The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer
Abstract
1. Introduction
2. Adipokines
2.1. Adipokines That Increase the Risk of Endometrial Cancer
2.1.1. Leptin
2.1.2. Visfatin
2.1.3. Galectins
2.2. Adipokines Which Reduce the Risk of Endometrial Cancer
2.2.1. Adiponectin
2.2.2. Omentin-1
2.2.3. Vaspin
| Adipokines | Direct Effect |
|---|---|
| Leptin | predominantly through JAK/STAT pathway which modulates PI3K/AKT3 signaling [62,63] |
| Visfatin | promotion of cell growth via NF-κB/Notch1 [64] |
| Galectin | MAPK family signal transduction and cell proliferation [65] |
| Adiponectin | inhibits cell proliferation via ERK1/2-MAPK pathway [66] |
| Omentin | Stimulates apoptosis through the activation of JAK/STAT signaling pathway [67] |
| Vaspin | inhibits proliferation and chemokinesis through the inhibition of NF-κB/Notch1 pathway [68] |
3. Adipose-Derived Inflammatory Factors
3.1. Interleukin-1β
3.2. Interleukin-6
3.3. Interleukin-8
3.4. Tumor Necrosis Factor α (TNFα)
4. Angiogenic Factors Secreted by the Adipose Tissue
4.1. VEGF
4.2. Fibroblast Growth Factor
4.3. Insulin Growth Factor-1 (IGF-1)
5. Conclusions
| Proposed Factors | Explanation | |
|---|---|---|
| Diagnostic factors | Adiponectin, Leptin, Leptin/Adiponectin Ratio Vaspin, Omentin | May serve as independent endometrial risk factors [51] Meet the criteria to be used as a good diagnostic test [20,75] |
| Prognostic factors | Visfatin, Resistin Galectin | Can possibly serve to predict patients’ staging [32,33] May have a prognostic value and aid in prediction of patients’ survival [127] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Berrino, F.; Key, T.; Rinaldi, S.; Dossus, L.; Biessy, C.; Secreto, G.; Amiano, P.; Bingham, S.; Boeing, H.; et al. Serum Sex Steroids in Premenopausal Women and Breast Cancer Risk Within the European Prospective Investigation into Cancer and Nutrition (EPIC). JNCI 2005, 97, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Cozzo, A.J.; Fuller, A.M.; Makowski, L. Contribution of adipose tissue to development of cancer. Compr. Physiol. 2018, 8, 237–282. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adipose tissue hormones. J. Endocrinol. Investig. 2002, 25, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Linkov, F.; Kokai, L.; Edwards, R.; Sheikh, M.A.; Freese, K.E.; Marra, K.G.; Rubin, J.P. The role of adipose-derived stem cells in endometrial cancer proliferation. Scand. J. Clin. Lab. Investig. 2014, 74, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Dornbush, S.; Aeddula, N.R. Physiology, Leptin; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative Review: The Role of Leptin in Human Physiology: Emerging Clinical Applications. Ann. Intern. Med. 2010, 152, 93. [Google Scholar] [CrossRef]
- Gao, J.; Tian, J.; Lv, Y.; Shi, F.; Kong, F.; Shi, H.; Zhao, L. Leptin induces functional activation of cyclooxygenase-2 through JAK2/ STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009, 100, 389–395. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Zhang, Y.; Lu, B. Serum leptin, adiponectin and endometrial cancer risk in Chinese women. J. Gynecol. Oncol. 2013, 24, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; He, X.Y.; Wang, R.; Wang, Z.; Wang, Y.G. High leptin level is an independent risk factor of endometrial cancer: A meta-analysis. Cell. Physiol. Biochem. 2014, 34, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Jagodzińska, A.; Pius-Sadowska, E.; Sompolska-Rzechuła, A.; Machaliński, B.; Menkiszak, J. Evaluation of biologically active substances promoting the development of or protecting against endometrial cancer. Onco. Targets. Ther. 2018, 11, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Li, C.; Ai, H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomarkers 2014, 14, 353–359. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Chai, Y.; Liu, Z. Leptin inhibits the apoptosis of endometrial carcinoma cells through activation of the nuclear factor κB-inducing kinase/IκB kinase pathway. Int. J. Gynecol. Cancer 2015, 25, 770–778. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Zheng, J.; Tang, G. Leptin promotes human endometrial carcinoma cell proliferation by enhancing aromatase (P450arom) expression and estradiol formation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.E.; Barron, G.A.; Bermano, G. Adipocytokines and their relationship to endometrial cancer risk: A systematic review and meta-analysis. Gynecol. Oncol. 2020, 158, 507–516. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, T.J.; Lee, W.J.; Chuang, L.M. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism 2010, 59, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M.; Janowska, J.; Kocełak, P.; Semik-Grabarczyk, E.; Holecki Michałand Dabrowski, P.; Skorupa, A. Serum concentration of visfatin in obese women. Metabolism 2007, 56, 1131–1134. [Google Scholar] [CrossRef]
- Berndt, J.; Klöting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Avcioglu, S.N.; Altinkaya, S.O.; Küçük, M.; Yüksel, H.; Ömürlü, I.K.; Yanik, S. Visfatin concentrations in patients with endometrial cancer. Gynecol. Endocrinol. 2015, 31, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhu, Y.; Wang, Y.; Teng, F.; Zhang, H.; Liu, G.; Ma, X.; Sun, D.; Rohan, T.; Xue, F. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol. Oncol. 2013, 129, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Sompolska-Rzechuła, A.; Machaliński, B.; Menkiszak, J. Circulating Serum Level of Visfatin in Patients with Endometrial Cancer. Biomed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, C.; Zhang, Y.; Gao, J.; Teng, F.; Tian, W.; Yang, W.; Yan, Y.; Xue, F. Visfatin stimulates endometrial cancer cell proliferation via activation of PI3K/Akt and MAPK/ERK1/2 signalling pathways. Gynecol. Oncol. 2016, 143, 168–178. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The Role of Galectins as Modulators of Metabolism and Inflammation. Mediators Inflamm. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Van Den Brûle, F.A.; Buicu, C.; Berchuck, A.; Bast, R.C.; Deprez, M.; Liu, F.T.; Cooper, D.N.W.; Pieters, C.; Sobel, M.E.; Castronovo, V. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum. Pathol. 1996, 27, 1185–1191. [Google Scholar] [CrossRef]
- Mylonas, I.; Mayr, D.; Walzel, H.; Shabani, N.; Dian, D.; Kuhn, C.; Kunze, S.; Jeschke, U.; Friese, K. Mucin 1, Thomsen-Friedenreich expression and galectin-1 binding in endometrioid adenocarcinoma: An immunohistochemical analysis. Anticancer Res. 2007, 27, 1975–1980. [Google Scholar]
- Sun, X.; Dai, S. The Significance of Galectin-1 and Galectin-9 Expression in Endometrial Carcinoma. Gynecol. Obstet. Investig. 2020, 85, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Brustmann, H.; Riss, D.; Naudé, S. Galectin-3 expression in normal, hyperplastic, and neoplastic endometrial tissues. Pathol. Res. Pract. 2003, 199, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Gargulińska, P.; Kwiatkowski, S.; Pius-Sadowska, E.; Machaliński, B. Could Galectin 3 Be a Good Prognostic Factor in Endometrial Cancer? Diagnostics 2020, 10, 635. [Google Scholar] [CrossRef]
- Menkhorst, E.; Griffiths, M.; van Sinderen, M.; Rainczuk, K.; Niven, K.; Dimitriadis, E. Galectin-7 is elevated in endometrioid (type I) endometrial cancer and promotes cell migration. Oncol. Lett. 2018, 16, 4721–4728. [Google Scholar] [CrossRef]
- Fujihara, S.; Mori, H.; Kobara, H.; Rafiq, K.; Niki, T.; Hirashima, M.; Masaki, T. Galectin-9 in Cancer Therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 130–137. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C. Galectin-9 in non-small cell lung cancer. Lung Cancer 2019, 136, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Brubel, R.; Bokor, A.; Pohl, A.; Schilli, G.K.; Szereday, L.; Bacher-Szamuel, R.; Rigo, J.; Polgar, B. Serum galectin-9 as a noninvasive biomarker for the detection of endometriosis and pelvic pain or infertility-related gynecologic disorders. Fertil. Steril. 2017, 108, 1016–1025.e2. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Schmandt, R.E.; Iglesias, D.A.; Co, N.N.; Lu, K.H. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am. J. Obstet. Gynecol. 2011, 205, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shi, J.; Long, Y.; Tian, H.; Li, X.; Zhao, A.Z.; Li, R.F.; Chen, T. Adiponectin and Endometrial Cancer: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2015, 36, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, N.; Yahata, T.; Quan, J.; Adachi, S.; Yoshihara, K.; Tanaka, K. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol. Oncol. 2010, 119, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Wu, Q.J.; Wang, Y.L.; Ma, X.X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int. J. Cancer 2015, 137, 1967–1978. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, X.L.; Yao, Y.; Han, W.Q.; Li, B. Circulating adiponectin levels and risk of endometrial cancer: Systematic review and meta-analysis. Exp. Ther. Med. 2016, 11, 2305–2313. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, X.; Kong, W. Association between adiponectin levels and endometrial carcinoma risk: Evidence from a dose–response meta-analysis. BMJ Open 2015, 5, e008541. [Google Scholar] [CrossRef]
- Yamauchi, N.; Takazawa, Y.; Maeda, D.; Hibiya, T.; Tanaka, M.; Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T.; Fukayama, M. Expression levels of adiponectin receptors are decreased in human endometrial adenocarcinoma tissues. Int. J. Gynecol. Pathol. 2012, 31, 352–357. [Google Scholar] [CrossRef]
- Heiker, J.T. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, Y.-B.; Long, J.-R.; Cai, H.; Cai, Q.; Cheng, J.; Wen, W.; Gao, Y.-T.; Zheng, W.; Shu, X.-O. Genetic polymorphisms in obesity-related genes and endometrial cancer risk. Cancer 2012, 118, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Barb, D.; Williams, C.J.; Neuwirth, A.K.; Mantzoros, C.S. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am. J. Clin. Nutr. 2007, 86, 858S–866S. [Google Scholar] [CrossRef]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose tissue-derived omentin-1 function and regulation. Compr. Physiol. 2017, 7, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Guo, L.; Li, Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 88, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Ouwens, D.M.; Carstensen, M.; Kowall, B.; Huth, C.; Meisinger, C.; Rathmann, W.; Roden, M.; Thorand, B. Adiponectin may mediate the association between omentin, circulating lipids and insulin sensitivity: Results from the KORA F4 study. Eur. J. Endocrinol. 2015, 172, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Bu, P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res. Clin. Pract. 2011, 93, 21–25. [Google Scholar] [CrossRef]
- Sperling, M.; Grzelak, T.; Pelczyńska, M.; Jasinska, P.; Bogdanski, P.; Pupek-Musialik, D.; Czyzewska, K. Concentrations of omentin and vaspin versus insulin resistance in obese individuals. Biomed. Pharmacother. 2016, 83, 542–547. [Google Scholar] [CrossRef]
- Zabetian-Targhi, F.; Mirzaei, K.; Keshavarz, S.A.; Hossein-Nezhad, A. Modulatory Role of Omentin-1 in Inflammation: Cytokines and Dietary Intake. J. Am. Coll. Nutr. 2016, 35, 670–678. [Google Scholar] [CrossRef]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Barbe, A.; Staub, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Vaspin in the pig ovarian follicles: Expression and regulation by different hormones. Reproduction 2019, 158, 137–148. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Dupont, J.; Rak, A. Role of vaspin in porcine ovary: Effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A. Biol. Reprod. 2020, 102, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Sezer, S.; Baser, E.; Gun-Eryilmaz, O.; Gungor, T.; Uysal, S.; Yilmaz, F.M. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr. Relat. Cancer 2013, 20, 669–675. [Google Scholar] [CrossRef]
- Cirillo, D.; Rachiglio, A.M.; la Montagna, R.; Giordano, A.; Normanno, N. Leptin signaling in breast cancer: An overview. J. Cell. Biochem. 2008, 105, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mukhopadhyay, P.; Pandit, K.; Chowdhury, S.; Dutta, D. Leptin and cancer: Pathogenesis and modulation. Indian J. Endocrinol. Metab. 2012, 16, 596. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Wang, Y.-Y.; Lo, S.; Tseng, L.-M.; Chen, D.-R.; Wu, Y.-C.; Hou, M.-F.; Yuan, S.-S.F. Visfatin Mediates Malignant Behaviors through Adipose-Derived Stem Cells Intermediary in Breast Cancer. Cancers 2019, 12, 29. [Google Scholar] [CrossRef]
- Gao, X.; Balan, V.; Tai, G.; Raz, A. Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway. Oncotarget 2014, 5, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef] [PubMed]
- Khadem Ansari, M.H.; Gholamnejad, M.; Meghrazi, K.; Khalkhali, H.R. Association of circulating omentin-1 level with lung cancer in smokers. Med. J. Islam. Repub. Iran 2018, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, W.; Zhuang, J.; Lu, Y.; Jian, W.; Wei, Y.; Li, W.; Xu, Y. Vaspin attenuates high glucose-induced vascular smooth muscle cells proliferation and chemokinesis by inhibiting the MAPK, PI3K/Akt, and NF-κB signaling pathways. Atherosclerosis 2013, 228, 61–68. [Google Scholar] [CrossRef]
- Harvey, A.E.; Lashinger, L.M.; Hursting, S.D. The growing challenge of obesity and cancer: An inflammatory issue. Ann. N. Y. Acad. Sci. 2011, 1229, 45–52. [Google Scholar] [CrossRef]
- Hefetz-Sela, S.; Scherer, P.E. Adipocytes: Impact on tumor growth and potential sites for therapeutic intervention. Pharmacol. Ther. 2013, 138, 197–210. [Google Scholar] [CrossRef]
- Litmanovich, A.; Khazim, K.; Cohen, I. The Role of Interleukin-1 in the Pathogenesis of Cancer and its Potential as a Therapeutic Target in Clinical Practice. Oncol. Ther. 2018, 6, 109–127. [Google Scholar] [CrossRef]
- Kolb, R.; Kluz, P.; Tan, Z.W.; Borcherding, N.; Bormann, N.; Vishwakarma, A.; Balcziak, L.; Zhu, P.; Davies, B.S.; Gourronc, F.; et al. Obesity-associated inflammation promotes angiogenesis and breast cancer via angiopoietin-like 4. Oncogene 2019, 38, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Duan, X.; Wang, Y.; Zhang, Z. Interleukin-1 receptor-associated kinase 1 correlates with metastasis and invasion in endometrial carcinoma. J. Cell. Biochem. 2018, 119, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.R. Leptin regulation of the interleukin-1 system in human endometrial cells. Mol. Hum. Reprod. 2003, 9, 151–158. [Google Scholar] [CrossRef]
- Wolvekamp, M.C.J.; Marquet, R.L. Interleukin-6: Historical background, genetics and biological significance. Immunol. Lett. 1990, 24, 1–9. [Google Scholar] [CrossRef]
- Che, Q.; Xiao, X.; Xu, J.; Liu, M.; Lu, Y.; Liu, S.; Dong, X. 17β-estradiol promotes endometrial cancer proliferation and invasion through IL-6 pathway. Endocr. Connect. 2019, 8, 961–968. [Google Scholar] [CrossRef]
- Che, Q.; Liu, B.Y.; Wang, F.Y.; He, Y.Y.; Lu, W.; Liao, Y.; Gu, W.; Wan, X.P. Interleukin 6 promotes endometrial cancer growth through an autocrine feedback loop involving ERK-NF-κB signaling pathway. Biochem. Biophys. Res. Commun. 2014, 446, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; Watts, K.; Cane’, S.; Palmieri, M.; Cannon, M.J.; Burnett, A.; Roman, J.J.; Pecorelli, S.; Santin, A.D. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol. Oncol. 2005, 98, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Polverini, P.; Kunkel, S.; Harlow, L.; DiPietro, L.; Elner, V.; Elner, S.; Strieter, R. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Tjiong, M.Y.; van der Vange, N.; ten Kate, F.J.W.; Tjong-A-Hung, S.P.; ter Schegget, J.; Burger, M.P.M.; Out, T.A. Increased IL-6 and IL-8 Levels in Cervicovaginal Secretions of Patients with Cervical Cancer. Gynecol. Oncol. 1999, 73, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ewington, L.; Taylor, A.; Sriraksa, R.; Horimoto, Y.; Lam, E.W.F.; El-Bahrawy, M.A. The expression of interleukin-8 and interleukin-8 receptors in endometrial carcinoma. Cytokine 2012, 59, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Green, V.L.; White, M.C.; Speirs, V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: Identification of interleukin-8 as a potential regulatory factor in breast tumours. Int. J. Cancer 1997, 72, 937–941. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Madan, A.K.; Pedersen, S.B.; Schiøtt, K.M.; Fain, J.N.; Richelsen, B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am. J. Physiol. Metab. 2004, 286, E8–E13. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, H.-S.; Kawada, T.; Kim, J.-H.; Lim, D.; Hubbard, N.E.; Kwon, B.-S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Fujimoto, J.; Aoki, I.; Khatun, S.; Toyoki, H.; Tamaya, T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann. Oncol. 2002, 13, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Kotowicz, B.; Fuksiewicz, M.; Jonska-Gmyrek, J.; Berezowska, A.; Radziszewski, J.; Bidzinski, M.; Kowalska, M. Clinical significance of pretreatment serum levels of VEGF and its receptors, IL- 8, and their prognostic value in type I and II endometrial cancer patients. PLoS ONE 2017, 12, e0184576. [Google Scholar] [CrossRef]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: An early target in anticancer therapeutic strategy. In Vivo 2007, 21, 147–161. [Google Scholar]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, F.; Roselli, M.; Martini, F.; Spila, A.; Riondino, S.; D’Alessandro, R.; Del Monte, G.; Formica, V.; Laudisi, A.; Portarena, I.; et al. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009, 29, 3321–3327. [Google Scholar] [PubMed]
- Strong, A.L.; Burow, M.E.; Gimble, J.M.; Bunnell, B.A. Concise Review: The Obesity Cancer Paradigm: Exploration of the Interactions and Crosstalk with Adipose Stem Cells. Stem Cells 2015, 33, 318–326. [Google Scholar] [CrossRef]
- Cao, Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat. Rev. Drug Discov. 2010, 9, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.A.; Merritt, W.M.; Coffey, D.; Lin, Y.G.; Patel, P.R.; Broaddus, R.; Nugent, E.; Han, L.Y.; Landen, C.N.; Spannuth, W.A.; et al. Clinical and Biological Significance of Vascular Endothelial Growth Factor in Endometrial Cancer. Clin. Cancer Res. 2007, 13, 7487–7495. [Google Scholar] [CrossRef] [PubMed]
- Sousa Moreira, I.; Alexandrino Fernandes, P.; Joao Ramos, M. Vascular Endothelial Growth Factor (VEGF) Inhibition—A Critical Review. Anticancer. Agents Med. Chem. 2007, 7, 223–245. [Google Scholar] [CrossRef]
- Salven, P.; Lymboussaki, A.; Heikkilä, P.; Jääskela-Saari, H.; Enholm, B.; Aase, K.; von Euler, G.; Eriksson, U.; Alitalo, K.; Joensuu, H. Vascular Endothelial Growth Factors VEGF-B and VEGF-C Are Expressed in Human Tumors. Am. J. Pathol. 1998, 153, 103–108. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Vaisman, N.; Gospodarowicz, D.; Neufeld, G. Characterization of the receptors for vascular endothelial growth factor. J. Biol. Chem. 1990, 265, 19461–19466. [Google Scholar] [CrossRef]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831. [Google Scholar] [CrossRef]
- Dobrzycka, B.; Terlikowski, S.J.; Kwiatkowski, M.; Garbowicz, M.; Kinalski, M.; Chyczewski, L. Prognostic significance of VEGF and its receptors in endometrioid endometrial cancer. Ginekol. Pol. 2010, 81, 422–425. [Google Scholar]
- Hirai, M.; Nakagawara, A.; Oosaki, T.; Hayashi, Y.; Hirono, M.; Yoshihara, T. Expression of Vascular Endothelial Growth Factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in Postmenopausal Uterine Endometrial Carcinoma. Gynecol. Oncol. 2001, 80, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Itoh, N. Roles of FGFs as Adipokines in Adipose Tissue Development, Remodeling, and Metabolism. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef]
- Winterhoff, B.; Konecny, G.E. Targeting fibroblast growth factor pathways in endometrial cancer. Curr. Probl. Cancer 2017, 41, 37–47. [Google Scholar] [CrossRef]
- Lee, P.S.; Secord, A.A. Targeting molecular pathways in endometrial cancer: A focus on the FGFR pathway. Cancer Treat. Rev. 2014, 40, 507–512. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Ozen, M.; Ittmann, M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr. Relat. Cancer 2004, 11, 709–724. [Google Scholar] [CrossRef]
- Soufla, G.; Sifakis, S.; Spandidos, D.A. FGF2 transcript levels are positively correlated with EGF and IGF-1 in the malignant endometrium. Cancer Lett. 2008, 259, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Hori, M.; Ichigo, S.; Tamaya, T. Expressions of the Fibroblast Growth Factor Family (FGF-1,-2 and-4)mRNA in Endometrial Cancers. Tumor Biol. 1996, 17, 226–233. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Gargulińska, P.; Chudecka-Głaz, A.; Kwiatkowski, S.; Pius-Sadowska, E.; Machaliński, B. The Suitability of FGF21 and FGF23 as New Biomarkers in Endometrial Cancer Patients. Diagnostics 2020, 10, 414. [Google Scholar] [CrossRef]
- Byron, S.A.; Gartside, M.; Powell, M.A.; Wellens, C.L.; Gao, F.; Mutch, D.G.; Goodfellow, P.J.; Pollock, P.M. FGFR2 Point Mutations in 466 Endometrioid Endometrial Tumors: Relationship with MSI, KRAS, PIK3CA, CTNNB1 Mutations and Clinicopathological Features. PLoS ONE 2012, 7, e30801. [Google Scholar] [CrossRef]
- Dieci, M.V.; Arnedos, M.; Andre, F.; Soria, J.C. Fibroblast Growth Factor Receptor Inhibitors as a Cancer Treatment: From a Biologic Rationale to Medical Perspectives. Cancer Discov. 2013, 3, 264–279. [Google Scholar] [CrossRef]
- Yu, H. Role of the Insulin-Like Growth Factor Family in Cancer Development and Progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Baczmańska, D.; Malinowski, A. Does IGF-1 play a role in the biology of endometrial cancer? Ginekol. Pol. 2016, 87, 598–604. [Google Scholar] [CrossRef] [PubMed]
- MURPHY, L.J.; GHAHARY, A. Uterine Insulin-Like Growth Factor-1: Regulation of Expression and Its Role in Estrogen-Induced Uterine Proliferation. Endocr. Rev. 1990, 11, 443–453. [Google Scholar] [CrossRef]
- Suvanto-Luukkonen, E.; Sundström, H.; Penttinen, J.; Kauppila, A.; Rutanen, E.-M. Insulin-like growth factor-binding protein-1: A biochemical marker of endometrial response to progestin during hormone replacement therapy. Maturitas 1995, 22, 255–262. [Google Scholar] [CrossRef]
- Rutanen, E. mRNA expression of insulin-like growth factor-I (IGF-I) is suppressed and those of IGF-II and IGF-binding protein-1 are constantly expressed in the endometrium during use of an intrauterine levonorgestrel system. Mol. Hum. Reprod. 1997, 3, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.; Ho, G.Y.F.; et al. A Prospective Evaluation of Insulin and Insulin-like Growth Factor-I as Risk Factors for Endometrial Cancer. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 921–929. [Google Scholar] [CrossRef]
- Rutanen, E.M.; Stenman, S.; Blum, W.; Kärkkäinen, T.; Lehtovirta, P.; Stenman, U.H. Relationship between carbohydrate metabolism and serum insulin-like growth factor system in postmenopausal women: Comparison of endometrial cancer patients with healthy controls. J. Clin. Endocrinol. Metab. 1993, 77, 199–204. [Google Scholar] [CrossRef]
- Lacey, J.V.; Potischman, N.; Madigan, M.P.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Barrett, R.J.; Wilbanks, G.D.; Lurain, J.R.; Fillmore, C.-M.; et al. Insulin-like growth factors, insulin-like growth factor-binding proteins, and endometrial cancer in postmenopausal women: Results from a U.S. case-control study. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 607–612. [Google Scholar] [PubMed]
- Weiderpass, E.; Brismar, K.; Bellocco, R.; Vainio, H.; Kaaks, R. Serum levels of insulin-like growth factor-I, IGF-binding protein 1 and 3, and insulin and endometrial cancer risk. Br. J. Cancer 2003, 89, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Dal Maso, L.; Franceschi, S.; Talamini, R.; Kendall, C.W.C.; Jenkins, D.J.A.; Vidgen, E.; La Vecchia, C. Association between Components of the Insulin-Like Growth Factor System and Endometrial Cancer Risk. Oncology 2004, 67, 54–59. [Google Scholar] [CrossRef]
- Bruchim, I.; Werner, H. Targeting IGF-1 signaling pathways in gynecologic malignancies. Expert Opin. Ther. Targets 2013, 17, 307–320. [Google Scholar] [CrossRef]
- Joehlin-Price, A.S.; Stephens, J.A.; Zhang, J.; Backes, F.J.; Cohn, D.E.; Suarez, A.A. Endometrial Cancer Insulin-Like Growth Factor 1 Receptor (IGF1R) Expression Increases with Body Mass Index and Is Associated with Pathologic Extent and Prognosis. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 438–445. [Google Scholar] [CrossRef]
- Mendivil, A.; Zhou, C.; Cantrell, L.A.; Gehrig, P.A.; Malloy, K.M.; Blok, L.J.; Burger, C.W.; Bae-Jump, V.L. AMG 479, a Novel IGF-1-R Antibody, Inhibits Endometrial Cancer Cell Proliferation Through Disruption of the PI3K/Akt and MAPK Pathways. Reprod. Sci. 2011, 18, 832–841. [Google Scholar] [CrossRef]
- Attias-Geva, Z.; Bentov, I.; Ludwig, D.L.; Fishman, A.; Bruchim, I.; Werner, H. Insulin-like growth factor-I receptor (IGF-IR) targeting with monoclonal antibody cixutumumab (IMC-A12) inhibits IGF-I action in endometrial cancer cells. Eur. J. Cancer 2011, 47, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Attias-Geva, Z.; Bentov, I.; Fishman, A.; Werner, H.; Bruchim, I. Insulin-like growth factor-I receptor inhibition by specific tyrosine kinase inhibitor NVP-AEW541 in endometrioid and serous papillary endometrial cancer cell lines. Gynecol. Oncol. 2011, 121, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Klöting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Bjørge, T.; Engeland, A.; Tretli, S.; Weiderpass, E. Body size in relation to cancer of the uterine corpus in 1 million Norwegian women. Int. J. Cancer 2007, 120, 378–383. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Patel, A.V.; Patel, R.; Rodriguez, C.; Feigelson, H.S.; Bandera, E.V.; Gansler, T.; Thun, M.J.; Calle, E.E. Body Mass and Endometrial Cancer Risk by Hormone Replacement Therapy and Cancer Subtype. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sato, Y.; Watanabe, J.; Kuramoto, H.; Kaba, S.; Fukuda, T. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol. Int. 2007, 57, 140–147. [Google Scholar] [CrossRef] [PubMed]


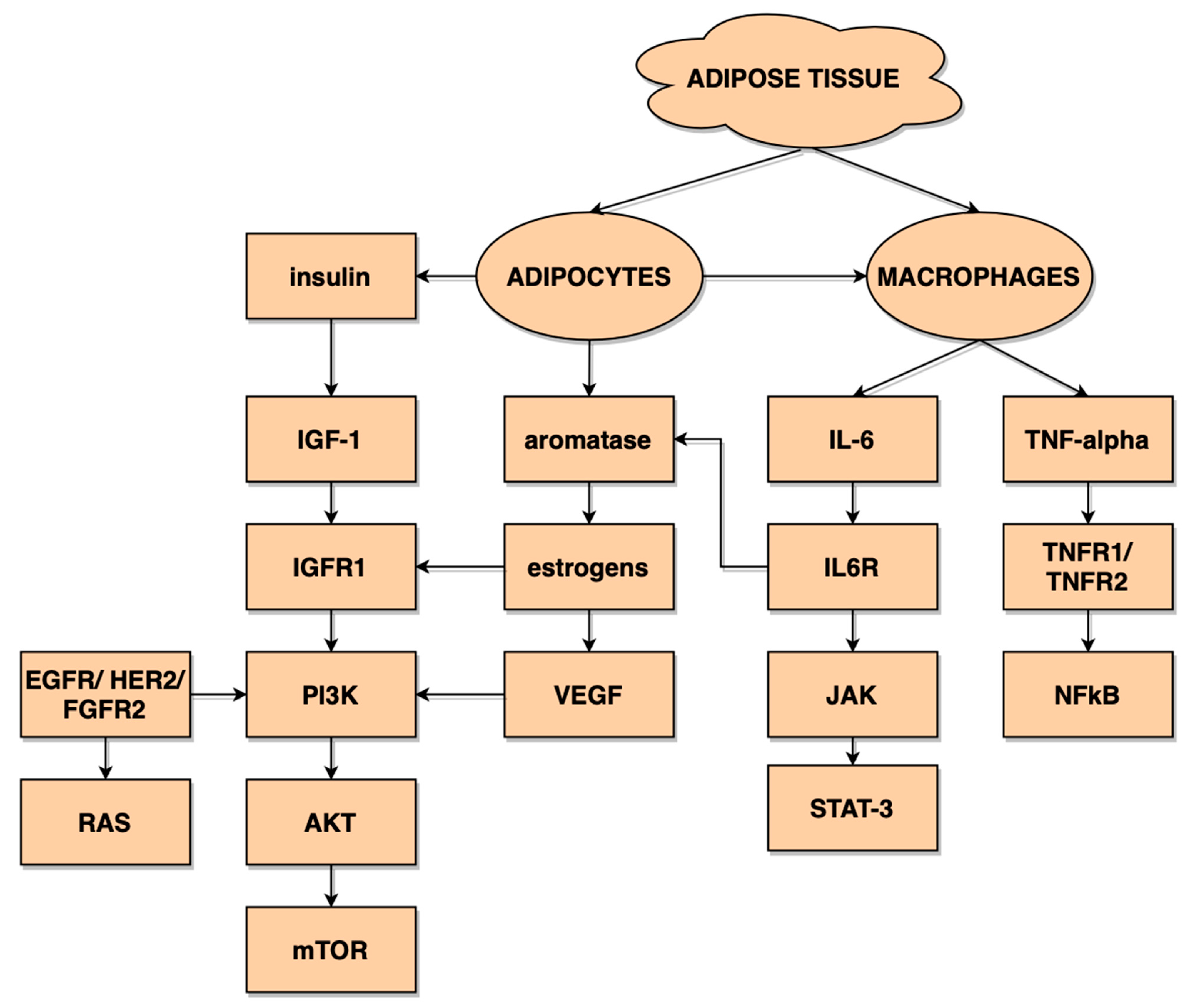
| Adipokines | Leptin Visfatin Galectin Adiponectin Vaspin Omentin Resistin Apelin |
| Cytokines | TNFα IL-1β IL-6 IL-8 |
| Angiogenic factors | VEGF FGF IGF-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalczyk, K.; Niklas, N.; Rychlicka, M.; Cymbaluk-Płoska, A. The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer. Diagnostics 2021, 11, 494. https://doi.org/10.3390/diagnostics11030494
Michalczyk K, Niklas N, Rychlicka M, Cymbaluk-Płoska A. The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer. Diagnostics. 2021; 11(3):494. https://doi.org/10.3390/diagnostics11030494
Chicago/Turabian StyleMichalczyk, Kaja, Natalia Niklas, Małgorzata Rychlicka, and Aneta Cymbaluk-Płoska. 2021. "The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer" Diagnostics 11, no. 3: 494. https://doi.org/10.3390/diagnostics11030494
APA StyleMichalczyk, K., Niklas, N., Rychlicka, M., & Cymbaluk-Płoska, A. (2021). The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer. Diagnostics, 11(3), 494. https://doi.org/10.3390/diagnostics11030494







