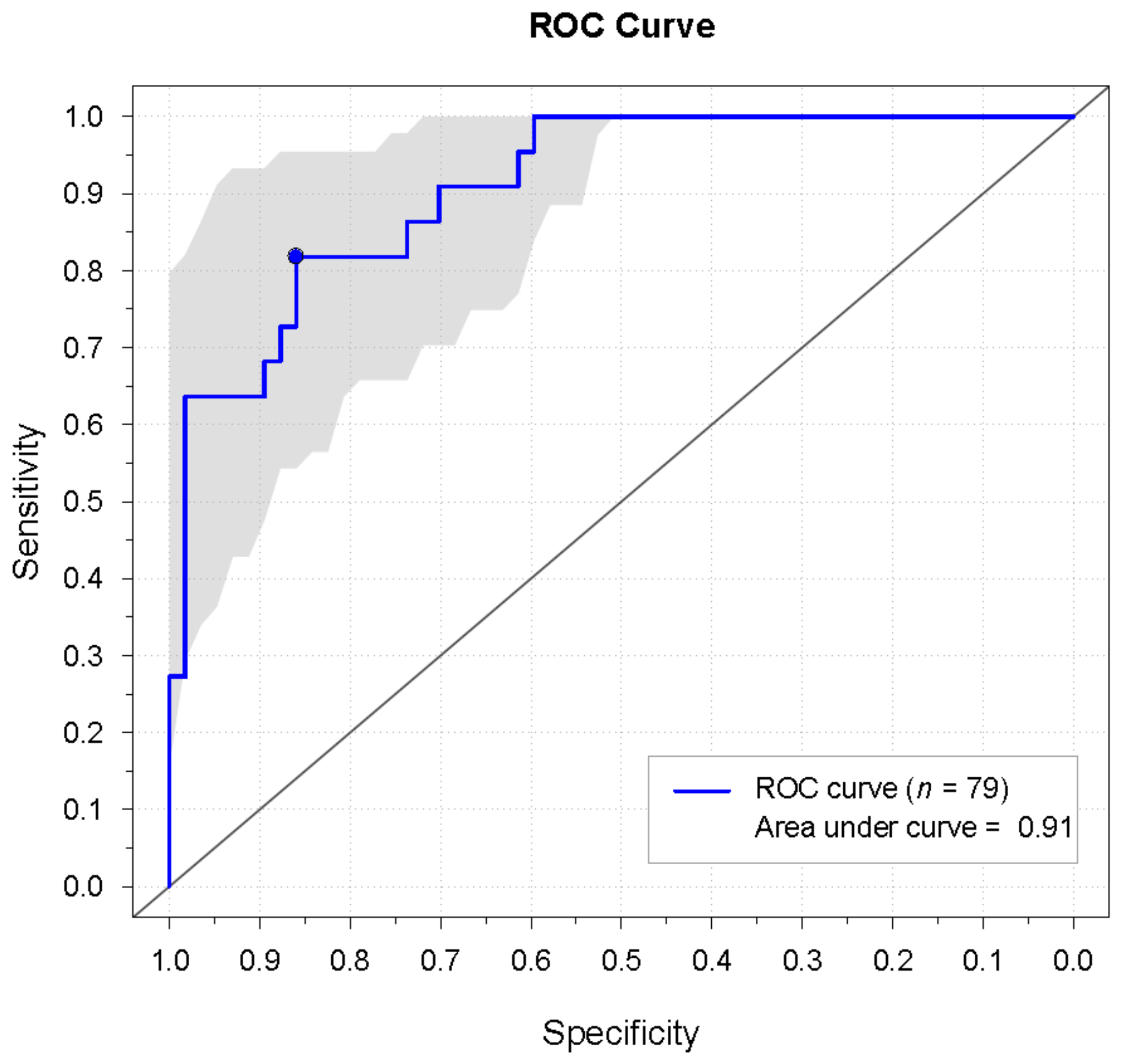
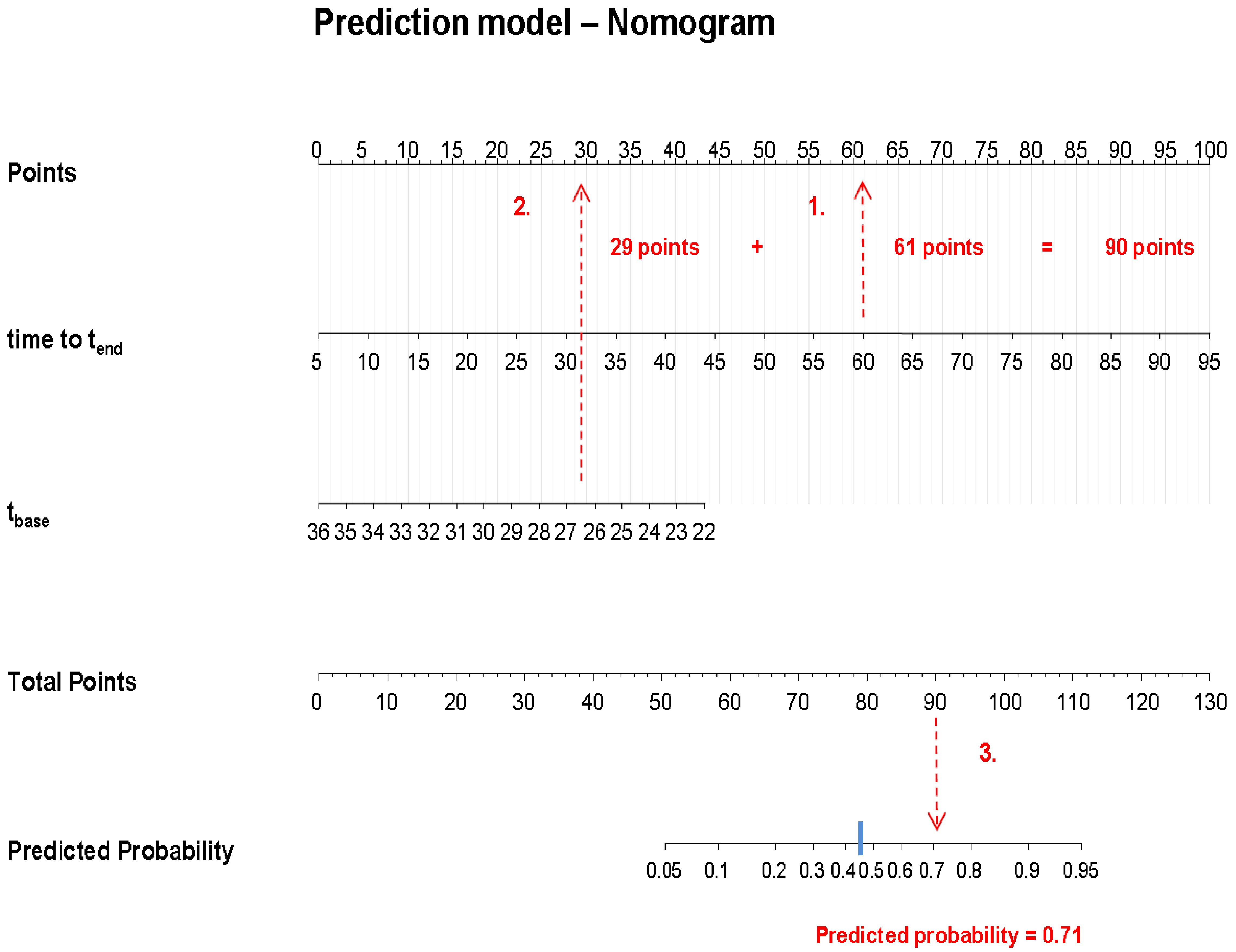
3.2. The Prediction Model
3.3. Cut-Off Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrick, A.L. The Pathogenesis, Diagnosis and Treatment of Raynaud Phenomenon. Nat. Rev. Rheumatol. 2012, 8, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L.; Clark, S. Quantifying Digital Vascular Disease in Patients with Primary Raynaud’s Phenomenon and Systemic Sclerosis. Ann. Rheum. Dis 1998, 57, 70–78. [Google Scholar] [CrossRef]
- Nielsen, S.L. Raynaud Phenomena and Finger Systolic Pressure during Cooling. Scand. J. Clin. Lab. Investig. 1978, 38, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.; Nielsen, S.L. Diagnosis of Raynaud’s Phenomenon in Quarrymen’s Traumatic Vasospastic Disease. Scand. J. Work Environ. Health 1979, 5, 249–256. [Google Scholar] [CrossRef]
- Olsen, N.; Nielsen, S.L.; Voss, P. Cold Response of Digital Arteries in Chain Saw Operators. Br. J. Ind. Med. 1982, 39, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N. Diagnostic Tests in Raynaud’s Phenomena in Workers Exposed to Vibration: A Comparative Study. Br. J. Ind. Med. 1988, 45, 426–430. [Google Scholar] [CrossRef]
- Pyykko, I.; Farkkila, M.; Korhonen, O.; Starck, J.; Jäntti, V. Cold Provocation Tests in the Evaluation of Vibration-Induced White Finger. Scand. J. Work Environ. Health 1986, 12, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ekenvall, L.; Lindblad, L.E. Vibration White Finger and Digital Systolic Pressure during Cooling. Br. J. Ind. Med. 1986, 43, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Corbin, D.; Wood, D.; Hously, E. An Evaluation of Finger Systolic-pressure Response to Local Cooling in the Diagnosis of Primary Raynaud’s Phenomenon. Clin. Physiol. Funct. Imaging 1985, 5, 383–392. [Google Scholar] [CrossRef]
- Leppert, J. Limitation of Finger Systolic Pressure Measurement as a Diagnostic Test for Primary Raynaud’s Phenomenon in a Female Population. Clin. Physiol. Funct. Imaging 1989, 9, 457–465. [Google Scholar] [CrossRef]
- Allen, J.; Devlin, M.; McGrann, S.; Doherty, C. An Objective Test for the Diagnosis and Grading of Vasospasm in Patients with Raynaud’s Syndrome. Clin. Sci. 1992, 82, 529–534. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Leggett, S.A.; Marjanovic, E.J.; Moore, T.L.; Anderson, M.E.; Britton, J.; Buch, M.H.; Del Galdo, F.; Denton, C.P.; Dinsdale, G.; et al. A Multicenter Study of the Validity and Reliability of Responses to Hand Cold Challenge as Measured by Laser Speckle Contrast Imaging and Thermography Outcome Measures for Systemic Sclerosis—Related Raynaud’s Phenomenon. Arthritis Rheumatol. 2018, 70, 903–911. [Google Scholar] [CrossRef]
- Anderson, M.E.; Moore, T.L.; Lunt, M.; Herrick, A.L. The “Distal-Dorsal Difference”: A Thermographic Parameter by Which to Differentiate between Primary and Secondary Raynaud’s Phenomenon. Rheumatology 2007, 46, 533–538. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Taylor, L.; El-Hadidy, K.; Jayson, M.I.V. Measurement of Cold Challenge Responses in Primary Raynaud’s Phenomenon and Raynaud’s Phenomenon Associated with Systemic Sclerosis. Ann. Rheum. Dis. 1992, 51, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, P.A.; Chetter, I.C.; Kent, P.J.; Kester, R.C. The Analysis of Sensitivity, Specificity, Positive Predictive Value and Negative Predictive Value of Cold Provocation Thermography in the Objective Diagnosis of the Hand-Arm Vibration Syndrome. Occup. Med. (Lond.) 2001, 51, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Patel, F.; Kronenberg, D.G.; Chung, L.; Fiorentino, D.; Allanore, Y.; Guiducci, S.; Hesselstrand, R.; Hummers, L.K.; Duong, C.; et al. International Consensus Criteria for the Diagnosis of Raynaud’s Phenomenon. J. Autoimmun. 2014, 48–49, 60–65. [Google Scholar] [CrossRef]
- Datta, S.; Satten, G.A. A Signed-Rank Test for Clustered Data. Biometrics 2008, 64, 501–507. [Google Scholar] [CrossRef]
- Steyerberg, E.W. Clinical Prediction Models. A Practical Approach to Development, Validation, and Updating, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Harrell, F. Regression Modeling Strategies. With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Nattino, G.; Finazzi, S.; Bertolini, G. A New Calibration Test and a Reappraisal of the Calibration Belt for the Assessment of Prediction Models Based on Dichotomous Outcomes. Stat. Med. 2014, 33, 2390–2407. [Google Scholar] [CrossRef]
- Herrick, A.L.; Dinsdale, G.; Murray, A. New Perspectives in the Imaging of Raynaud’s Phenomenon. Eur. J. Rheumatol. 2020, 7, 212–221. [Google Scholar] [CrossRef]
- Mahbub, M.; Harada, N. Review of Different Quantification Methods for the Diagnosis of Digital Vascular Abnormalities in Hand-Arm Vibration Syndrome. J. Occup. Health 2011, 53, 241–249. [Google Scholar] [CrossRef]
- Schuhfried, O.; Vacariu, G.; Lang, T.; Korpan, M.; Kiener, H.P.; Fialka-Moser, V. Thermographic Parameters in the Diagnosis of Secondary Raynaud’s Phenomenon. Arch. Phys. Med. Rehabil. 2000. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, L.F.; Carter, L.; Spector, T.D.; Howell, K.J.; Black, C.M.; MacGregor, A.J. Use of Thermographic Criteria to Identify Raynaud’s Phenomenon in a Population Setting. J. Rheumatol. 2003, 30, 720–722. [Google Scholar]
- Lim, M.J.; Kwon, S.R.; Jung, K.; Joo, K.; Park, S.; Park, W. Digital Thermography of the Fingers and Toes in Raynaud’s Phenomenon. J. Korean Med. Sci. 2014, 29, 502–506. [Google Scholar] [CrossRef] [PubMed]
- von Bierbrauer, A.; Schilk, I.; Lucke, C.; Schmidt, J.A. Infrared Thermography in the Diagnosis of Raynaud’s Phenomenon in Vibration-Induced White Finger. VASA 1998, 27, 94–99. [Google Scholar]
- House, R.; Holness, L.; Taraschuk, I.; Nisenbaum, R. Infrared Thermography in the Hands and Feet of Hand-Arm Vibration Syndrome (HAVS) Cases and Controls. Int. J. Ind. Ergon. 2017, 62, 70–76. [Google Scholar] [CrossRef]
- Ye, Y.; Griffin, M.J. Effect of Room Temperature on Tests for Diagnosing Vibration-Induced White Finger: Finger Rewarming Times and Finger Systolic Blood Pressures. Int. Arch. Occup. Environ. Health 2017, 90, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Cappella, M.; Culpo, R.; Vittadello, F.; Sprocati, M.; Zulian, F. Infrared Thermography in Children: A Reliable Tool for Differential Diagnosis of Peripheral Microvascular Dysfunction and Raynaud’s Phenomenon? Pediatr. Rheumatol. 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, K.L. Evaluation of Hand Skin Temperature—Infrared Thermography in Combination with Cold Stress Tests; Luleå University of Technology: Luleå, Sweden, 2017. [Google Scholar]
- Ruaro, B.; Smith, V.; Sulli, A.; Pizzorni, C.; Tardito, S.; Patané, M.; Paolino, S.; Cutolo, M.; Chighizola, C.B. Innovations in the Assessment of Primary and Secondary Raynaud’s Phenomenon. Front. Pharmacol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Magalhaes, C.; Mendes, J.; Vardasca, R. Meta-Analysis and Systematic Review of the Application of Machine Learning Classifiers in Biomedical Applications of Infrared Thermography. Appl. Sci. 2021, 11, 842. [Google Scholar] [CrossRef]
- Umapathy, S.; Vasu, S.; Gupta, N. Computer Aided Diagnosis Based Hand Thermal Image Analysis: A Potential Tool for the Evaluation of Rheumatoid Arthritis. J. Med. Biol. Eng. 2018, 38, 666–677. [Google Scholar] [CrossRef]
- Bandalakunta Gururajarao, S.; Venkatappa, U.; Shivaram, J.M.; Sikkandar, M.Y.; Al Amoudi, A. Infrared Thermography and Soft Computing for Diabetic Foot Assessment. Mach. Learn. Bio-Signal Anal. Diagn. Imaging 2009, 73–97. [Google Scholar] [CrossRef]
- Sathish, D.; Kamath, S.; Prasad, K.; Kadavigere, R. Role of Normalization of Breast Thermogram Images and Automatic Classification of Breast Cancer. Vis. Comput. 2019, 35, 57–70. [Google Scholar] [CrossRef]
- Jesenšek Papež, B.; Palfy, M.; Mertik, M.; Turk, Z. Infrared Thermography Based on Artificial Intelligence as a Screening Method for Carpal Tunnel Syndrome Diagnosis. J. Int. Med. Res. 2009, 37, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Kacmaz, S.; Ercelebi, E. International Symposium on Multidisciplinary Studies and Innovative Technologies. In The Thermal Imaging System Design in the Diagnosis and Follow-Up of Raynaud’s Phenomenon; IEEE: New York, NY, USA, 2018; pp. 299–302. [Google Scholar]
- Shaikh, F.; Dehmeshki, J.; Bisdas, S.; Roettger-Dupont, D.; Kubassova, O.; Aziz, M.; Awan, O. Artificial Intelligence-Based Clinical Decision Support Systems Using Advanced Medical Imaging and Radiomics. Curr. Probl. Diagn. Radiol. 2021, 50, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, T.J.M.; Fennis, J.F.M.; Van’t Laar, A. Finger Temperature after a Finger-Cooling Test: Influence of Air Temperature and Smoking. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 1167–1171. [Google Scholar] [CrossRef] [PubMed]


| Patients, pRP | Controls | p-Value | ||
|---|---|---|---|---|
| Number of participants | 22 | 57 | ||
| Gender | Female | 19 (86.4) | 24 (42.1) | <0.001 |
| Male | 3 (13.6) | 33 (57.9) | ||
| Age | 57.2 (10.0) | 57.8 (12.5) | 0.82 | |
| Smoking status | Never | 11 (50.0) | 35 (61.4) | 0.51 |
| Current | 0 (0.0) | 1 (1.8) | ||
| Former | 11 (50.0) | 21 (36.8) | ||
| Tobacco (pack-years) | 0 (0;5) | 0 (0;3) | 0.81 | |
| Alcohol (units/week) | 3 (1;7) | 4 (2;7) | 0.83 |
| Predictor | Estimate | Std. Error | Wald χ2 | p-Value |
|---|---|---|---|---|
| Intercept | 2.4 | 4.9 | 0.50 | 0.62 |
| time to tend | 0.11 | 0.04 | 2.9 | 0.004 |
| tbase | –0.30 | 0.15 | –2.0 | 0.04 |
| Final Model | Original | Bootstrap Corrected |
|---|---|---|
| Calibration intercept | 0.00 | –0.01 |
| Calibration slope | 1.00 | 0.89 |
| Brier score | 0.11 | 0.13 |
| Concordance statistic/AUC | 0.91 (0.84–0.98) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindberg, L.; Kristensen, B.; Thomsen, J.F.; Eldrup, E.; Jensen, L.T. Characteristic Features of Infrared Thermographic Imaging in Primary Raynaud’s Phenomenon. Diagnostics 2021, 11, 558. https://doi.org/10.3390/diagnostics11030558
Lindberg L, Kristensen B, Thomsen JF, Eldrup E, Jensen LT. Characteristic Features of Infrared Thermographic Imaging in Primary Raynaud’s Phenomenon. Diagnostics. 2021; 11(3):558. https://doi.org/10.3390/diagnostics11030558
Chicago/Turabian StyleLindberg, Lotte, Bent Kristensen, Jane F. Thomsen, Ebbe Eldrup, and Lars T. Jensen. 2021. "Characteristic Features of Infrared Thermographic Imaging in Primary Raynaud’s Phenomenon" Diagnostics 11, no. 3: 558. https://doi.org/10.3390/diagnostics11030558
APA StyleLindberg, L., Kristensen, B., Thomsen, J. F., Eldrup, E., & Jensen, L. T. (2021). Characteristic Features of Infrared Thermographic Imaging in Primary Raynaud’s Phenomenon. Diagnostics, 11(3), 558. https://doi.org/10.3390/diagnostics11030558






