Detectability on Plain CT Is an Effective Discriminator between Carcinoma and Benign Disorder for a Polyp >10 mm in the Gallbladder
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Patients
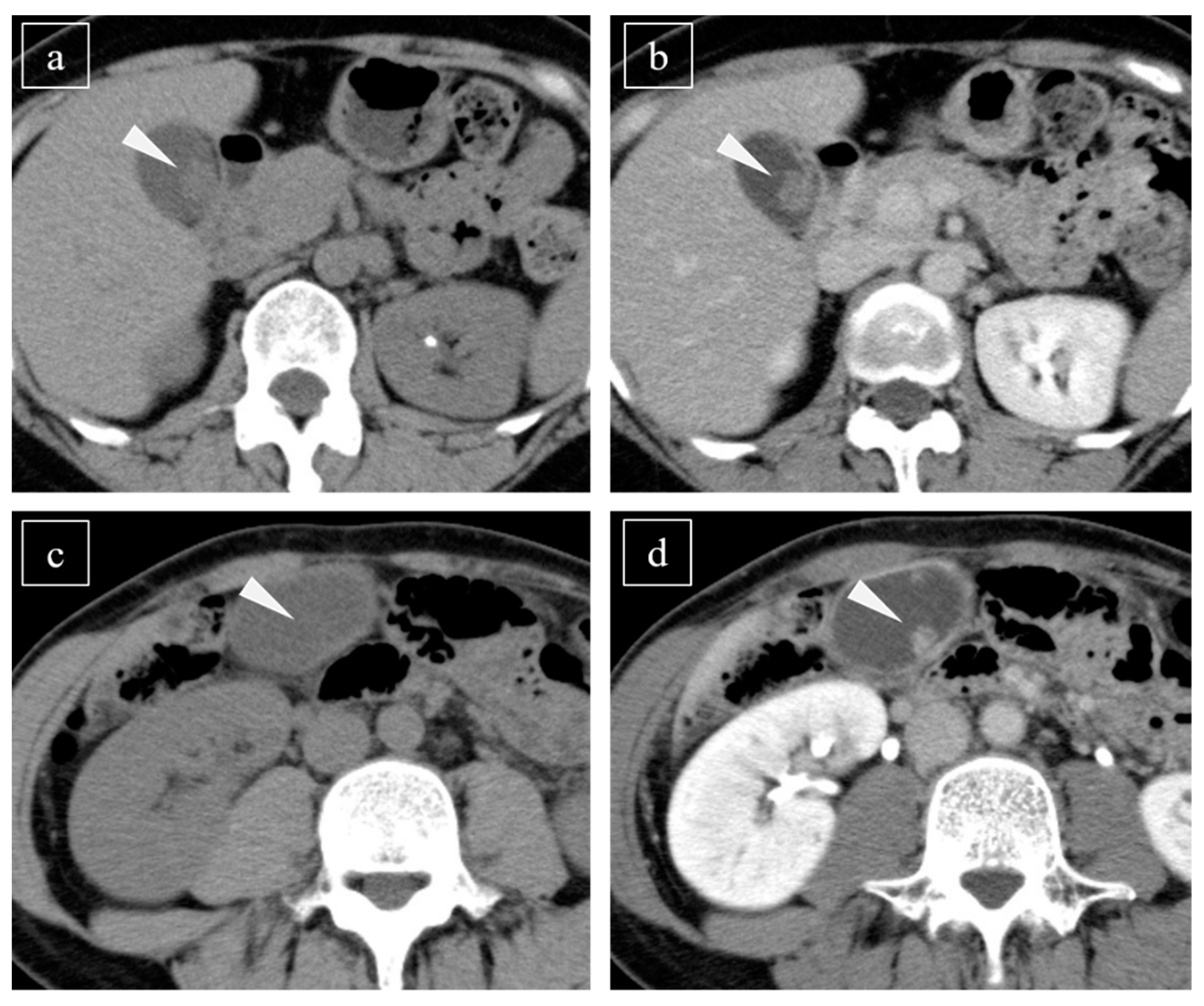
2.3. Evaluation of the CT Scan
2.4. Histopathological Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Detectability of Lesions Using Plain CT and CE-CT
3.3. Multivariate Analysis
3.4. Histopathological Evaluation
4. Discussion
4.1. Need for Differentiation between GC and Noncancerous GP
4.2. Methods of the Differential Diagnosis for GPs
4.3. Correlation between Histopathological Results and CT Findings
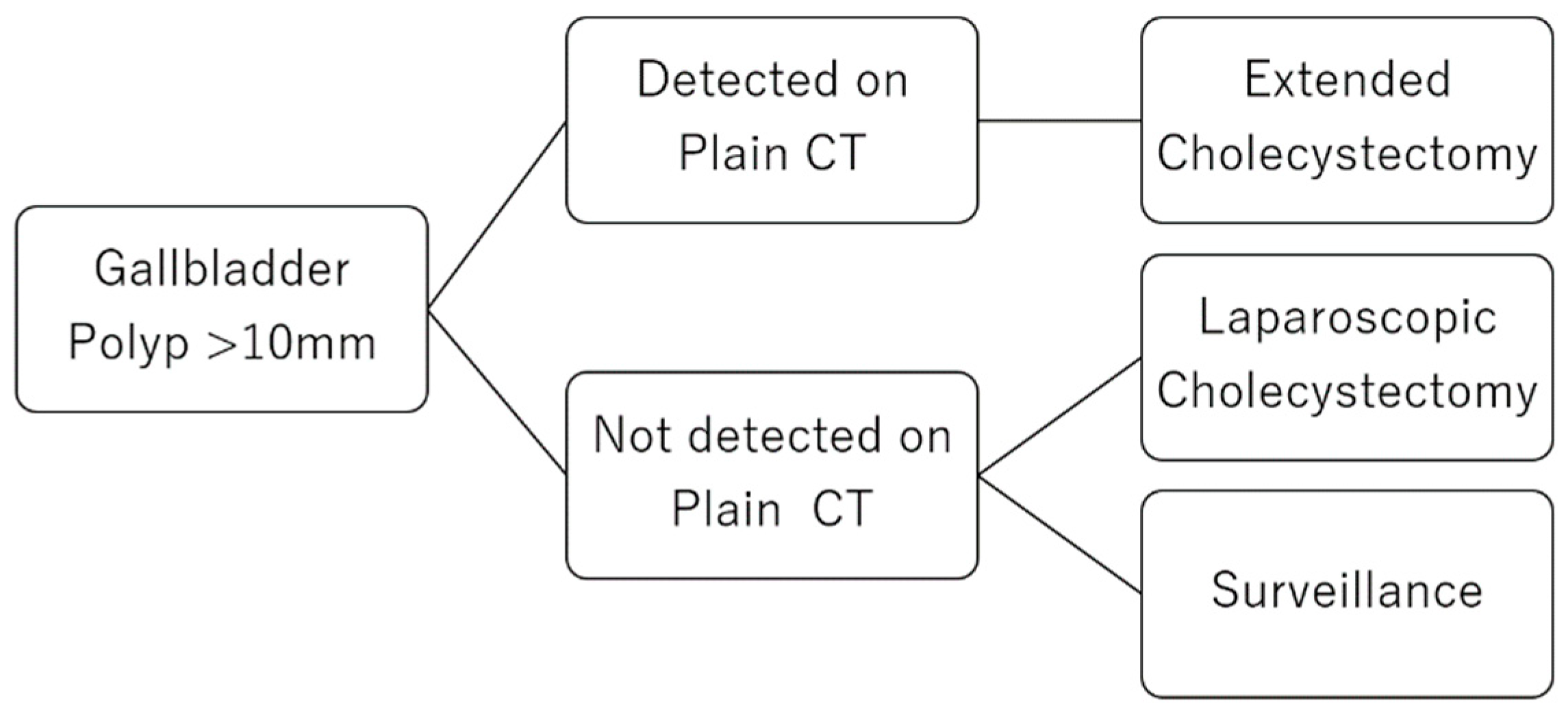
4.4. A Clinical Strategy for GPs
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattopadhyay, D.; Lochan, R.; Balupuri, S.; Gopinath, B.R.; Wynne, K.S. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: A nine year experience. World J. Gastroenterol. 2005, 11, 2171–2173. [Google Scholar] [CrossRef] [PubMed]
- Andrén-Sandberg, A. Diagnosis and management of gallbladder polyps. N. Am. J. Med. Sci. 2012, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.H.; Ishak, K.G. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch. Pathol. 1970, 90, 423–432. [Google Scholar]
- Piehler, J.M.; Crichlow, R.W. Primary carcinoma of the gallbladder. Surg. Gynecol. Obstet. 1978, 147, 929–942. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.Y.; Break, J.H.; Eun, H.W.; Kim, Y.J.; Han, J.K.; Choi, B.I. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am. J. Roentgenol. 2015, 204, W150–W159. [Google Scholar] [CrossRef]
- Zheng, S.G.; Xu, H.X.; Liu, L.N.; Lu, M.D.; Xie, X.Y.; Wang, W.P.; Hu, B.; Yan, K.; Ding, H.; Tang, S.S.; et al. Contrast-enhanced ultrasound versus conventional ultrasound in the diagnosis of polypoid lesion of gallbladder: A multi-center study of dynamic microvascularization. Clin. Hemorheol. Microcirc. 2013, 55, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Park, J.Y.; Kim, Y.J.; Kim, H.M.; Kim, H.J.; Hong, S.P.; Park, S.W.; Chung, J.B.; Song, S.Y.; Bang, S. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest Endosc. 2009, 69, 1244–12450. [Google Scholar] [CrossRef]
- Sugiyama, M.; Xie, X.Y.; Atomi, Y.; Saito, M. Differential diagnosis of small polypoid lesions of the gallbladder: The value of endoscopic ultrasonography. Ann. Surg. 1999, 229, 498–504. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Choi, J.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013, 78, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Kamochi, N.; Nojiri, J.; Egashira, Y.; Sasaguri, K.; Kudo, S. High b-value diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions. Acta Radiol. 2011, 52, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, M.; Kim, K.S.; Lee, J.D.; Kim, C.K. Risk stratification of gallbladder polyps (1–2 cm) for surgical intervention with 18F-FDG PET/CT. J. Nucl. Med. 2012, 53, 353–358. [Google Scholar] [CrossRef]
- Itsuki, H.; Serikawa, M.; Sasaki, T.; Ishii, Y.; Tsushima, K.; Furukawa, Y.; Murakami, Y.; Arihiro, K.; Chayama, K. Indication and usefulness of bile juice cytology for diagnosis of gallbladder cancer. Gastroenterol. Res. Pract. 2018, 2018, 5410349. [Google Scholar] [CrossRef]
- Furukawa, H.; Kosuge, T.; Shimada, K.; Yamamoto, J.; Kanai, Y.; Mukai, K.; Iwata, R.; Ushio, K. Small polypoid lesions of the gallbladder: Differential diagnosis and surgical indications by helical computed tomography. Arch. Surg 1998, 133, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography). 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and multi-energy CT: Principles, technical approaches, and clinical applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Chijiiwa, K.; Tanaka, M. Polypoid lesion of the gallbladder: Indications of carcinoma and outcome after surgery for malignant polypoid lesion. Int. Surg. 1994, 79, 106–109. [Google Scholar] [PubMed]
- Kubota, K.; Bandai, Y.; Noie, T.; Ishizaki, Y.; Teruya, M.; Makuuchi, M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery 1995, 117, 481–487. [Google Scholar] [CrossRef]
- Park, J.K.; Yoon, Y.B.; Kim, Y.T.; Ryu, J.K.; Yoon, W.J.; Lee, S.H.; Yu, S.J.; Kang, H.Y.; Lee, J.Y.; Park, M.J. Management strategies for gallbladder polyps: Is it possible to predict malignant gallbladder polyps? Gut Liver 2008, 2, 88–94. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ohtsuka, M.; Miyakawa, S.; Nagino, M.; Yamamoto, M.; Kokubo, N.; Sano, K.; Endo, I.; Unno, M.; Chijiiwa, K.; et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3rd English edition. J. Hepatobiliary Pancreat Sci. 2015, 22, 181–196. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yoshitomi, H.; Miyakawa, S.; Uesaka, K.; Unno, M.; Endo, I.; Ota, T.; Ohtsuka, M.; Kinoshita, H.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J. Hepatobiliary Pancreat Sci. 2015, 22, 249–273. [Google Scholar] [CrossRef]
- Wullstein, C.; Woeste, G.; Barkhausen, S.; Gross, E.; Hopt, U.T. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc. 2002, 16, 828–832. [Google Scholar] [CrossRef]
- Jin, K.; Lan, H.; Zhu, T.; He, K.; Teng, L. Gallbladder carcinoma incidentally encountered during laparoscopic cholecystectomy: How to deal with it. Clin. Transl. Oncol. 2011, 13, 25–33. [Google Scholar] [CrossRef]
- Reddy, Y.P.; Sheridan, W.G. Port-site metastasis following laparoscopic cholecystectomy: A review of the literature and a case report. Eur. J. Surg. Oncol. 2000, 26, 95–102. [Google Scholar] [CrossRef] [PubMed]
- You, D.D.; Lee, H.G.; Paik, K.Y.; Heo, J.S.; Choi, S.H.; Choi, D.W. What is an adequate extent of resection for Tz gallbladder cancers? Ann. Surg. 2008, 247, 835–838. [Google Scholar] [CrossRef]
- Jensen, E.H.; Abraham, A.; Jarosek, S.; Habermann, E.B.; AI-Refaie, W.B.; Vickers, S.A.; Virnig, B.A.; Tuttle, T.M. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery 2009, 146, 706–713. [Google Scholar] [CrossRef]
- Martin, E.; Gill, R.; Debru, E. Diagnostic accuracy of transabdominal ultrasonography for gallbladder polyp: Systemtic review. Can. J. Surg. 2018, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wennmacker, S.Z.; Lamberts, M.P.; Di Martino, M.; Drenth, J.P.; Gurusamy, K.S.; van Laarhoven, C.J. Transabdominal ultrasound and endoscopic ultrasound for diagnosis of gallbladder polyps. Cochrane Database Syst. Rev. 2018, 8, CD012233. [Google Scholar] [CrossRef] [PubMed]

| GC (n = 11) | GChP (n = 10) | p Value | |
|---|---|---|---|
| Age, years | 63 (40–90) | 60.5 (45–76) | 0.78 |
| Male, number | 3 (27) | 3 (30) | 1.0 |
| Indication for workup | |||
| Symptoms | 3 (27) | 1 (10) | 0.59 |
| Incidental | 8 (72) | 9 (90) | 0.59 |
| Medical checkup/screening | 5 (45) | 4 (40) | |
| Initial examination for other disease | 1 (9) | 3 (30) | |
| Surveillance for other disease | 2 (18) | 2 (20) | |
| History of follow up | 4 (36) | 6 (60) | 0.40 |
| Tumor size, mm | 18 (10–28) | 14.5 (10–20) | 0.23 |
| CEA, ng/mL | 3.0 (0.9–17) | 2.2 (0.8–49) | 0.65 |
| CA19-9, U/mL | 15 (2–58) | 11 (2–45) | 0.50 |
| GC (n = 11) | GChP (n = 10) | p Value | |
|---|---|---|---|
| Detected on plain CT | 8 (73) | 1 (10) | <0.01 |
| Detected on CE-CT | 11 (100) | 10 (100) | 1.0 |
| CT value of the lesions on plain CT | 28.5 (10–37.5) | 12 (4.5–23) | <0.01 |
| CT value of the lesions on CE-CT | 80 (40.5–116) | 67.25 (32–106) | 0.35 |
| CT value of bile around the lesion | 11 (7–20.5) | 12 (8.5–26) | 0.34 |
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Cutoff | GC | GChP | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Age (years) | >63 | 3 | 5 | 1.94 | 0.32–11.8 | 0.47 | 0.58 | 0.035–9.52 | 0.70 |
| ≤63 | 7 | 5 | 1 | 1 | |||||
| CEA (ng/mL) | >2.9 | 6 | 3 | 2.80 | 0.46–16.9 | 0.26 | 1.97 | 0.10–37.4 | 0.65 |
| ≤2.9 | 5 | 7 | 1 | 1 | |||||
| CA19-9 (U/mL) | >10 | 8 | 5 | 2.67 | 0.43–16.4 | 0.29 | 1.27 | 0.089–18.2 | 0.86 |
| ≤10 | 3 | 5 | 1 | 1 | |||||
| Detected on PCT | Yes | 8 | 1 | 24.0 | 2.06–280 | 0.011 | 27.1 | 1.07–685 | 0.045 |
| No | 3 | 9 | 1 | 1 | |||||
| CT value on CE-CT | >68 | 8 | 5 | 2.67 | 0.43–16.4 | 0.29 | 0.86 | 0.034–21.8 | 0.93 |
| ≤68 | 3 | 5 | 1 | 1 | |||||
| GC | GChP | p Value | |
|---|---|---|---|
| Vessel area *, μm2 | 52,737 (4292–635,267) | 31,906 (9594–370,033) | 0.51 |
| Number of vessels * | 24 (9–32) | 13.5 (7–35) | 0.72 |
| Cell densities *, n/μm2 | 0.015 (0.0084–0.024) | 0.0080 (0.0050–0.018) | <0.01 |
| Detected GPs | Undetected GPs | p Value | |
|---|---|---|---|
| Vessel area *, μm2 | 52,165 (13955–635,267) | 48,053 (4292–370,033) | 0.86 |
| Number of vessels * | 24 (9–32) | 13.5 (7–39) | 0.52 |
| Cell densities *, n/μm2 | 0.015 (0.0085–0.024) | 0.0083 (0.0050–0.018) | <0.01 |
| ρ | p Value | |
|---|---|---|
| Vessel area | −0.029 | 0.90 |
| Number of vessels | −0.053 | 0.82 |
| Cell densities | 0.45 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, T.; Kikuyama, M.; Sasaki, K.; Ishiwatari, H.; Kawaguchi, S.; Sato, J.; Kaneko, J.; Matsubayashi, H. Detectability on Plain CT Is an Effective Discriminator between Carcinoma and Benign Disorder for a Polyp >10 mm in the Gallbladder. Diagnostics 2021, 11, 388. https://doi.org/10.3390/diagnostics11030388
Satoh T, Kikuyama M, Sasaki K, Ishiwatari H, Kawaguchi S, Sato J, Kaneko J, Matsubayashi H. Detectability on Plain CT Is an Effective Discriminator between Carcinoma and Benign Disorder for a Polyp >10 mm in the Gallbladder. Diagnostics. 2021; 11(3):388. https://doi.org/10.3390/diagnostics11030388
Chicago/Turabian StyleSatoh, Tatsunori, Masataka Kikuyama, Keiko Sasaki, Hirotoshi Ishiwatari, Shinya Kawaguchi, Junya Sato, Junichi Kaneko, and Hiroyuki Matsubayashi. 2021. "Detectability on Plain CT Is an Effective Discriminator between Carcinoma and Benign Disorder for a Polyp >10 mm in the Gallbladder" Diagnostics 11, no. 3: 388. https://doi.org/10.3390/diagnostics11030388
APA StyleSatoh, T., Kikuyama, M., Sasaki, K., Ishiwatari, H., Kawaguchi, S., Sato, J., Kaneko, J., & Matsubayashi, H. (2021). Detectability on Plain CT Is an Effective Discriminator between Carcinoma and Benign Disorder for a Polyp >10 mm in the Gallbladder. Diagnostics, 11(3), 388. https://doi.org/10.3390/diagnostics11030388







