Diverse Adiposity and Atrio-Ventricular Dysfunction across Obesity Phenotypes: Implication of Epicardial Fat Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Data, Cardiac Biomarkers and HOMA-IR Analysis
2.3. Definition of Metabolic Health and Obesity Phenotype
- (1)
- Systolic blood pressure ≥ 13.0 mmHg, diastolic blood pressure ≥ 85 mmHg;
- (2)
- Triglycerides (TG) ≥150 mg/dL;
- (3)
- High density lipoprotein (HDL-c) <40 mg/dL in men and <50 mg/dL in women;
- (4)
2.4. Assessment of Epicardial Adipose Tissue (EAT)
2.5. Body Fat Composition Assessment
2.6. Conventional Echocardiography and Diastolic Functional Indices
2.7. Two-Dimensional Speckle-Tracking Analysis Protocol
2.8. Determination of Clinical Endpoints
2.9. Statistical Analysis
3. Results
3.1. Baseline Demographics, Metabolic Profiles and Adiposity Measures
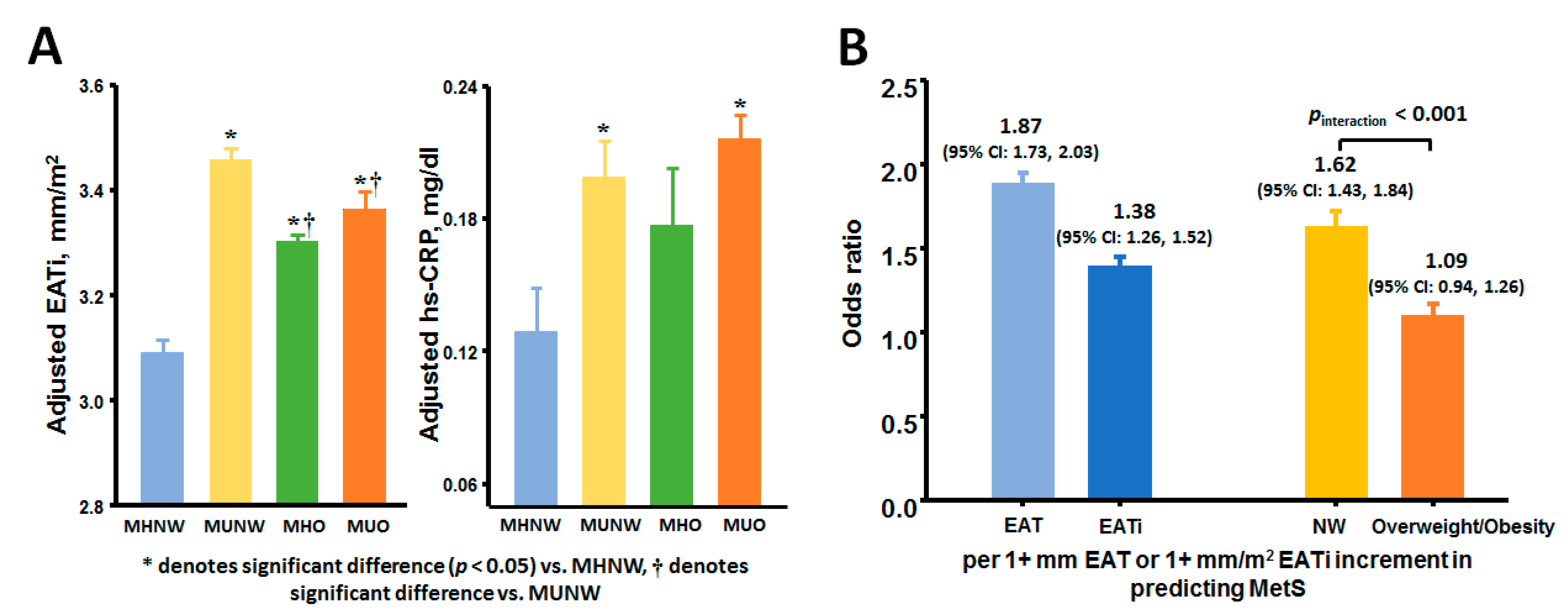
3.2. Associations of EAT and Metabolic Abnormalities with Atrio-Eentricular Mechanics
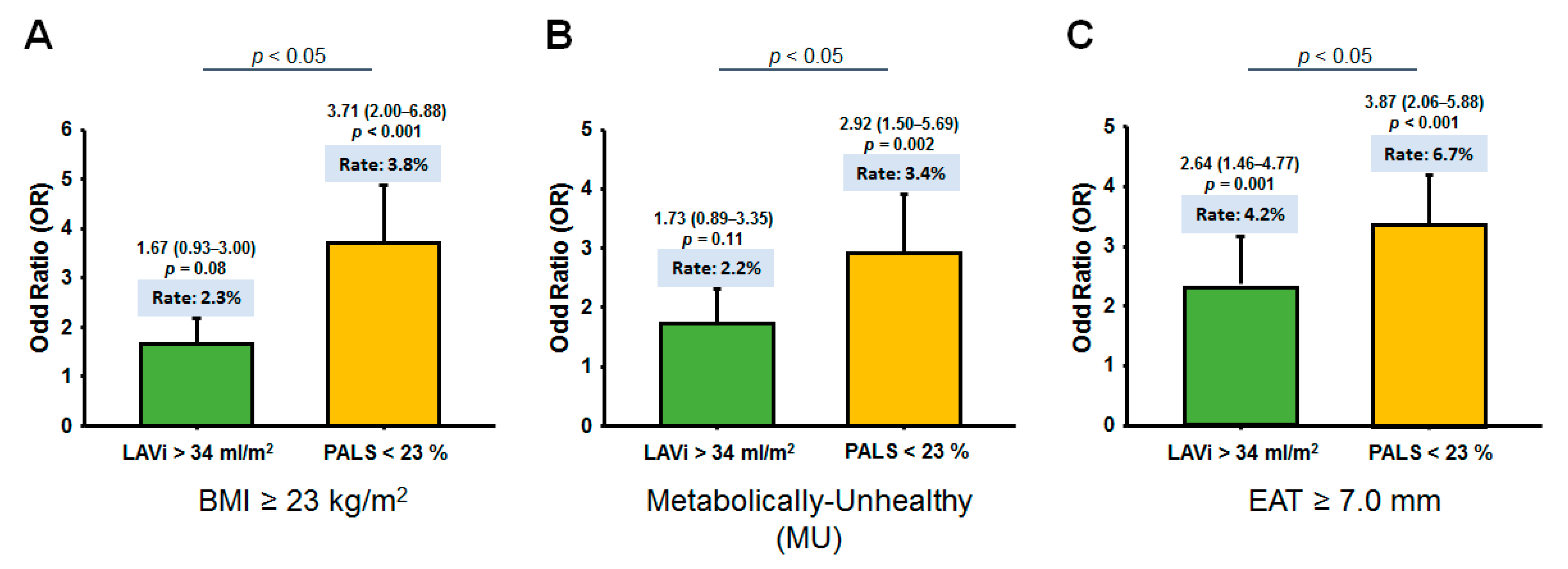
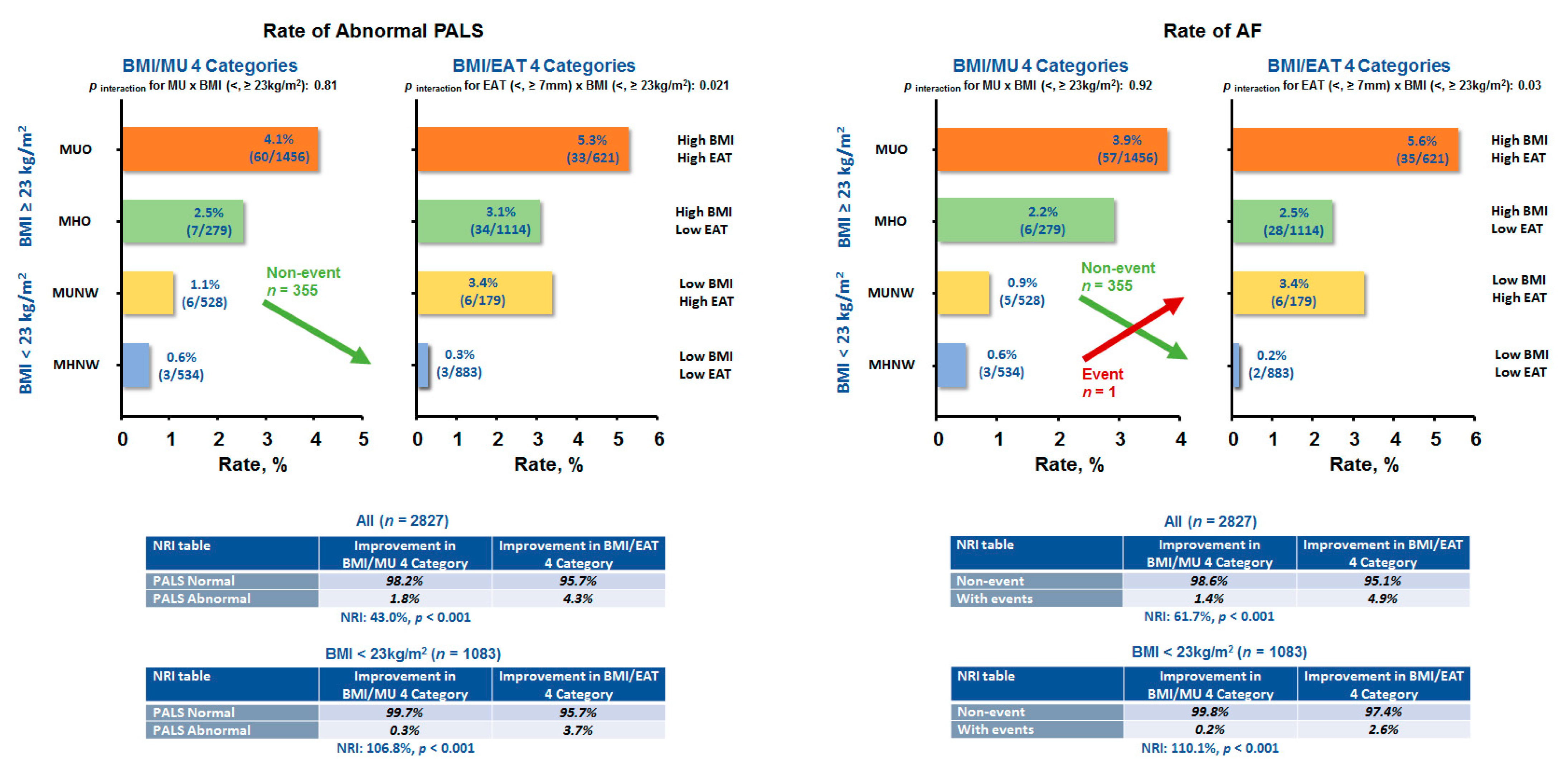
3.3. Association of Obesity Phenotypes and EAT with Clinical Outcomes
4. Discussion
4.1. Summary of Study Results
4.2. Utilization of Visceral Adiposity in Redefining Cardiometabolic Obesity
4.3. Effect of EAT Burden on Ventricular Remodeling and Dysfunction
4.4. Effect of EAT Burden on Atrial Remodeling and Dysfunction
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aurigemma:, G.P.; De Simone, G.; Fitzgibbons, T.P. Cardiac Remodeling in Obesity. Circ. Cardiovasc. Imaging 2013, 6, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Litwin, S.E. Cardiac remodeling in obesity: Time for a new paradigm. JACC Cardiovasc. Imaging. 2010, 3, 275–277. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Sanders, P.; Kottkamp, H.; Kalman, J.M. The role of obesity in atrial fibrillation. Eur. Heart J. 2016, 37, 1565–1572. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Liu, M.-E.; Su, C.-H.; Yun, C.-H.; Liu, C.-Y.; Hou, C.J.-Y.; Hu, K.-C.; Hung, C.-L.; Yeh, H.-I.; Lam, C.S. Obesity-Related Changes in Cardiac Structure and Function Among Asian Men and Women. J. Am. Coll. Cardiol. 2017, 69, 2876–2878. [Google Scholar] [CrossRef]
- Bello, N.A.; Cheng, S.; Claggett, B.; Shah, A.M.; Ndumele, C.E.; Roca, G.Q.; Santos, A.B.; Gupta, D.; Vardeny, O.; Aguilar, D.; et al. Association of Weight and Body Composition on Cardiac Structure and Function in the ARIC Study (Atherosclerosis Risk in Communities). Circ. Heart Fail. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Bank, I.E.; Gijsberts, C.M.; Teng, T.-H.K.; Benson, L.; Sim, D.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Leong, G.K.; Ling, L.H.; et al. Prevalence and Clinical Significance of Diabetes in Asian Versus White Patients With Heart Failure. JACC Hearth Fail. 2017, 5, 14–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO expert consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Tromp, J.; Tay, W.T.; Ouwerkerk, W.; Teng, T.K.; Yap, J.; MacDonald, M.R.; Leineweber, K.; McMurray, J.J.V.; Zile, M.R.; Anand, I.S.; et al. Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry. PLoS Med. 2018, 15, e1002541. [Google Scholar]
- Schulze, M.B. Metabolic health in normal-weight and obese individuals. Diabetology 2019, 62, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, C.; Tentolouris, N.; Dilaveris, P.; Tousoulis, D.; Katsilambros, N.; Stefanadis, C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J. Am. Coll. Cardiol. 2011, 58, 1343–1350. [Google Scholar] [CrossRef]
- Fitzgibbons, T.P.; Czech, M.P. Epicardial and Perivascular Adipose Tissues and Their Influence on Cardiovascular Disease: Basic Mechanisms and Clinical Associations. J. Am. Hearth Assoc. 2014, 3, e000582. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Hearth J. 2016, 38, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Yamagishi, M.; Tanaka, N.; Uematsu, M.; Yamazaki, N.; Mine, Y.; Sano, A.; Hirama, M. New method for evaluating left ven-tricular wall motion by color-coded tissue Doppler imaging: In vitro and in vivo studies. J. Am. Coll. Cardiol. 1995, 25, 717–724. [Google Scholar] [CrossRef]
- Leong, D.P.; Joyce, E.; Debonnaire, P.; Katsanos, S.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Dysfunction in the Pathogenesis of Cryptogenic Stroke: Novel Insights from Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 71–79.e1. [Google Scholar] [CrossRef]
- Hung, C.-L.; Gonçalves, A.; Lai, Y.-J.; Lai, Y.-H.; Sung, K.-T.; Lo, C.-I.; Liu, C.-C.; Kuo, J.-Y.; Hou, C.J.-Y.; Chao, T.-F.; et al. Light to Moderate Habitual Alcohol Consumption Is Associated with Subclinical Ventricular and Left Atrial Mechanical Dysfunction in an Asymptomatic Population: Dose-Response and Propensity Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 1043–1051.e4. [Google Scholar] [CrossRef]
- Chiang, C.-E.; Wang, T.-D.; Lin, T.-H.; Yeh, H.-I.; Liu, P.-Y.; Cheng, H.-M.; Chao, T.-H.; Chen, C.-H.; Shyu, K.-G.; Ueng, K.-C.; et al. The 2017 Focused Update of the Guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the Management of Hypertension. Acta Cardiol. Sin. 2017, 33, 213–225. [Google Scholar] [CrossRef]
- Lavie, C.J.; Laddu, D.; Arena, R.; Ortega, F.B.; Alpert, M.A.; Kushner, R.F. Healthy weight and obesity prevention: JACC health pro-motion series. J. Am. Coll. Cardiol. 2018, 72, 1506–1531. [Google Scholar] [CrossRef]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497.
- National Department of Health. Definition of Metabolic Syndrome. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=221 (accessed on 18 January 2020).
- Lai, Y.-H.; Yun, C.-H.; Yang, F.-S.; Liu, C.-C.; Wu, Y.-J.; Kuo, J.-Y.; Yeh, H.-I.; Lin, T.-Y.; Bezerra, H.G.; Shih, S.-C.; et al. Epicardial Adipose Tissue Relating to Anthropometrics, Metabolic Derangements and Fatty Liver Disease Independently Contributes to Serum High-Sensitivity C-Reactive Protein Beyond Body Fat Composition: A Study Validated with Computed Tomography. J. Am. Soc. Echocardiogr. 2012, 25, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H., 3rd; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, L.P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiog-raphy and the European Association of Cardiovascular Imaging. Eur. Hearth J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.; Tedesco, M.A.; Mocerino, R.; De Simone, V.; Di Marco, G.M.; Aronne, L.; Credendino, M.; Siniscalchi, C.; Calabrò, P.; Cotrufo, M.; et al. Visceral adiposity and arterial stiffness: Echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 2009, 10, 549–555. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Jerosch-Herold, M.; Lima, J.A.C.; Ding, J. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA study. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef]
- Hasebe, H.; Yoshida, K.; Iida, M.; Hatano, N.; Muramatsu, T.; Nogami, A.; Aonuma, K. Differences in the structural characteristics and distribution of epicardial adipose tissue between left and right atrial fibrillation. Europace 2017, 20, 435–442. [Google Scholar] [CrossRef]
- Van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; Van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Hearth Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef]
- Gaborit, B.; Kober, F.; Jacquier, A.; Moro, P.J.; Flavian, A.; Quilici, J.; Cuisset, T.; Simeoni, U.; Cozzone, P.; Alessi, M.-C.; et al. Epicardial Fat Volume Is Associated with Coronary Microvascular Response in Healthy Subjects: A Pilot Study. Obesity 2012, 20, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Burgess, M.I.; Sprung, V.S.; Irwin, A.J.; Hamer, M.; A Jones, J.; Daousi, C.; Adams, V.; Kemp, G.J.; Shojaee-Moradie, F.; et al. Metabolically healthy and unhealthy obesity: Differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int. J. Obes. 2016, 40, 153–161. [Google Scholar] [CrossRef]
- Madamanchi, C.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef]
- Alpert, M.A. Obesity Cardiomyopathy: Pathophysiology and Evolution of the Clinical Syndrome. Am. J. Med. Sci. 2001, 321, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Kato, T.; Haruna, T.; Izumi, T.; Miyamoto, S.; Nakane, E.; Inoko, M. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Leonetti, F.; Singh, N.; Sharma, A.M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007, 115, 272–273. [Google Scholar] [CrossRef]
- Nakamori, S.; Nezafat, M.; Ngo, L.H.; Manning, W.J.; Nezafat, R. Left Atrial Epicardial Fat Volume Is Associated With Atrial Fibrillation: A Prospective Cardiovascular Magnetic Resonance 3D Dixon Study. J. Am. Hearth Assoc. 2018, 7, e008232. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.K.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity with Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e004378. [Google Scholar] [CrossRef]
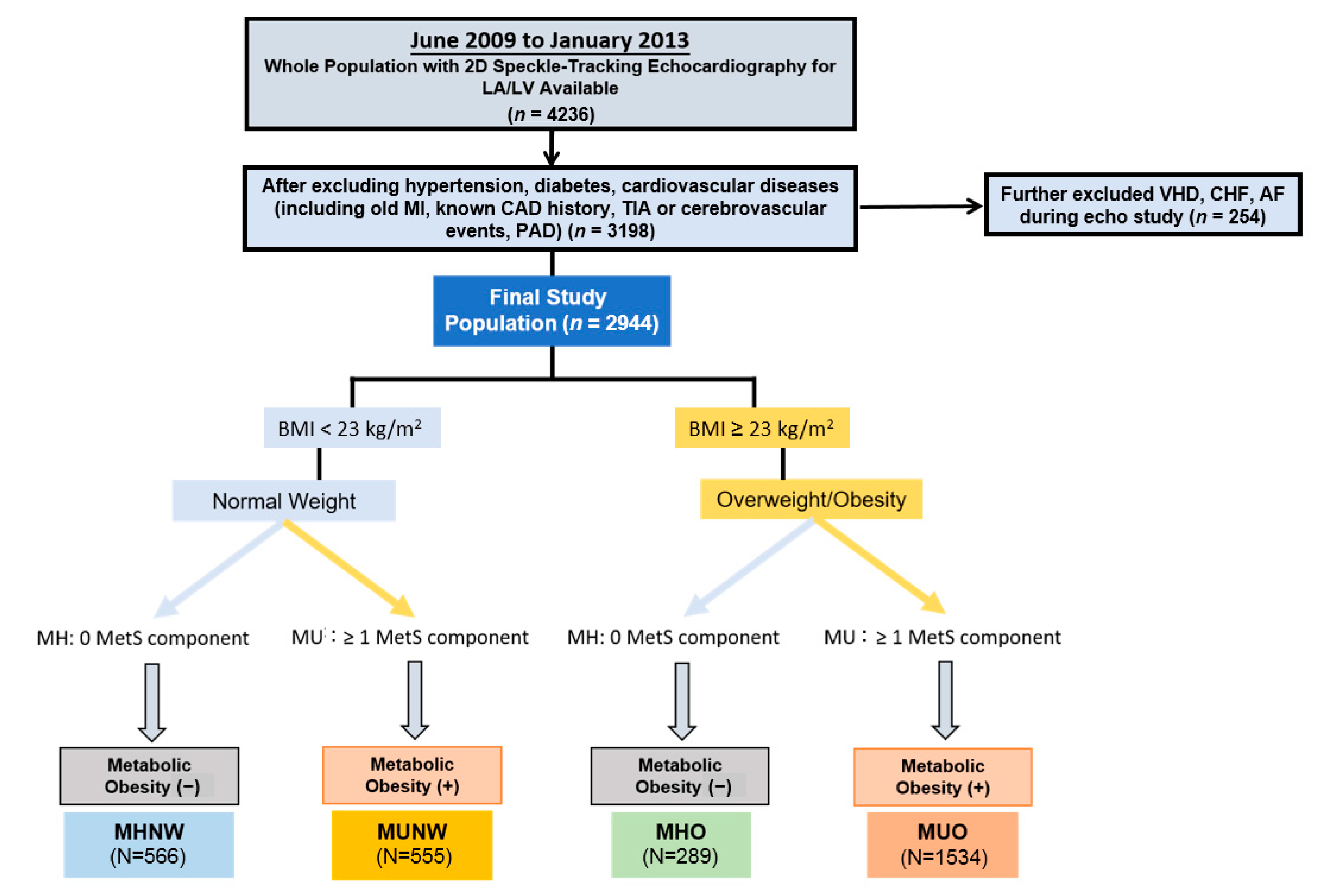

| Total (n = 2944) | Normal-Weight (BMI < 23 kg/m2) | Overweight/Obese (BMI ≥ 23 kg/m2) | p-Value | ||
|---|---|---|---|---|---|
| MHNW (n = 566) | MUNW (n = 555) | MHO (n = 289) | MUO (n = 1534) | ||
| Demographic/Anthropometric Data | |||||
| Age, years | 44.55 ± 9.88 | 49.25 ± 10.13 * | 45.36 ± 9.09† | 48.17 ± 9.81 *# | <0.001 |
| Males (%) | 217 (38.3%) | 332 (59.8%) | 204 (70.6%) | 1209 (78.8%) | <0.001 |
| Weight, kg | 55.9 ± 7.1 | 58.8 ± 7.5 * | 68.1 ± 7.8 *† | 73.8 ± 10.7 *†# | <0.001 |
| BMI, kg/m2 | 20.83 ± 1.26 | 21.45 ± 1.26 * | 24.46 ± 1.62 *† | 26.23 ± 2.7 *†# | <0.001 |
| WC, cm | 73.17 ± 5.55 | 77.4 ± 6.05 * | 84.71 ± 5.59 *† | 88.14 ± 8.08 *†# | <0.001 |
| Abnormal WC (%) | 36 (6.4%) | 39 (7.0%) | 108 (37.4%) | 715 (46.6%) | <0.001 |
| WH ratio | 0.81 ± 0.06 | 0.85 ± 0.06 * | 0.85 ± 0.05 * | 0.90 ± 0.06 *†# | <0.001 |
| WHt ratio | 0.45 ± 0.03 | 0.46 ± 0.03 * | 0.51 ± 0.04 *† | 0.53 ± 0.04 *† | <0.001 |
| BF (%) | 22.77 ± 4.82 | 22.84 ± 5.08 | 24.87 ± 5.03 *† | 27.91 ± 6.3 *†# | <0.001 |
| BSA (m2) | 1.71 ± 0.15 | 1.76 ± 0.15 * | 1.91 ± 0.15 *† | 1.99 ± 0.18 *†# | <0.001 |
| SBP, mmHg | 109.22 ± 10.55 | 121.2 ± 15.45 * | 114.03 ± 9.58 *† | 124.97 ± 15.52 *†# | <0.001 |
| DBP, mmHg | 66.9 ± 7.69 | 73.77 ± 10.09 * | 70.57 ± 7.26 *† | 77.14 ± 9.98 *†# | <0.001 |
| Pulse rate, beats/min | 71.41 ± 11.24 | 74.58 ± 10.84 * | 70.29 ± 11.01† | 74 ± 10.5 *# | <0.001 |
| Active Smoking (%) | 26 (4.6%) | 51 (9.2%) | 20 (6.9%) | 158 (10.3%) | <0.001 |
| Laboratory Data | |||||
| Fasting sugar, mg/dl | 89.8 ± 5.86 | 98.72 ± 15.14 * | 91.96 ± 5.23† | 101.21 ± 16.79 *†# | <0.001 |
| HOMA-IR | 1.14 ± 0.6 | 1.54 ± 1.0 * | 1.42 ± 0.79 | 2.25 ± 1.63 *†# | <0.001 |
| Total cholesterol, mg/dl | 197.18 ± 34.36 | 204.53 ± 39.63 * | 201.83 ± 32.62 | 207.01 ± 36.61 * | <0.001 |
| TG, mg/dl | 79.95 ± 26.63 | 130.87 ± 103.17 * | 91.56 ± 26.62 † | 159.12 ± 91.09 *†# | <0.001 |
| LDL-c, mg/dl | 121.5 ± 31.63 | 131.8 ± 35.11 * | 132.11 ± 31.8 * | 137.68 ± 32.85 *† | <0.001 |
| HDL-c, mg/dl | 66.98 ± 14.82 | 56.17 ± 15.02 * | 59.23 ± 12.1 *† | 48.99 ± 12.78 *†# | <0.001 |
| eGFR, ml/min/m2 | 94.36 ± 18.25 | 91.67 ± 17.82 * | 89.33 ± 13.17 * | 87.84 ± 15.85 *† | <0.001 |
| Biomarkers | |||||
| NT-proBNP, pg/ml | 39.56 ± 32.77 | 43.48 ± 70.98 | 30.17 ± 27.58 *† | 31.36 ± 30.3 *† | <0.001 |
| hs-CRP, mg/dl | 0.10 ± 0.17 | 0.19 ± 0.39 * | 0.16 ± 0.32 | 0.23 ± 0.34 *# | <0.001 |
| Total (n = 2944) | Normal-Weight (BMI <23 kg/m2) | Overweight/Obese (BMI ≥ 23 kg/m2) | p-Value | ||
|---|---|---|---|---|---|
| MHNW (n = 566) | MUNW (n = 555) | MHO (n = 289) | MUO (n = 1534) | ||
| EAT, mm | 5.35 ± 1.13 | 6.18 ± 1.01 * | 6.25 ± 0.99 * | 6.48 ± 0.93 *†# | <0.001 |
| EATi, mm/m2 | 3.14 ± 0.70 | 3.53 ± 0.65 * | 3.29 ± 0.56 *† | 3.28 ± 0.55 *† | <0.001 |
| LVST, mm | 8.2 ± 1.1 | 8.5 ± 1.2 * | 8.8 ± 1.1 *† | 9.2 ± 1.2 *†# | <0.001 |
| LVPT, mm | 8.14 ± 1.01 | 8.5 ± 1.08 * | 8.82 ± 1.03 *† | 9.16 ± 1.12 *†# | <0.001 |
| LVEDV, ml | 66.36 ± 14.99 | 69.08 ± 15.5 * | 79.14 ± 16.66 *† | 81.44 ± 16.68 *† | <0.001 |
| LVM, gm | 117.7 ± 27.46 | 126.63 ± 30.44 * | 142.06 ± 28.9 4*† | 150.55 ± 33.13 *†# | <0.001 |
| LVMi (Ht2.7), gm/m2.7 | 31.2 ± 7.15 | 32.73 ± 8.19 * | 35.88 ± 7.54 *† | 37.65 ± 9.18 *†# | <0.001 |
| LAV(max), ml | 23.56 ± 8.21 | 26.53 ± 9.6 * | 29.3 ± 8.93 *† | 34.77 ± 12.5 *†# | <0.001 |
| LAV(min), ml | 9.63 ± 4.02 | 10.87 ± 4.57 * | 12.32 ± 4.8 *† | 14.87 ± 6.35 *†# | <0.001 |
| LAEF (%) | 59.1 ± 12.61 | 58.39 ± 12.65 | 58.08 ± 11.6 | 57.33 ± 12.4 * | 0.03 |
| LAVi (BSA), ml/m2 | 14.4 ± 4.9 | 14.9 ± 5.7 | 15.9 ± 4.6 * | 16.9 ± 6.0 *† | <0.001 |
| LAVi > 34 mL/m2 (%) | 6 (1.2%) | 10 (1.7%) | 4 (1.5%) | 37 (2.4%) | 0.29 |
| DT, ms | 201.8 ± 33.42 | 202.42 ± 32.96 | 201.1 ± 35.74 | 201.59 ± 34.13 | 0.83 |
| IVRT, ms | 85.19 ± 12.84 | 88.08 ± 12.64 * | 87.85 ± 11.9 * | 89.5 ± 14.25* | <0.001 |
| E/A ratio | 1.52 ± 0.44 | 1.3 ± 0.42 * | 1.38 ± 0.44 *† | 1.21 ± 0.37 *†# | <0.001 |
| LV e’, cm/sec | 11.05 ± 2.29 | 9.9 ± 2.22 * | 10.26 ± 2.12 * | 9.2 ± 2.11 *†# | <0.001 |
| LV s’, cm/sec | 8.68 ± 1.5 | 8.49 ± 1.49 | 8.49 ± 1.36 | 8.41 ± 1.42 * | 0.005 |
| LV E/e’ ratio | 6.95 ± 1.86 | 7.51 ± 2.25 * | 7.03 ± 1.8 † | 7.75 ± 2.36 *# | <0.001 |
| GLS (%) | 21.35 ± 1.89 | 20.6 ± 1.74 * | 20.43 ± 1.77 * | 19.84 ± 1.68 *†# | <0.001 |
| GLS < 18% (%) | 15 (2.7%) | 26 (4.7%) | 11 (3.8%) | 163 (10.6%) | <0.001 |
| PALS (%) | 42.18 ± 7.45 | 39.21 ± 7.54 * | 37.8 ± 7.39 * | 36.32 ± 7.8 *†# | <0.001 |
| PALS < 23% (%) | 3 (0.5%) | 8 (1.4%) | 7 (2.4%) | 62 (4.0%) | <0.001 |
| ALSRsyst | 1.85 ± 0.37 | 1.77 ± 0.39 * | 1.64 ± 0.34 *† | 1.62 ± 0.36 *† | <0.001 |
| ALSRearly | 2.21 ± 0.51 | 1.94 ± 0.54 * | 1.91 ± 0.48 * | 1.63 ± 0.47 *†# | <0.001 |
| ALSRlate | 1.97 ± 0.48 | 2.06 ± 0.47 * | 1.93 ± 0.43 † | 2.02 ± 0.49 # | 0.001 |
| LAstiff | 0.17 ± 0.06 | 0.20 ± 0.09 * | 0.20 ± 0.07 * | 0.23 ± 0.11 *†# | <0.001 |
| LV Indices | LV s’ | LV e’ | E/e’ | GLS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s R | −0.08 | −0.27 | 0.14 | −0.27 | |||||||||
| Regression models | Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | |
| Uni-variate | −0.11 | −0.16; −0.06 | <0.001 | −0.58 | −0.66; −0.5 | <0.001 | 0.3 | 0.22; 0.37 | <0.001 | −0.47 | −0.53; −0.41 | <0.001 | |
| Multi-variate | |||||||||||||
| Model 1 (+ Age) | −0.02 | −0.07; 0.03 | 0.44 | −0.33 | −0.39; −0.26 | <0.001 | 0.13 | 0.06; 0.2 | <0.001 | −0.46 | −0.53; −0.4 | <0.001 | |
| BMI-based | Age + BMI | 0.01 | −0.04; 0.06 | 0.73 | −0.15 | −0.22; −0.09 | <0.001 | 0.08 | 0.003; 0.16 | 0.04 | −0.3 | −0.36; −0.23 | <0.001 |
| Age + BMI + CV | −0.003 | −0.05; 0.05 | 0.92 | −0.13 | −0.19; −0.06 | <0.001 | 0.09 | 0.02; 0.17 | 0.02 | −0.25 | −0.32; −0.19 | <0.001 | |
| Age + BMI + CV + LVM | 0.01 | −0.04; 0.06 | 0.79 | −0.12 | −0.18; −0.05 | 0.001 | 0.08 | 0.01; 0.16 | 0.03 | −0.25 | −0.31; −0.19 | <0.001 | |
| WC-based | Age + WC | −0.01 | −0.07; 0.04 | 0.61 | −0.17 | −0.23; −0.1 | <0.001 | 0.12 | 0.05; 0.2 | 0.002 | −0.28 | −0.35; −0.22 | <0.001 |
| Age + WC + CV | −0.01 | −0.06; 0.04 | 0.72 | −0.14 | −0.2; −0.07 | <0.001 | 0.1 | 0.03; 0.18 | 0.007 | −0.26 | −0.32; −0.2 | <0.001 | |
| Age + WC + CV + LVM | 0.004 | −0.05; 0.06 | 0.88 | −0.12 | −0.19; −0.06 | <0.001 | 0.09 | 0.01; 0.16 | 0.02 | −0.25 | −0.32; −0.19 | <0.001 | |
| BF-based | Age + BF | 0.03 | −0.03; 0.08 | 0.33 | −0.27 | −0.33; −0.2 | <0.001 | 0.06 | −0.02; 0.13 | 0.13 | −0.45 | −0.51; −0.38 | <0.001 |
| Age + BF + CV | −0.01 | −0.06; 0.05 | 0.84 | −0.13 | −0.19; −0.06 | <0.001 | 0.11 | 0.03; 0.18 | 0.005 | −0.24 | −0.31; −0.18 | <0.001 | |
| Age + BF + CV + LVM | 0.01 | −0.04,0.06 | 0.74 | −0.11 | −0.18; −0.04 | 0.001 | 0.09 | 0.02,0.17 | 0.02 | −0.24 | −0.3; −0.17 | <0.001 | |
| LA Indices | PALS | ALSRsyst | ALSRearly | ALSRlate | LAstiff | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s R | −0.26 | −0.17 | −0.33 | 0.04 | 0.24 | |||||||||||
| Regression models | Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | |
| Uni-variate | −1.99 | −2.26; −1.72 | <0.001 | −0.06 | −0.07; −0.05 | <0.001 | −0.17 | −0.18; −0.15 | <0.001 | 0.02 | 0.003; 0.04 | 0.02 | 0.022 | 0.018,0.025 | <0.001 | |
| Multi-variate | ||||||||||||||||
| Model 1 (+Age) | −1.68 | −1.95; −1.4 | <0.001 | −0.05 | −0.06; −0.04 | <0.001 | −0.11 | −0.13; −0.1 | <0.001 | 0.01 | −0.01; 0.03 | 0.31 | 0.015 | 0.011; 0.018 | <0.001 | |
| BMI-based | Age + BMI | −1.05 | −1.33; −0.76 | <0.001 | −0.02 | −0.03; −0.004 | 0.01 | −0.05 | −0.07; −0.03 | <0.001 | 0.01 | −0.01; 0.03 | 0.21 | 0.009 | 0.006; 0.012 | <0.001 |
| Age + BMI + CV | −0.98 | −1.27; −0.7 | <0.001 | −0.02 | −0.03; −0.01 | 0.005 | −0.04 | −0.06; −0.03 | <0.001 | 0.005 | −0.01; 0.02 | 0.63 | 0.009 | 0.006; 0.012 | <0.001 | |
| Age + BMI + CV + LAV(max) | −0.88 | −1.16; −0.59 | <0.001 | −0.01 | −0.03; −0.001 | 0.04 | −0.04 | −0.06; −0.03 | <0.001 | 0.01 | −0.01; 0.03 | 0.21 | 0.007 | 0.004; 0.01 | <0.001 | |
| WC-based | Age + WC | −1.13 | −1.41; −0.85 | <0.001 | −0.03 | −0.04; −0.02 | <0.001 | −0.06 | −0.08; −0.04 | <0.001 | 0.004 | −0.01; 0.02 | 0.69 | 0.011 | 0.008; 0.014 | <0.001 |
| Age + WC + CV | −1.05 | −1.33; −0.77 | <0.001 | −0.03 | −0.04; −0.01 | <0.001 | −0.05 | −0.07; −0.04 | <0.001 | 0.001 | −0.02; 0.02 | 0.9 | 0.01 | 0.007; 0.013 | <0.001 | |
| Age + WC + CV + LAV(max) | −0.91 | −1.19; −0.63 | <0.001 | −0.02 | −0.03; −0.01 | 0.006 | −0.05 | −0.06; −0.03 | <0.001 | 0.01 | −0.01; 0.03 | 0.28 | 0.007 | 0.004; 0.011 | <0.001 | |
| BF-based | Age + BF | −1.45 | −1.72; −1.17 | <0.001 | −0.03 | −0.05; −0.02 | <0.001 | −0.09 | −0.11; −0.08 | <0.001 | 0.02 | 0.01; 0.04 | 0.008 | 0.01 | 0.007; 0.013 | <0.001 |
| Age + BF + CV | −1.02 | −1.3; −0.73 | <0.001 | −0.02 | −0.04; −0.01 | 0.001 | −0.05 | −0.07; −0.04 | <0.001 | 0.01 | −0.01; 0.02 | 0.53 | 0.009 | 0.005; 0.012 | <0.001 | |
| Age + BF + CV + LAV(max) | −0.88 | −1.17; −0.59 | <0.001 | −0.02 | −0.03; −0.002 | 0.02 | −0.05 | −0.06; −0.03 | <0.001 | 0.01 | −0.01; 0.03 | 0.16 | 0.006 | 0.003; 0.01 | <0.001 | |
| Models | Univariate Model | Multivariate Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate Model 1 | Multivariate Model 2 | Multivariate Model 3 | Multivariate Model 4 | |||||||
| LV e’ | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | −1.18 | <0.001 | −1.03 | <0.001 | −0.9 | <0.001 | −0.49 | <0.001 | −0.36 | <0.001 |
| Sugar Abnormality | −0.83 | <0.001 | −0.58 | <0.001 | −0.45 | <0.001 | −0.07 | 0.37 | −0.02 | 0.77 |
| TG Abnormality | −0.91 | <0.001 | −0.7 | <0.001 | −0.57 | <0.001 | −0.66 | <0.001 | −0.63 | <0.001 |
| HDL Abnormality | −0.46 | <0.001 | −0.19 | 0.1 | −0.1 | 0.37 | −0.2 | 0.03 | −0.13 | 0.14 |
| EAT | −0.58 | <0.001 | — | — | −0.45 | <0.001 | −0.22 | <0.001 | −0.18 | <0.001 |
| E/e’ | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | 0.67 | <0.001 | 0.64 | <0.001 | 0.57 | <0.001 | 0.45 | <0.001 | 0.39 | <0.001 |
| Sugar Abnormality | 0.34 | <0.001 | 0.23 | 0.02 | 0.16 | 0.09 | 0.06 | 0.5 | 0.06 | 0.5 |
| TG Abnormality | 0.17 | 0.08 | −0.03 | 0.8 | −0.1 | 0.34 | 0.25 | 0.008 | 0.26 | 0.005 |
| HDL Abnormality | 0.39 | <0.001 | 0.39 | 0.001 | 0.34 | 0.002 | 0.3 | 0.003 | 0.25 | 0.02 |
| EAT | 0.3 | <0.001 | — | — | 0.24 | <0.001 | 0.15 | <0.001 | 0.12 | 0.001 |
| GLS, % | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | −0.72 | <0.001 | −0.62 | <0.001 | −0.5 | <0.001 | −0.37 | <0.001 | −0.27 | 0.01 |
| Sugar Abnormality | −0.54 | <0.001 | −0.35 | <0.001 | −0.25 | 0.001 | −0.11 | 0.15 | −0.07 | 0.38 |
| TG Abnormality | −0.84 | <0.001 | −0.68 | <0.001 | −0.57 | <0.001 | −0.32 | <0.001 | −0.29 | <0.001 |
| HDL Abnormality | −0.5 | <0.001 | −0.23 | 0.01 | −0.16 | 0.08 | −0.25 | 0.005 | −0.21 | 0.02 |
| EAT | −0.47 | <0.001 | — | — | −0.38 | <0.001 | −0.32 | <0.001 | −0.3 | <0.001 |
| Models | Univariate Model | Multivariate Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate Model 1 | Multivariate Model 2 | Multivariate Model 3 | Multivariate Model 4 | |||||||
| PALS, % | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | −2.34 | <0.001 | −1.98 | <0.001 | −1.47 | <0.001 | −0.91 | 0.006 | −0.86 | 0.01 |
| Sugar Abnormality | −1.88 | <0.001 | −1.28 | <0.001 | −0.8 | 0.02 | −0.28 | 0.4 | −0.21 | 0.53 |
| TG Abnormality | −2.59 | <0.001 | −2.08 | <0.001 | −1.58 | <0.001 | −1.55 | <0.001 | −1.48 | <0.001 |
| HDL Abnormality | −1.48 | <0.001 | −0.65 | 0.12 | −0.3 | 0.45 | −0.47 | 0.23 | −0.36 | 0.37 |
| EAT | −1.99 | <0.001 | — | — | −1.72 | <0.001 | −1.42 | <0.001 | −1.21 | <0.001 |
| ALSRearly | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | −0.24 | <0.001 | −0.24 | <0.001 | −0.2 | <0.001 | −0.12 | <0.001 | −0.1 | <0.001 |
| Sugar Abnormality | −0.24 | <0.001 | −0.19 | <0.001 | −0.15 | <0.001 | −0.08 | <0.001 | −0.07 | <0.001 |
| TG Abnormality | −0.22 | <0.001 | −0.16 | <0.001 | −0.13 | <0.001 | −0.14 | <0.001 | −0.12 | <0.001 |
| HDL Abnormality | −0.15 | <0.001 | −0.08 | 0.003 | −0.05 | 0.04 | −0.08 | 0.001 | −0.06 | 0.006 |
| EAT | −0.16 | <0.001 | — | — | −0.13 | <0.001 | −0.09 | <0.001 | −0.07 | <0.001 |
| LAstiff | Coef. | p | Coef. | p | Coef. | p | Coef. | p | Coef. | p |
| BP Abnormality | 0.03 | <0.001 | 0.03 | <0.001 | 0.02 | <0.001 | 0.02 | <0.001 | 0.01 | 0.001 |
| Sugar Abnormality | 0.02 | <0.001 | 0.02 | <0.001 | 0.01 | 0.005 | 0.005 | 0.23 | 0.004 | 0.23 |
| TG Abnormality | 0.02 | <0.001 | 0.01 | 0.07 | 0.003 | 0.54 | 0.01 | 0.002 | 0.01 | 0.006 |
| HDL Abnormality | 0.02 | <0.001 | 0.02 | <0.001 | 0.01 | 0.003 | 0.01 | 0.001 | 0.01 | 0.004 |
| EAT | 0.02 | <0.001 | — | — | 0.02 | <0.001 | 0.01 | <0.001 | 0.01 | <0.001 |
| Event Types | AF Event | HF Event | ||||||
|---|---|---|---|---|---|---|---|---|
| Obesity Phenotypes by BMI vs. Metabolic status | Rate (%) | aHR | (95% CI) | p-value | Rate (%) | aHR | (95% CI) | p-value |
| Low BMI/metabolically healthy (MHNW) | 0.4 | 1 | (reference) | — | 0.6 | 1 | (reference) | — |
| Low BMI/metabolically unhealthy (MUNW) | 1.1 | 1.3 | (0.2–7.0) | 0.75 | 2.1 | 3.2 | (1.0–11.9) | 0.04 |
| High BMI/metabolically healthy (MHO) | 2.3 | 5.4 | (1.2–31.5) | 0.04 | 0.8 | 1.8 | (0.4–9.3) | 0.46 |
| High BMI/metabolically unhealthy (MUO) | 3.8 | 5.7 | (1.7–30.6) | 0.02 | 3.6 | 5.3 | (1.5–18.5) | 0.01 |
| Obesity Phenotypes by BMI vs. Visceral Fat | Rate (%) | aHR | (95% CI) | p-value | Rate (%) | aHR | (95% CI) | p-value |
| Low BMI/low EAT (<7.0 mm) | 0.2 | 1 | (reference) | — | 0.8 | 1 | (reference) | — |
| Low BMI/high EAT (≥7.0 mm) | 3.2 | 9.3 | (1.8−48.1) | 0.008 | 4.3 | 3.6 | (1.2−10.4) | 0.018 |
| High BMI/low EAT (<7.0 mm) | 2.5 | 7.4 | (1.7–31.7) | 0.007 | 2.2 | 2.3 | (0.9–5.7) | 0.065 |
| High BMI/high EAT (≥7.0 mm) | 5.6 | 13.8 | (3.3–59.9) | <0.001 | 4.8 | 4.2 | (1.7–10.2) | 0.002 |
| NRI table for abnormal GLS (<18%) | ||
| All, n = 2827 | Improvement in BMI/MU 4 Category | Improvement in BMI/EAT 4 Category |
| GLS Normal | 93.7% | 91.1% |
| GLS Abnormal | 6.3% | 8.1% |
| NRI: 13.2%, p = 0.065 | ||
| BMI ≥ 23 kg/m2, n = 1744 | Improvement in BMI/MU 4 Category | Improvement in BMI/EAT 4 Category |
| GLS Normal | 92.1% | 88.7% |
| GLS Abnormal | 7.9% | 11.3% |
| NRI: 19.9%, p = 0.01 | ||
| NRI table for HF event | ||
| All, n = 2827 | Improvement in BMI/MU 4 Category | Improvement in BMI/EAT 4 Category |
| Non-event | 97.8% | 97.2% |
| With events | 2.2% | 2.8% |
| NRI: 12.5%, p = 0.29 | ||
| BMI ≥ 23 kg/m2, n = 1744 | Improvement in BMI/MU 4 Category | Improvement in BMI/EAT 4 Category |
| Non-event | 97.3% | 96.1% |
| With events | 2.7% | 3.9% |
| NRI: 19.9%, p = 0.15 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-H.; Liu, L.Y.-m.; Sung, K.-T.; Tsai, J.-P.; Huang, W.-H.; Yun, C.-H.; Lin, J.-L.; Chen, Y.-J.; Su, C.-H.; Hung, T.-C.; et al. Diverse Adiposity and Atrio-Ventricular Dysfunction across Obesity Phenotypes: Implication of Epicardial Fat Analysis. Diagnostics 2021, 11, 408. https://doi.org/10.3390/diagnostics11030408
Lai Y-H, Liu LY-m, Sung K-T, Tsai J-P, Huang W-H, Yun C-H, Lin J-L, Chen Y-J, Su C-H, Hung T-C, et al. Diverse Adiposity and Atrio-Ventricular Dysfunction across Obesity Phenotypes: Implication of Epicardial Fat Analysis. Diagnostics. 2021; 11(3):408. https://doi.org/10.3390/diagnostics11030408
Chicago/Turabian StyleLai, Yau-Huei, Lawrence Yu-min Liu, Kuo-Tzu Sung, Jui-Peng Tsai, Wen-Hung Huang, Chun-Ho Yun, Jiun-Lu Lin, Ying-Ju Chen, Cheng-Huang Su, Ta-Chuan Hung, and et al. 2021. "Diverse Adiposity and Atrio-Ventricular Dysfunction across Obesity Phenotypes: Implication of Epicardial Fat Analysis" Diagnostics 11, no. 3: 408. https://doi.org/10.3390/diagnostics11030408
APA StyleLai, Y.-H., Liu, L. Y.-m., Sung, K.-T., Tsai, J.-P., Huang, W.-H., Yun, C.-H., Lin, J.-L., Chen, Y.-J., Su, C.-H., Hung, T.-C., Hung, C.-L., & Kuo, J.-Y. (2021). Diverse Adiposity and Atrio-Ventricular Dysfunction across Obesity Phenotypes: Implication of Epicardial Fat Analysis. Diagnostics, 11(3), 408. https://doi.org/10.3390/diagnostics11030408







