Females and Males Show Differences in Early-Stage Transcriptomic Biomarkers of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Feature Selection and Classification Algorithms
2.3. Performance Evaluation Metrics
2.4. Programming and Running Environments
2.5. Workflow of This Study
3. Results
3.1. Baseline Summary of the Two Lung Cancer Subtypes
3.2. Evaluation of the Classifiers on the Ttest-Ranked Features
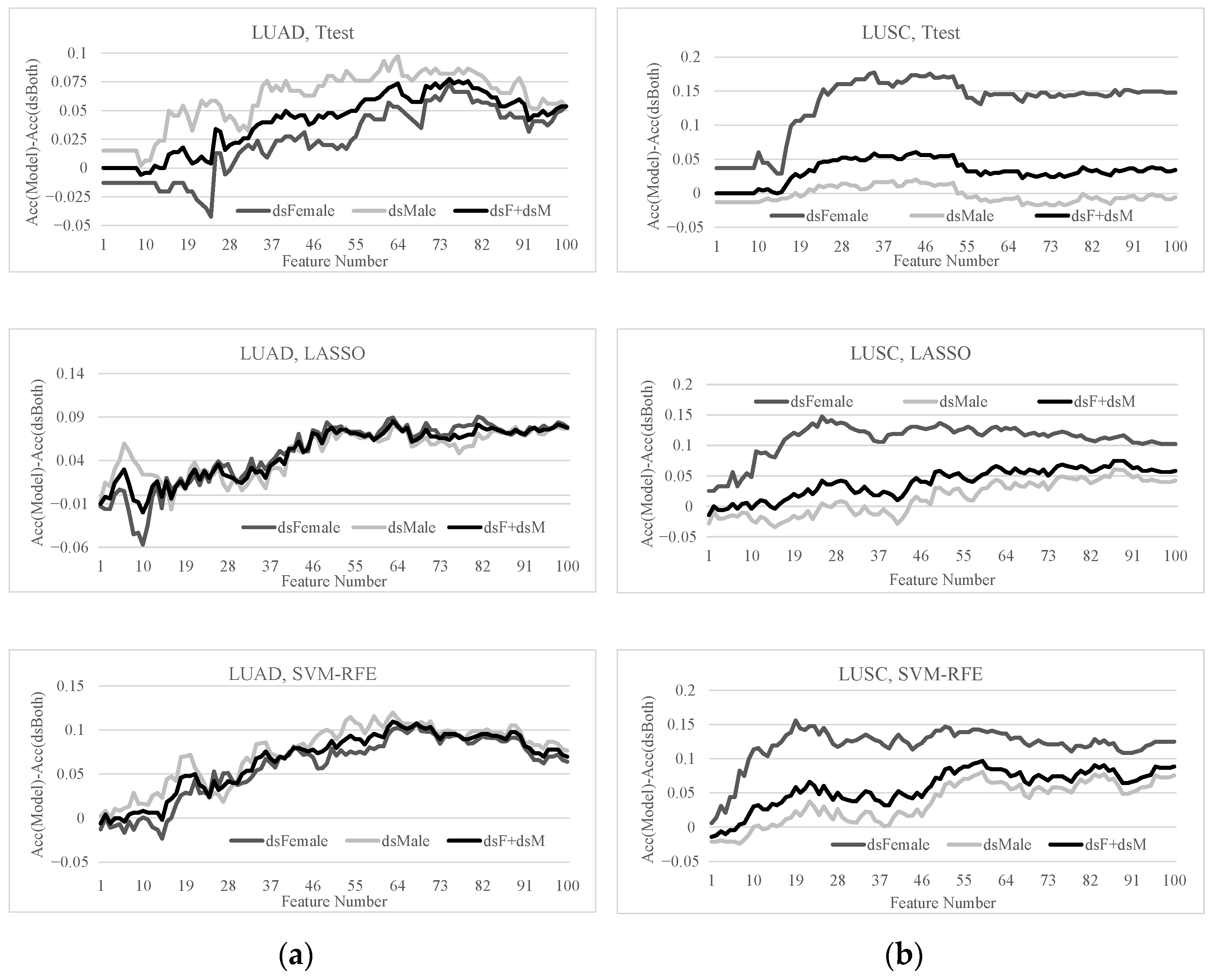
3.3. Sex Disparities Using the Ttest-Ranked Biomarkers
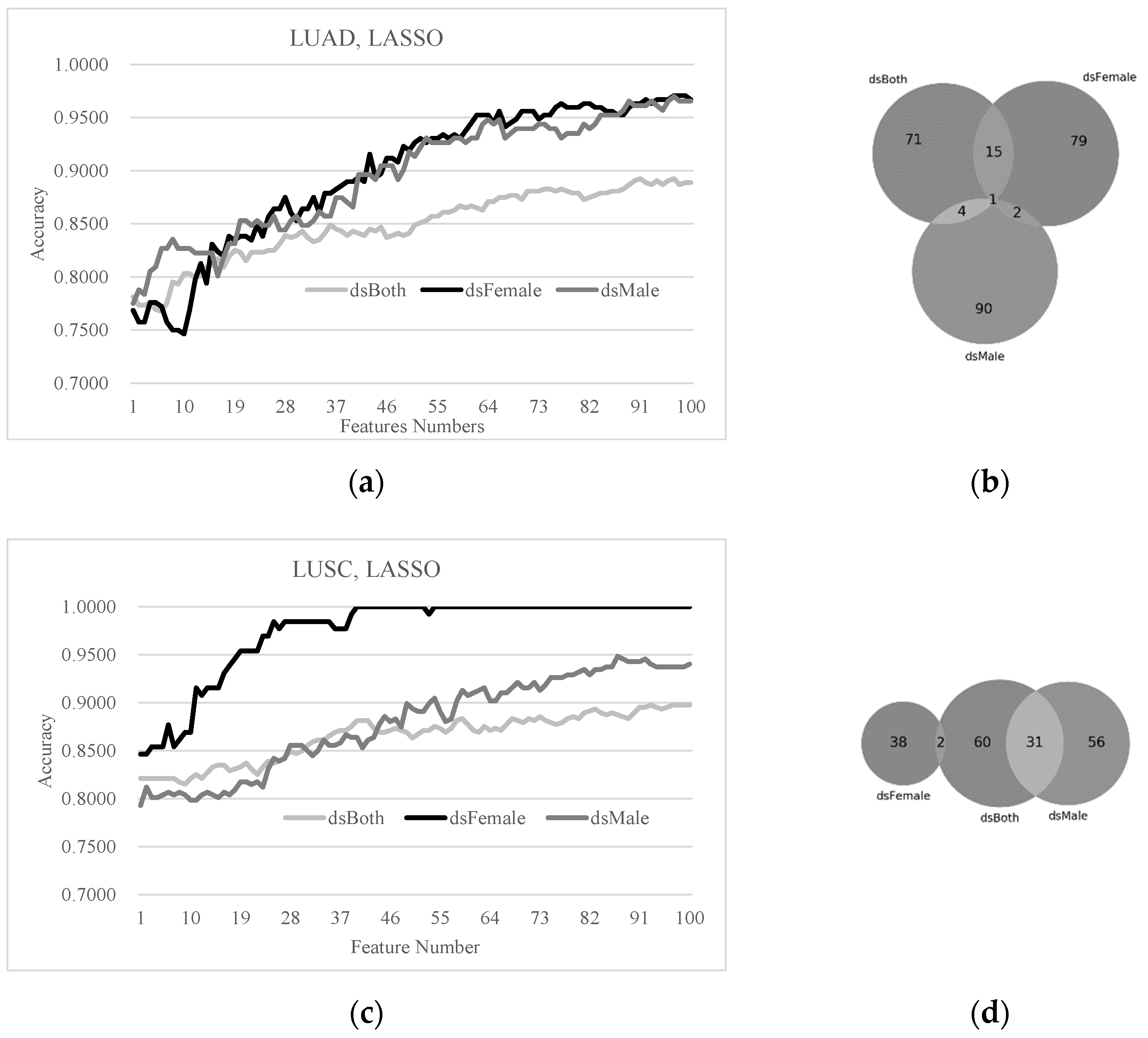
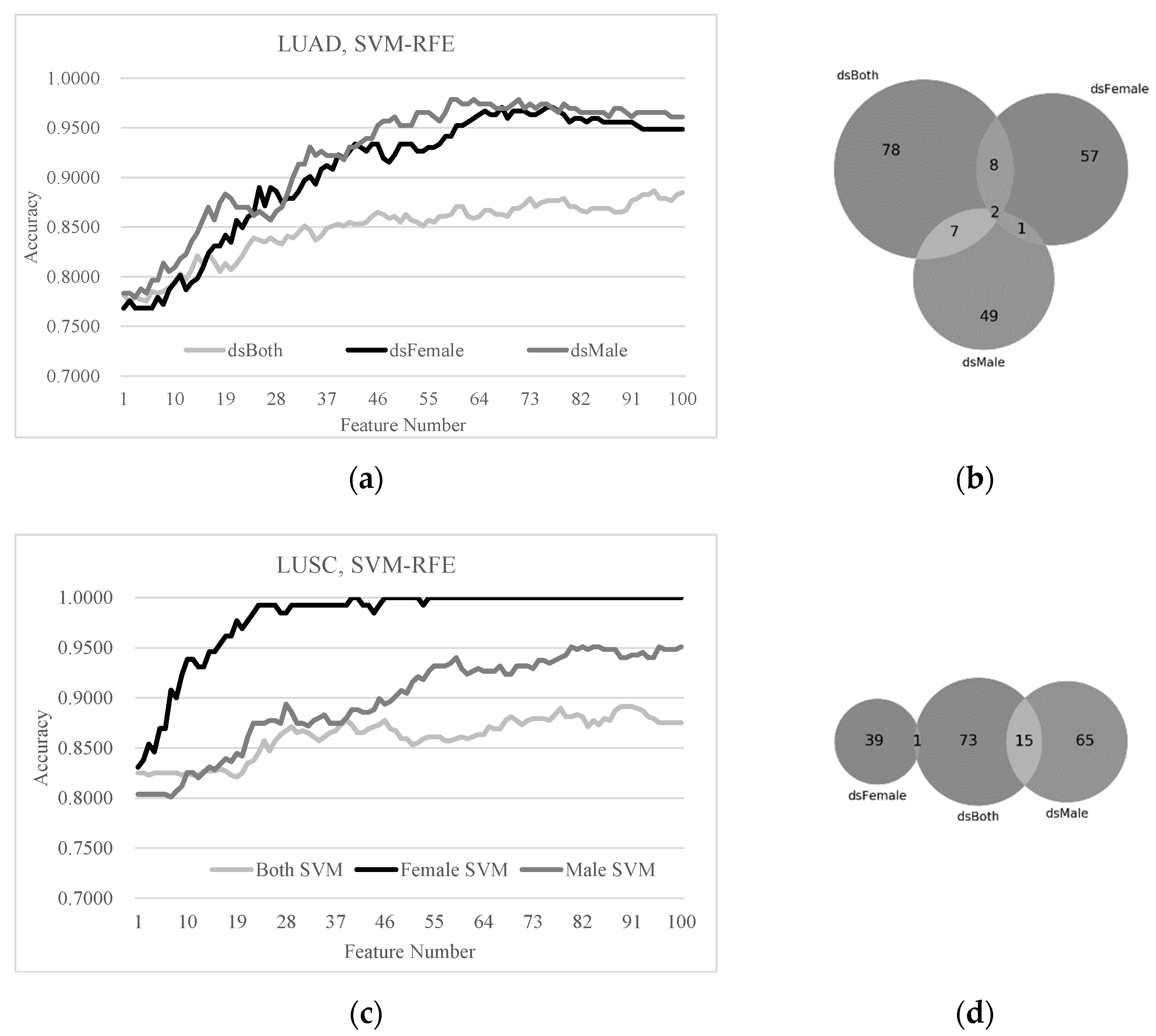
3.4. Sex Disparities in the Biomarkers Ranked by LASSO and SVM-RFE
3.5. Sex-Specific Models May Improve Early-Stage Lung Cancer Detection
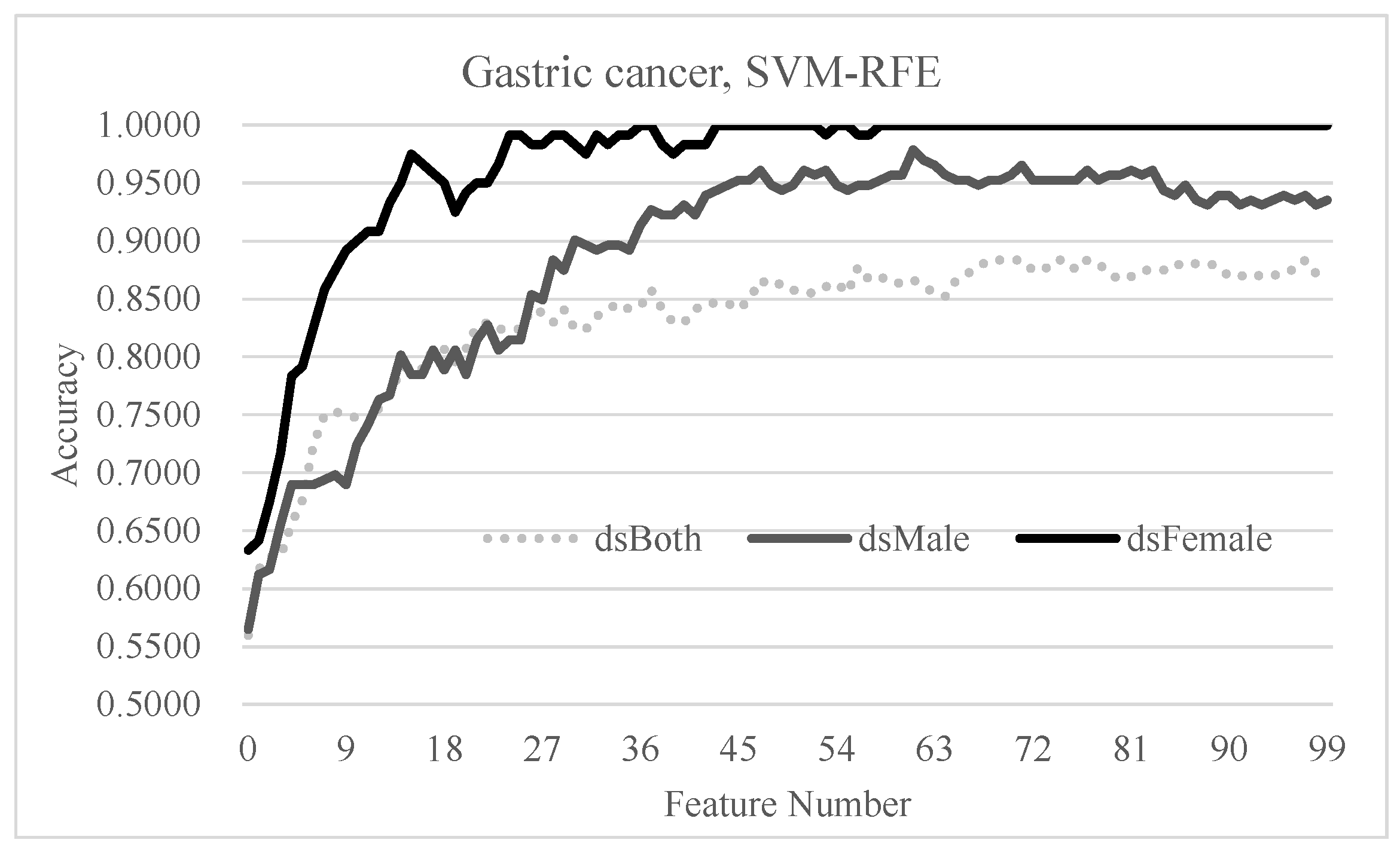
3.6. Independent Evaluation of the Hypothesis on Gastric Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhakar, B.; Shende, P.; Augustine, S. Current trends and emerging diagnostic techniques for lung cancer. Biomed. Pharmacother. 2018, 106, 1586–1599. [Google Scholar] [CrossRef]
- Tomasetti, M.; Amati, M.; Neuzil, J.; Santarelli, L. Circulating epigenetic biomarkers in lung malignancies: From early diagnosis to therapy. Lung Cancer 2017, 107, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.B.; Li, J.; Lai, X.N.; Jiang, R.; Zhao, R.C.; Xiong, L.X. Multifaceted Roles of Caveolin-1 in Lung Cancer: A New Investigation Focused on Tumor Occurrence, Development and Therapy. Cancers 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Lababede, O.; Meziane, M.; Rice, T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: Quick reference chart and diagrams. Chest 2011, 139, 183–189. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Liu, B. Exploring and comparing of the gene expression and methylation differences between lung adenocarcinoma and squamous cell carcinoma. J. Cell. Physiol. 2019, 234, 4454–4459. [Google Scholar] [CrossRef]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef]
- Serke, M.; Schönfeld, N. Diagnosis and staging of lung cancer. Dtsch. Med. Wochenschr. 2007, 132, 1165–1169. [Google Scholar] [CrossRef]
- Nesbitt, J.C.; Putnam, J.B., Jr.; Walsh, G.L.; Roth, J.A.; Mountain, C.F. Survival in early-stage non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 466–472. [Google Scholar] [CrossRef]
- Smith, C.B.; Bonomi, M.; Packer, S.; Wisnivesky, J.P. Disparities in lung cancer stage, treatment and survival among American Indians and Alaskan Natives. Lung Cancer 2011, 72, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.K.; Tsang, K.W.; Ip, M.S. Chemotherapy for advanced (stage IIIB and stage IV) non-small cell lung cancer: The Hong Kong perspective. Respirology 1998, 3, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Tolwin, Y.; Gillis, R.; Peled, N. Gender and lung cancer-SEER-based analysis. Ann. Epidemiol. 2020, 46, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Augustine, S.; Prabhakar, B.; Gaud, R.S. Advanced multimodal diagnostic approaches for detection of lung cancer. Expert Rev. Mol. Diagn. 2019, 19, 409–417. [Google Scholar] [CrossRef]
- Cykert, S.; Eng, E.; Walker, P.; Manning, M.A.; Robertson, L.B.; Arya, R.; Jones, N.S.; Heron, D.E. A system-based intervention to reduce Black-White disparities in the treatment of early stage lung cancer: A pragmatic trial at five cancer centers. Cancer Med. 2019, 8, 1095–1102. [Google Scholar] [CrossRef]
- Doria-Rose, V.P.; White, M.C.; Klabunde, C.N.; Nadel, M.R.; Richards, T.B.; McNeel, T.S.; Rodriguez, J.L.; Marcus, P.M. Use of lung cancer screening tests in the United States: Results from the 2010 National Health Interview Survey. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1049–1059. [Google Scholar] [CrossRef]
- Taylor, K.L.; Deros, D.E.; Fallon, S.; Stephens, J.; Kim, E.; Lobo, T.; Davis, K.M.; Luta, G.; Jayasekera, J.; Meza, R.; et al. Study protocol for a telephone-based smoking cessation randomized controlled trial in the lung cancer screening setting: The lung screening, tobacco, and health trial. Contemp. Clin. Trials 2019, 82, 25–35. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Tufman, A.; Huber, R.M. Biological markers in lung cancer: A clinician’s perspective. Cancer Biomark. Sect. A Dis. Markers 2010, 6, 123–135. [Google Scholar] [CrossRef]
- Sears, C.R.; Mazzone, P.J. Biomarkers in Lung Cancer. Clin. Chest Med. 2020, 41, 115–127. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Xue, X.; Asuquo, I.; Hong, L.; Gao, J.; Dong, Z.; Pang, L.; Jiang, T.; Meng, M.; Fan, J.; Wen, J.; et al. Catalog of Lung Cancer Gene Mutations Among Chinese Patients. Front. Oncol. 2020, 10, 1251. [Google Scholar] [CrossRef]
- Bryan, S.; Masoud, H.; Weir, H.K.; Woods, R.; Lockwood, G.; Smith, L.; Brierley, J.; Gospodarowicz, M.; Badets, N. Cancer in Canada: Stage at diagnosis. Health Rep. 2018, 29, 21–25. [Google Scholar]
- Campanella, A.; De Summa, S.; Tommasi, S. Exhaled breath condensate biomarkers for lung cancer. J. Breath Res. 2019, 13, 044002. [Google Scholar] [CrossRef]
- Yousefi, M.; Ghaffari, P.; Nosrati, R.; Dehghani, S.; Salmaninejad, A.; Abarghan, Y.J.; Ghaffari, S.H. Prognostic and therapeutic significance of circulating tumor cells in patients with lung cancer. Cell. Oncol. 2020, 43, 31–49. [Google Scholar] [CrossRef] [PubMed]
- NCI’s Genome Characterization Pipeline. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/genomic-pipeline#collection-processing. (accessed on 12 February 2021).
- Zhao, J.; Bao, W.; Cai, W. Immune Infiltration Landscape in Lung Squamous Cell Carcinoma Implications. Biomed. Res. Int. 2020, 2020, 5981870. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Yin, Z.; Xue, X.; Zhou, B. An eight-miRNA signature as a potential biomarker for predicting survival in lung adenocarcinoma. J. Transl. Med. 2014, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Sathipati, S.Y.; Ho, S.Y. Novel miRNA signature for predicting the stage of hepatocellular carcinoma. Sci. Rep. 2020, 10, 14452. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Qi, Y.; Gao, Z.; Lin, X. A new feature selection algorithm based on relevance, redundancy and complementarity. Comput. Biol. Med. 2020, 119, 103667. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, J.; Li, H.; Chen, H.; Li, F.; Liu, Q.; You, Z.H.; Zhou, F. Age Is Important for the Early-Stage Detection of Breast Cancer on Both Transcriptomic and Methylomic Biomarkers. Front. Genet. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.P.; Su, N.; Wang, L.; Huang, W.J.; Liu, Y.H.; Zhang, X.; Huang, H.Q.; Lin, T.Y.; Ma, S.Y.; Rao, H.L.; et al. A CpG Methylation Classifier to Predict Relapse in Adults with T-Cell Lymphoblastic Lymphoma. Clin. Cancer Res. 2020, 26, 3760–3770. [Google Scholar] [CrossRef]
- Kang, C.; Huo, Y.; Xin, L.; Tian, B.; Yu, B. Feature selection and tumor classification for microarray data using relaxed Lasso and generalized multi-class support vector machine. J. Theor. Biol. 2019, 463, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Ma, Z.L.; Liu, Z.Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Song, H.; Gao, R.; Yang, J.; Dong, W.; Dang, W. Classification of schizophrenia using general linear model and support vector machine via fNIRS. Phys. Eng. Sci. Med. 2020, 43, 1151–1160. [Google Scholar] [CrossRef]
- Giannini, V.; Rosati, S.; Defeudis, A.; Balestra, G.; Vassallo, L.; Cappello, G.; Mazzetti, S.; De Mattia, C.; Rizzetto, F.; Torresin, A.; et al. Radiomics predicts response of individual HER2-amplified colorectal cancer liver metastases in patients treated with HER2-targeted therapy. Int. J. Cancer 2020, 147, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- LaValley, M.P. Logistic regression. Circulation 2008, 117, 2395–2399. [Google Scholar] [CrossRef]
- Bonte, C.; Vercauteren, F. Privacy-preserving logistic regression training. BMC Med. Genom. 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar] [CrossRef]
- Sanz, H.; Valim, C.; Vegas, E.; Oller, J.M.; Reverter, F. SVM-RFE: Selection and visualization of the most relevant features through non-linear kernels. BMC Bioinform. 2018, 19, 432. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Mukherjee, D.P.; Das, P.; Gangopadhyay, A.; Chintha, A.R.; Kundu, S. Improved Random Forest for Classification. IEEE Trans. Image Process. 2018, 27, 4012–4024. [Google Scholar] [CrossRef]
- Zhang, P.B.; Yang, Z.X. A Novel AdaBoost Framework with Robust Threshold and Structural Optimization. IEEE Trans. Cybern. 2018, 48, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Chandak, T.; Mayginnes, J.P.; Mayes, H.; Wong, C.F. Using machine learning to improve ensemble docking for drug discovery. Proteins 2020, 88, 1263–1270. [Google Scholar] [CrossRef]
- Tejera, E.; Carrera, I.; Jimenes-Vargas, K.; Armijos-Jaramillo, V.; Sánchez-Rodríguez, A.; Cruz-Monteagudo, M.; Perez-Castillo, Y. Cell fishing: A similarity based approach and machine learning strategy for multiple cell lines-compound sensitivity prediction. PLoS ONE 2019, 14, e0223276. [Google Scholar] [CrossRef]
- Haenssle, H.A.; Winkler, J.K.; Fink, C.; Toberer, F.; Enk, A.; Stolz, W.; Deinlein, T.; Hofmann-Wellenhof, R.; Kittler, H.; Tschandl, P.; et al. Skin lesions of face and scalp—Classification by a market-approved convolutional neural network in comparison with 64 dermatologists. Eur. J. Cancer 2020, 144, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Csutak, C.; Ștefan, P.A.; Lupean, R.A.; Lenghel, L.M.; Mihu, C.M.; Lebovici, A. Computed tomography in the diagnosis of intraperitoneal effusions: The role of texture analysis. Bosn. J. Basic Med. Sci. 2020, 1–7. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-specificity in lung cancer risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.H.; Alam, F.; Alam, K.; Gow, J. Gender-specific differences in care-seeking behaviour among lung cancer patients: A systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.; Wirtz, H. Lung cancer: Current diagnosis and treatment. Dtsch. Arztebl. Int. 2009, 106, 809–820. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Cote, M.L. Epidemiology of Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 21–41. [Google Scholar] [CrossRef]
- de Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Manzione, L.; Shan, L.; King, J. Trends in Smoking Behaviors Among US Adolescent Cigarette Smokers. Pediatrics 2020, 145, e20193047. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef]
- Peters, S.; Kromhout, H.; Olsson, A.C.; Wichmann, H.E.; Brüske, I.; Consonni, D.; Landi, M.T.; Caporaso, N.; Siemiatycki, J.; Richiardi, L.; et al. Occupational exposure to organic dust increases lung cancer risk in the general population. Thorax 2012, 67, 111–116. [Google Scholar] [CrossRef]
- Suraya, A.; Nowak, D.; Sulistomo, A.W.; Icksan, A.G.; Berger, U.; Syahruddin, E.; O’Reilly, S.B. Excess Risk of Lung Cancer Among Agriculture and Construction Workers in Indonesia. Ann. Glob. Health 2021, 87, 8. [Google Scholar] [CrossRef]
- Eguchi, H.; Wada, K.; Prieto-Merino, D.; Smith, D.R. Lung, gastric and colorectal cancer mortality by occupation and industry among working-aged men in Japan. Sci. Rep. 2017, 7, 43204. [Google Scholar] [CrossRef]



| N0 | N1 | N2 | N3 | M1a | M1b | |
|---|---|---|---|---|---|---|
| T1 | I | II | III | III | IV | IV |
| T1a | I | II | III | III | IV | IV |
| T1b | I | II | III | III | IV | IV |
| T2 | I | II | III | III | IV | IV |
| T2a | I | II | III | III | IV | IV |
| T2b | II | II | III | III | IV | IV |
| T3 | II | III | III | III | IV | IV |
| T4 | III | III | III | III | IV | IV |
| Stage I | Stage II | Stage III | Stage IV | ||
|---|---|---|---|---|---|
| LUAD | Male | 113 | 66 | 38 | 14 |
| Female | 160 | 54 | 46 | 12 | |
| LUSC | Male | 175 | 122 | 64 | 6 |
| Female | 69 | 40 | 20 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, Y.; Duan, M.; Fan, Y.; Pan, X.; Liu, S.; Yu, Q.; Huang, L.; Zhou, F. Females and Males Show Differences in Early-Stage Transcriptomic Biomarkers of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Diagnostics 2021, 11, 347. https://doi.org/10.3390/diagnostics11020347
Liu Q, Wang Y, Duan M, Fan Y, Pan X, Liu S, Yu Q, Huang L, Zhou F. Females and Males Show Differences in Early-Stage Transcriptomic Biomarkers of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Diagnostics. 2021; 11(2):347. https://doi.org/10.3390/diagnostics11020347
Chicago/Turabian StyleLiu, Quewang, Yueying Wang, Meiyu Duan, Yusi Fan, Xingyuan Pan, Shuai Liu, Qiong Yu, Lan Huang, and Fengfeng Zhou. 2021. "Females and Males Show Differences in Early-Stage Transcriptomic Biomarkers of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma" Diagnostics 11, no. 2: 347. https://doi.org/10.3390/diagnostics11020347
APA StyleLiu, Q., Wang, Y., Duan, M., Fan, Y., Pan, X., Liu, S., Yu, Q., Huang, L., & Zhou, F. (2021). Females and Males Show Differences in Early-Stage Transcriptomic Biomarkers of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Diagnostics, 11(2), 347. https://doi.org/10.3390/diagnostics11020347







