Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Biological Sample Collection
2.2. Materials
2.3. Urinary Extracellular Vesicles (UEv) Isolation
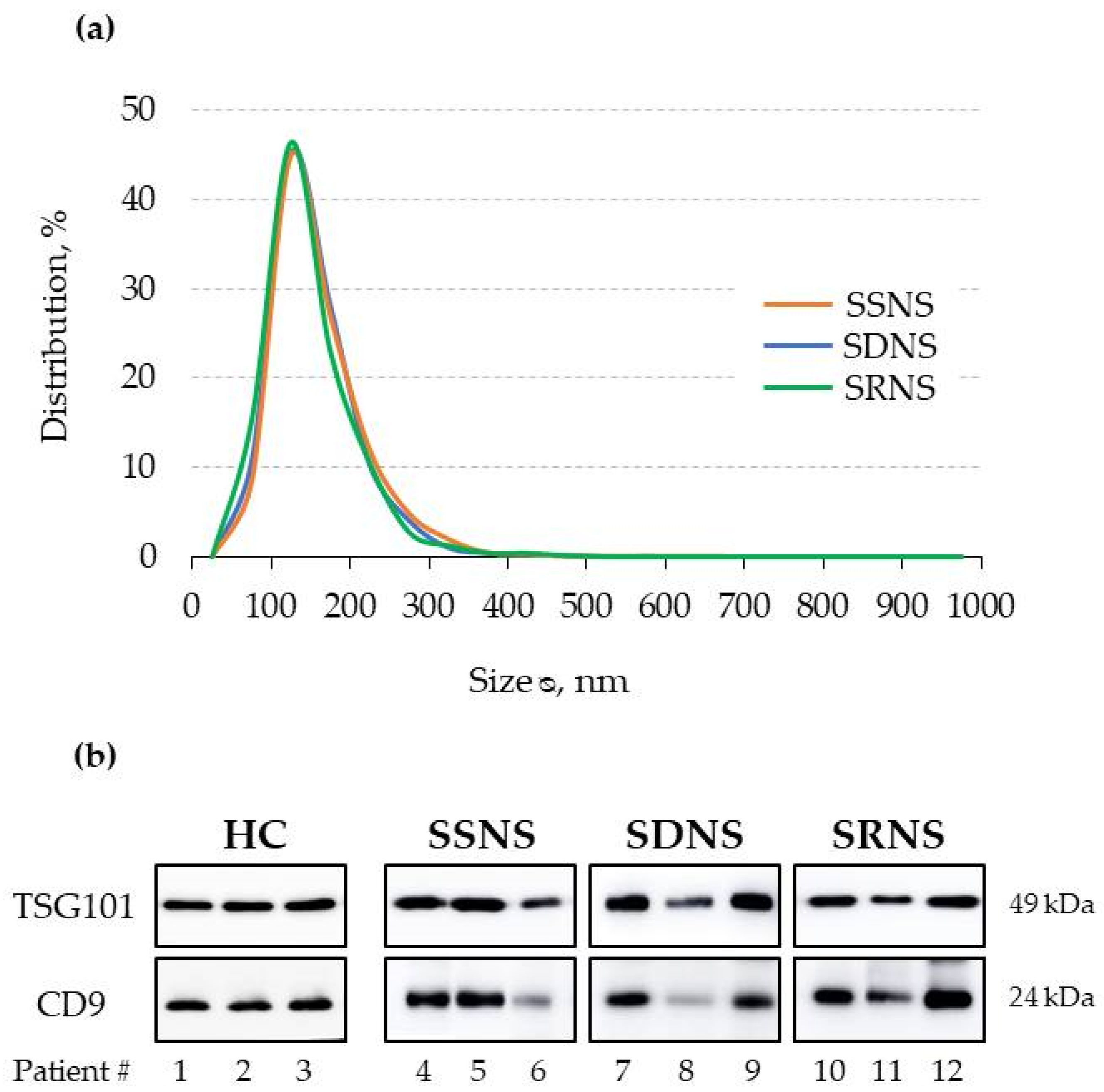
2.4. Nanoparticle Tracking Analysis
2.5. Electrophoresis and Western Blotting
2.6. Statistical Analysis
3. Results
3.1. UEv Characterisation: Nanoparticle Tracking Analysis (NTA) and Marker Enrichment
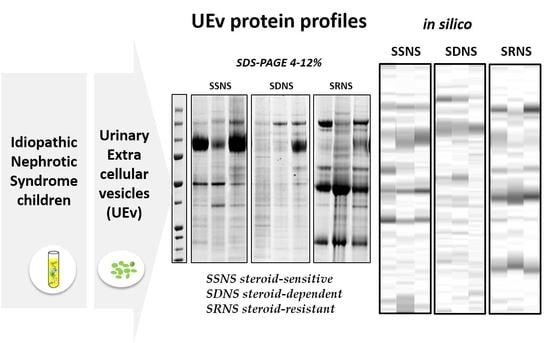
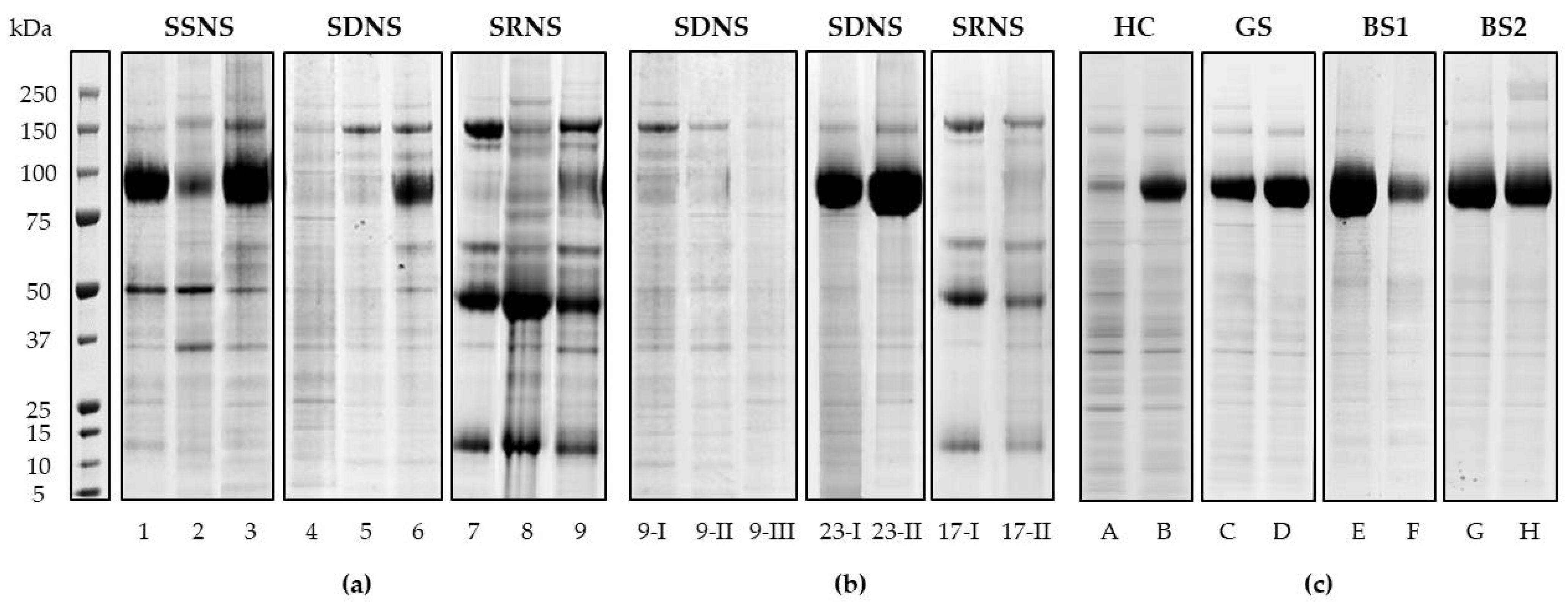
3.2. UEv Protein Profiling
3.3. Hierarchical Clustering and Classification
3.3.1. Qualitative Evaluation
3.3.2. Quantitative Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Colucci, M.; Corpetti, G.; Emma, F.; Vivarelli, M. Immunology of idiopathic nephrotic syndrome. Pediatr. Nephrol. 2018, 33, 573–584. [Google Scholar] [CrossRef]
- Dossier, C.; Jamin, A.; Deschenes, G. Idiopathic nephrotic syndrome: The EBV hypothesis. Pediatr. Res. 2017, 81, 233–239. [Google Scholar] [CrossRef]
- McCarthy, E.T.; Sharma, M.; Savin, V.J. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2010, 5, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Cuzzoni, E.; Franca, R.; De Iudicibus, S.; Marcuzzi, A.; Lucafò, M.; Pelin, M.; Favretto, D.; Monti, E.; Morello, W.; Ghio, L.; et al. MIF plasma level as a possible tool to predict steroid responsiveness in children with idiopathic nephrotic syndrome. Eur. J. Clin. Pharmacol. 2019, 75, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Morello, W.; Puvinathan, S.; Puccio, G.; Ghiggeri, G.M.; Dello Strologo, L.; Peruzzi, L.; Murer, L.; Cioni, M.; Guzzo, I.; Cocchi, E.; et al. Post-transplant recurrence of steroid resistant nephrotic syndrome in children: The Italian experience. J. Nephrol. 2020, 33, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, P.; Sabaratnam, R.; Jensen, B.L. Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol. 2020, 228, e13346. [Google Scholar] [CrossRef]
- Moon, P.G.; You, S.; Lee, J.E.; Hwang, D.; Baek, M.C. Urinary exosomes and proteomics. Mass Spectrom. Rev. 2011, 30, 1185–1202. [Google Scholar] [CrossRef]
- Słomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Zekanowska, E.; Kornek, M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018, 13, 2723. [Google Scholar] [CrossRef]
- Raimondo, F.; Morosi, L.; Chinello, C.; Magni, F.; Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 2011, 11, 709–720. [Google Scholar] [CrossRef]
- Chen, T.; Wang, C.; Yu, H.; Ding, M.; Zhang, C.; Lu, X.; Zhang, C.Y.; Zhang, C. Increased urinary exosomal microRNAs in children with idiopathic nephrotic syndrome. EBioMedicine 2019, 39, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, B.; Geeurickx, E.; Tulkens, J.; Van Deun, J.; Vergauwen, G.; Lippens, L.; Miinalainen, I.; Rappu, P.; Heino, J.; Ost, P.; et al. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J. Extracell. Vesicles 2020, 9, 1736935. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Benetti, E.; Conti, G.; Ghio, L.; Lepore, M.; Massella, L.; Molino, D.; Peruzzi, L.; Emma, F.; Fede, C.; et al. The Italian Society for Pediatric Nephrology (SINePe) consensus document on the management of nephrotic syndrome in children: Part I—Diagnosis and treatment of the first episode and the first relapse. Ital. J. Pediatr. 2017, 43, 41. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Dymicka-Piekarska, V.; Tomaszewska, J.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit. Rev. Clin. Lab. Sci. 2020, 57, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.; Morosi, L.; Corbetta, S.; Chinello, C.; Brambilla, P.; Della Mina, P.; Villa, A.; Albo, G.; Battaglia, C.; Bosari, S.; et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Biosyst. 2013, 9, 1220–1233. [Google Scholar] [CrossRef]
- Liu, Z.; Cauvi, D.M.; Bernardino, E.M.A.; Lara, B.; Lizardo, R.E.; Hawisher, D.; Bickler, S.; De Maio, A. Isolation and characterization of human urine extracellular vesicles. Cell Stress Chaperones 2018, 23, 943–953. [Google Scholar] [CrossRef]
- Corbetta, S.; Raimondo, F.; Tedeschi, S.; Syren, M.L.; Rebora, P.; Savoia, A.; Baldi, L.; Bettinelli, A.; Pitto, M. Urinary exosomes in the diagnosis of Gitelman and Bartter syndromes. Nephrol. Dial. Transplant. 2015, 30, 621–630. [Google Scholar] [CrossRef]
- Wang, S.; Kojima, K.; Mobley, J.A.; West, A.B. Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine 2019, 45, 351–361. [Google Scholar] [CrossRef]
- Dear, J.W.; Street, J.M.; Bailey, M.A. Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 2013, 13, 1572–1580. [Google Scholar] [CrossRef]
- Raimondo, F.; Cerra, D.; Magni, F.; Pitto, M. Urinary proteomics for the study of genetic kidney diseases. Expert Rev. Proteom. 2016, 13, 309–324. [Google Scholar] [CrossRef]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol. Ren. Physiol. 2014, 306, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Pei, F.; Zeng, C.; Yao, Y.; Liao, W.; Zhao, Z. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. Biomed. Res. Int. 2021, 11, 6611244. [Google Scholar] [CrossRef]
- Lee, H.; Han, K.H.; Lee, S.E.; Kim, S.H.; Kang, H.G.; Cheong, H.I. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr. Nephrol. 2012, 27, 317–320. [Google Scholar] [CrossRef]
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary exosomal Wilms′ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Ren. Physiol. 2013, 305, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Rood, I.M.; Deegens, J.K.; Merchant, M.L.; Tamboer, W.P.; Wilkey, D.W.; Wetzels, J.F.; Klein, J.B. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010, 78, 810–816. [Google Scholar] [CrossRef]
- Saleem, M.A. Molecular stratification of idiopathic nephrotic syndrome. Nat. Rev. Nephrol. 2019, 15, 750–765. [Google Scholar] [CrossRef]

| Patient ID | Sex | Age * (Years) | uPr/uCr (mg/mg) | Ongoing Therapy (Months) § | |||
|---|---|---|---|---|---|---|---|
| PRED | CYCL | MMF | TACR | ||||
| SSNS | |||||||
| 16 | M | 5 | 0.13 | 2 | |||
| 21 | M | 3 | 1.67 | ||||
| 24 | M | 4 | 0.27 | 1 | |||
| 43 | M | 17 | 0.08 | ||||
| 44 | F | 16 | 0.13 | ||||
| 67 | F | 11 | 0.16 | ||||
| 73 | M | 10 | 0.12 | 1 | |||
| 74 | M | 11 | 0.12 | <1 | |||
| SDNS | |||||||
| 5 | M | 5 | 0.24 | <1 | 33 | 3 | |
| 6 | M | 10 | 0.14 | 15 | |||
| 7 | M | 12 | 0.60 | 2 | 96 | ||
| 8 | M | 5 | 0.23 | 26 | 6 | ||
| 9 | M | 7 (I) | 0.18 | 26 | |||
| +4 months (II) | 0.18 | 30 | |||||
| +10 months (III) | 0.18 | 36 | |||||
| 10 | M | 18 | 0.08 | 5 | |||
| 13 | F | 4 | 0.19 | 29 | |||
| 14 | F | 8 | 0.18 | 8 | |||
| 18 | M | 8 | 0.15 | 28 | |||
| 20 | M | 5 (I) | 0.17 | 38 | |||
| +10 months (II) | 0.13 | 48 | |||||
| 23 | F | 2 (I) | 0.34 | 5 | |||
| +5 months (II) | 0.30 | 10 | |||||
| 30 | F | 6 | 0.16 | 1 | |||
| 32 | F | 8 | 0.15 | 2 | 1 | ||
| 38 | M | 15 | 0.27 | <1 | <1 | ||
| 53 | M | 10 | 0.84 | 49 | |||
| 55 | M | 5 | 0.74 | 38 | 39 | ||
| 58 | M | 12 | 0.15 | 32 | |||
| 61 | M | 7 | 0.25 | 14 | |||
| 66 | F | 6 | 0.14 | 30 | |||
| 69 | F | 15 | 0.15 | 130 | |||
| SRNS | |||||||
| 11 | F | 14 | 0.10 | 33 | 32 | ||
| 12 | F | 8 | 0.11 | 33 | |||
| 17 | M | 14 (I) | 0.44 | 17 | 6 | ||
| +8 months (II) | 0.32 | 14 | |||||
| 39 | F | 12 | 0.14 | <1 | |||
| 70 | F | 11 | 0.13 | ||||
| Subgroup Reference Profile | ||||||
|---|---|---|---|---|---|---|
| SSNS | SDNS | SRNS | GS | HC | ||
| True Subgroup | SSNS (n = 8) | 79.1 (10.6) | 70.4 (11.7) | 59.8 (10.6) | 58.6 (15.1) | 61.5 (14.0) |
| 6 | 2 | 0 | 0 | 0 | ||
| SDNS (n = 32) | 63.6 (14.8) | 72.5 (10.2) | 59.6 (12.3) | 63.0 (10.4) | 65.6 (14.0) | |
| 5 | 21 | 2 | 1 * | 3 * | ||
| SRNS (n = 6) | 61.6 (10.2) | 66.0 (14.0) | 83.4 (4.2) | 61.5 (11.9) | 58.5 (12.5) | |
| 0 | 1 | 5 | 0 | 0 | ||
| GS (n = 5) | 55.8 (9.4) | 69.5 (8.5) | 59.3 (12.5) | 82.2 (5.2) | 72.7 (7.0) | |
| 0 | 0 | 0 | 5 | 0 | ||
| HC (n = 7) | 62.2 (4.5) | 71.3 (8.5) | 59.2 (3.0) | 75.6 (11.0) | 85.6 (5.1) | |
| 0 | 0 | 0 | 0 | 7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santorelli, L.; Morello, W.; Barigazzi, E.; Capitoli, G.; Tamburello, C.; Ghio, L.; Crapella, B.; Galimberti, S.; Montini, G.; Pitto, M.; et al. Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome. Diagnostics 2021, 11, 456. https://doi.org/10.3390/diagnostics11030456
Santorelli L, Morello W, Barigazzi E, Capitoli G, Tamburello C, Ghio L, Crapella B, Galimberti S, Montini G, Pitto M, et al. Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome. Diagnostics. 2021; 11(3):456. https://doi.org/10.3390/diagnostics11030456
Chicago/Turabian StyleSantorelli, Lucia, William Morello, Elisa Barigazzi, Giulia Capitoli, Chiara Tamburello, Luciana Ghio, Barbara Crapella, Stefania Galimberti, Giovanni Montini, Marina Pitto, and et al. 2021. "Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome" Diagnostics 11, no. 3: 456. https://doi.org/10.3390/diagnostics11030456
APA StyleSantorelli, L., Morello, W., Barigazzi, E., Capitoli, G., Tamburello, C., Ghio, L., Crapella, B., Galimberti, S., Montini, G., Pitto, M., & Raimondo, F. (2021). Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome. Diagnostics, 11(3), 456. https://doi.org/10.3390/diagnostics11030456