Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment of Participants
2.2. Sample Size
2.3. Data Collection
2.4. Candidate miRNAs
2.5. Quantification of Expression Levels of Candidate miRNAs
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics, Laboratory Results, and Atherosclerosis Data of Participants
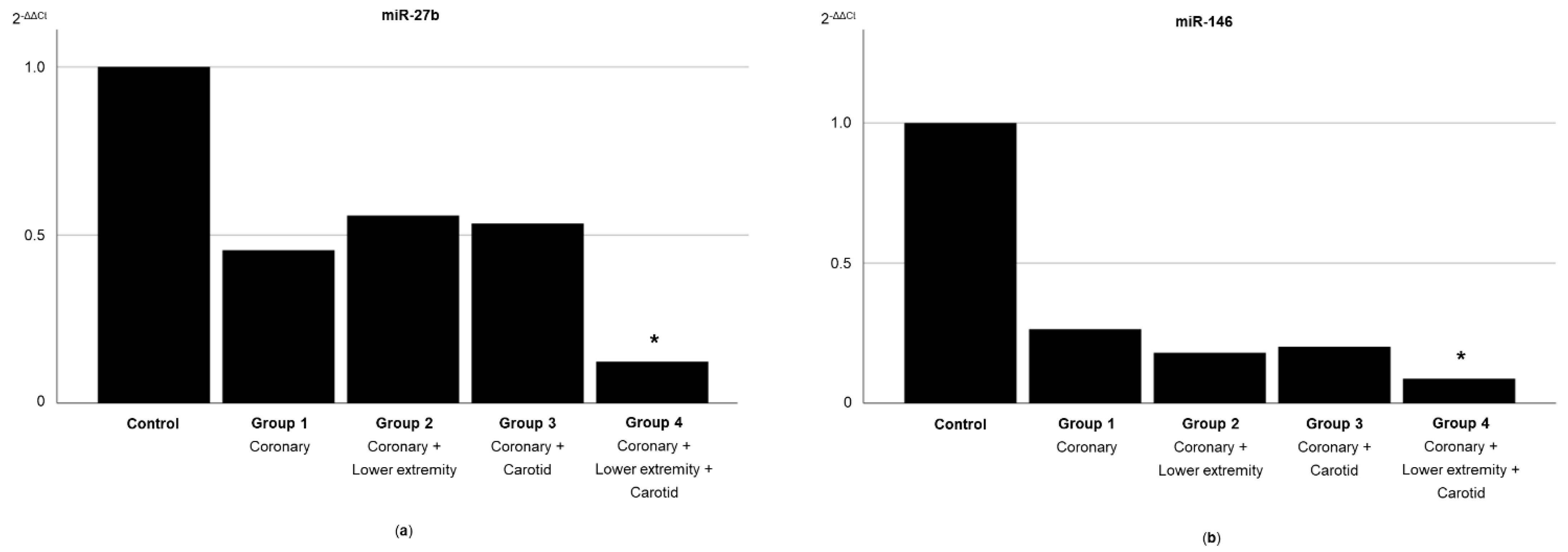
3.2. Expression of Circulating miRNAs According to the Systemic Extent of Atherosclerosis
3.3. Expression of Circulating miRNAs According to the Atherosclerosis Severity in Different Territories
3.4. Atherosclerosis Severity and miR-27b and miR-146 Expression Levels in Multivariate Analysis
3.5. miR-27b and miR-146 as Predictors of Polyvascular Atherosclerosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothwell, P.M. The Interrelation between carotid, femoral and coronary artery disease. Eur. Heart J. 2001, 22, 11–14. [Google Scholar] [CrossRef][Green Version]
- Fruchart, J.C.; Nierman, M.C.; Stroes, E.S.; Kastelein, J.J.; Duriez, P. New risk factors for atherosclerosis and patient risk assessment. Circulation 2004, 109, III15–III19. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, M.T. Genetic evaluation for coronary artery disease. Genet. Med. 2003, 5, 269–285. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Coutinho Cruz, M.; Carrusca, C.; Cruz Ferreira, R.; Napoleão, P.; Mota Carmo, M. Circulating microRNA profiles in different arterial territories of stable atherosclerotic disease: A systematic review. Am. J. Cardiovasc. Dis. 2018, 8, 1–13. [Google Scholar]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef]
- Alberts, M.J.; Bhatt, D.L.; Mas, J.L.; Ohman, E.M.; Hirsch, A.T.; Röther, J.; Salette, G.; Goto, S.; Smith, S.C.; Liau, C.S.; et al. Three-year follow-up and event rates in the international Reduction of Atherothrombosis for Continued Health Registry. Eur. Heart J. 2009, 30, 2318–2326. [Google Scholar] [CrossRef]
- Hwang, J.Y. Doppler ultrasonography of the lower extremity arteries: Anatomy and scanning guidelines. Ultrasonography 2017, 36, 111–119. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef]
- Collins, R.; Burch, J.; Cranny, G.; Aguiar-Ibáñez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: Systematic review. BMJ 2007, 334, 1257. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.A.; Kappetein, A.P.; Morice, M.C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Nylaende, M.; Kroese, A.; Stranden, E.; Morken, B.; Sandbaek, G.; Lindahl, A.K.; Arnesen, H.; Seljeflot, I. Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc. Med. 2006, 11, 21–28. [Google Scholar] [CrossRef]
- Andreou, I.; Sun, X.; Stone, P.H.; Edelman, E.R.; Feinberg, M.W. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol. Med. 2015, 21, 307–318. [Google Scholar] [CrossRef]
- Chen, L.J.; Lim, S.H.; Yeh, Y.T.; Lien, S.C.; Chiu, J.J. Roles of microRNAs in atherosclerosis and restenosis. J. Biomed. Sci. 2012, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Deo, A.; Carlsson, J.; Lindlöf, A. How to choose a normalization strategy for miRNA quantitative real-time (qPCR) arrays. J. Bioinform. Comput. Biol. 2011, 9, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Wolfinger, R.D.; Beedanagari, S.; Boitier, E.; Chen, T.; Couttet, P.; Ellinger-Ziegelbauer, H.; Guillemain, G.; Mariet, C.; Mouritzen, P.; O’Lone, R.; et al. Two approaches for estimating the lower limit of quantitation (LLOQ) of microRNA levels assayed as exploratory biomarkers by RT-qPCR. BMC Biotechnol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, S.; Geng, H.; Yu, Y.; Wang, C.; Liu, Z.; Yu, C.; Jiang, X.; Deng, Y.; Gao, L.; et al. Expression profiles of six circulating microRNAs critical to atherosclerosis in patients with subclinical hypothyroidism: A clinical study. J. Clin. Endocrinol. Metab. 2014, 99, E766–E774. [Google Scholar] [CrossRef]
- Kumar, D.; Narang, R.; Sreenivas, V.; Rastogi, V.; Bhatia, J.; Saluja, D.; Srivastava, K. Circulatory miR-133b and miR-21 as Novel Biomarkers in Early Prediction and Diagnosis of Coronary Artery Disease. Genes 2020, 11, 164. [Google Scholar] [CrossRef]
- Vegter, E.L.; Ovchinnikova, E.S.; van Veldhuisen, D.J.; Jaarsma, T.; Berezikov, E.; van der Meer, P.; Voors, A.A. Low circulating microRNA levels in heart failure patients are associated with atherosclerotic disease and cardiovascular-related rehospitalizations. Clin. Res. Cardiol. 2017, 106, 598–609. [Google Scholar] [CrossRef][Green Version]
- Stather, P.W.; Sylvius, N.; Sidloff, D.A.; Dattani, N.; Verissimo, A.; Wild, J.B.; Butt, H.Z.; Choke, E.; Sayers, R.D.; Bown, M.J. Identification of microRNAs associated with abdominal aortic aneurysms and peripheral arterial disease. Br. J. Surg. 2015, 102, 755–766. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Li, J.; Chen, J.Y.; Zhou, Y.L.; Cai, A.P.; Huang, C.; Feng, Y.Q. The Association of Circulating MiR-29b and Interleukin-6 with Subclinical Atherosclerosis. Cell. Physiol. Biochem. 2017, 44, 1537–1544. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Santulli, G.; Pascale, V.; Finelli, R.; Visco, V.; Giannotti, R.; Massari, A.; Morisco, C.; Ciccarelli, M.; Illario, M.; Iaccarino, G.; et al. We are What We Eat: Impact of Food from Short Supply Chain on Metabolic Syndrome. J. Clin. Med. 2019, 8, 61. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. The choice of methods in determining the optimal cut-off value for quantitative diagnostic test evaluation. Stat. Methods Med. Res. 2018, 27, 2374–2383. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Das, S. MicroRNAs in heart failure: Is the picture becoming less miRky? Circ. Heart Fail. 2014, 7, 203–214. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Aday, A.W.; Patel, M.R.; Jones, W.S. Polyvascular Disease: Reappraisal of the Current Clinical Landscape. Circ. Cardiovasc. Interv. 2019, 12, e007385. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; Steg, P.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.C.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients With Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728. [Google Scholar] [CrossRef]
- Anand, S.S.; Eikelboom, J.W.; Dyal, L.; Bosch, J.; Neumann, C.; Widimsky, P.; Avezum, A.A.; Probstfield, J.; Cook Bruns, N.; Fox, K.A.A.; et al. Rivaroxaban Plus Aspirin Versus Aspirin in Relation to Vascular Risk in the COMPASS Trial. J. Am. Coll. Cardiol. 2019, 73, 3271–3280. [Google Scholar] [CrossRef]
- Franzone, A.; Piccolo, R.; Gargiulo, G.; Ariotti, S.; Marino, M.; Santucci, A.; Baldo, A.; Magnani, G.; Moschovitis, A.; Windecker, S.; et al. Prolonged vs Short Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With or Without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 795–803. [Google Scholar] [CrossRef]
- Jukema, J.W.; Szarek, M.; Zijlstra, L.E.; de Silva, H.A.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; et al. Alirocumab in Patients With Polyvascular Disease and Recent Acute Coronary Syndrome: ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 74, 1167–1176. [Google Scholar] [CrossRef]
- Xie, W.; Li, L.; Zhang, M.; Cheng, H.P.; Gong, D.; Lv, Y.C.; Yao, F.; He, P.P.; Ouyang, X.P.; Lan, G.; et al. MicroRNA-27 Prevents Atherosclerosis by Suppressing Lipoprotein Lipase-Induced Lipid Accumulation and Inflammatory Response in Apolipoprotein E Knockout Mice. PLoS ONE 2016, 11, e0157085. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef]
- Liang, S.; Song, Z.; Wu, Y.; Gao, Y.; Gao, M.; Liu, F.; Wang, F.; Zhang, Y. MicroRNA-27b Modulates Inflammatory Response and Apoptosis during. J. Immunol. 2018, 200, 3506–3518. [Google Scholar] [CrossRef]
- Huang, K.D.; Shen, Y.; Wei, X.; Zhang, F.Q.; Liu, Y.Y.; Ma, L. Inhibitory effect of microRNA-27b on interleukin 17 (IL-17)-induced monocyte chemoattractant protein-1 (MCP1) expression. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Veliceasa, D.; Biyashev, D.; Qin, G.; Misener, S.; Mackie, A.R.; Kishore, R.; Volpert, O.V. Therapeutic manipulation of angiogenesis with miR-27b. Vasc. Cell 2015, 7, 6. [Google Scholar] [CrossRef]
- Boon, R.A.; Hergenreider, E.; Dimmeler, S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb. Haemost. 2012, 108, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ching, D.; Luk, F.S.; Raffai, R.L. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ. Res. 2015, 117, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Olejníčková, V.; Tkáčová, N.; Santulli, G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv. Exp. Med. Biol. 2015, 887, 79–100. [Google Scholar] [CrossRef]
- Infante-Menéndez, J.; López-Pastor, A.R.; González-López, P.; Gómez-Hernández, A.; Escribano, O. The Interplay between Oxidative Stress and miRNAs in Obesity-Associated Hepatic and Vascular Complications. Antioxidants 2020, 9, 607. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Sun, X.; Belkin, N.; Feinberg, M.W. Endothelial microRNAs and atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 372. [Google Scholar] [CrossRef]
- Santulli, G. MicroRNAs and Endothelial (Dys) Function. J. Cell. Physiol. 2016, 231, 1638–1644. [Google Scholar] [CrossRef]
- Stather, P.W.; Sylvius, N.; Wild, J.B.; Choke, E.; Sayers, R.D.; Bown, M.J. Differential microRNA expression profiles in peripheral arterial disease. Circ. Cardiovasc. Genet. 2013, 6, 490–497. [Google Scholar] [CrossRef]
- Takahashi, Y.; Satoh, M.; Minami, Y.; Tabuchi, T.; Itoh, T.; Nakamura, M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin. Sci. (Lond) 2010, 119, 395–405. [Google Scholar] [CrossRef]
- Rizzacasa, B.; Morini, E.; Mango, R.; Vancheri, C.; Budassi, S.; Massaro, G.; Maletta, S.; Macrini, M.; D’Annibale, S.; Romeo, F.; et al. MiR-423 is differentially expressed in patients with stable and unstable coronary artery disease: A pilot study. PLoS ONE 2019, 14, e0216363. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Y.; Song, D.; Liu, B. Reduced Plasma miR-146a Is a Predictor of Poor Coronary Collateral Circulation in Patients with Coronary Artery Disease. Biomed. Res. Int. 2016, 2016, 4285942. [Google Scholar] [CrossRef]
- Calais, F.; Eriksson Östman, M.; Hedberg, P.; Rosenblad, A.; Leppert, J.; Fröbert, O. Incremental prognostic value of coronary and systemic atherosclerosis after myocardial infarction. Int. J. Cardiol. 2018, 261, 6–11. [Google Scholar] [CrossRef]

| Study Group | Control | Group 1 | Group 2 | Group 3 | Group 4 | p-Value |
|---|---|---|---|---|---|---|
| Territories of atherosclerosis | None | Coronary | Coronary + LE | Coronary + Carotid | Coronary + LE + Carotid | |
| n | 26 | 20 | 18 | 12 | 18 | |
| Clinical Characteristics | ||||||
| Age, years | 59 (53–69) | 65 (56–70) | 67 (57–72) | 59 (51–73) | 69 (60–75) | 0.079 |
| Male, n (%) | 23 (88.5) | 18 (90.0) | 16 (88.9) | 10 (83.3) | 17 (94.4) | 0.912 |
| Hypertension, n (%) | 14 (53.8) | 17 (85.0) a | 18 (100.0) a | 11 (91.7) a | 18 (100.0) a | <0.001 |
| SBP, mmHg | 132 (14) | 130 (20) | 133 (21) | 135 (21) | 130 (18) | 0.939 |
| DBP, mmHg | 73 (10) | 72 (11) | 72 (11) | 73 (12) | 65 (9) | 0.175 |
| Dyslipidemia, n (%) | 18 (69.2) | 19 (95.0) a | 18 (100.0) a | 11 (91.7) | 17 (94.4) a | 0.010 |
| Diabetes mellitus, n (%) | 3 (11.5) | 6 (30.0) | 8 (44.4) a | 6 (50.0) a | 9 (50.0) a | 0.036 |
| Smoking history, n (%) | 6 (23.1) | 9 (45.0) | 12 (66.7) a | 4 (33.3) | 12 (66.7) a | 0.014 |
| LVEF > 50%, n (%) | 26 (100.0) | 20 (100.0) | 18 (100.0) | 12 (100.0) | 18 (100.0) | – |
| Antiplatelet therapy, n (%) | 6 (23.1) | 20 (100.0) a | 17 (94.4) a | 11 (91.7) a | 18 (100) a | <0.001 |
| Statin therapy, n (%) | 13 (50.0) | 18 (90.0) a | 16 (94.1) a | 11 (91.7) a | 16 (88.9) a | 0.001 |
| Laboratory Parameters | ||||||
| Hemoglobin, g/dL | 13.9 (12.9–15.0) | 14.53 (10.0–15.1) | 14.1 (13.2–14.6) | 12.0 (11.4–13.4) b | 12.9 (12.1–14.2) | 0.017 |
| Leukocyte count, 109/L | 6.4 (1.7) | 7.4 (1.9) | 7.3 (1.7) | 7.5 (2.2) | 8.1 (1.7) | 0.080 |
| Neutrophil count, 109/L | 3.2 (2.5–4.8) | 4.1 (3.4–5.2) | 3.9 (3.4–4.8) | 4.0 (3.4–6.7) | 4.7 (3.6–6.0) a | 0.043 |
| Lymphocyte count, 109/L | 1.9 (1.7–2.2) | 2.1 (1.6–2.4) | 2.1 (1.6–2.8) | 1.7 (1.2–2.3) | 2.2 (1.6–2.6) | 0.401 |
| Platelet count, 109/L | 242 (191–274) | 209 (176–269) | 219 (195–264) | 229 (137–251) | 227 (203–263) | 0.854 |
| Fasting glycaemia, mg/dL | 89 (80–98) | 94 (86–129) | 94 (83–125) | 99 (84–157) | 85 (75–123) | 0.385 |
| Percentage of glycosylated hemoglobin | 5.6 (5.2–5.9) | 5.9 (5.6–6.7) | 5.9 (5.5–6.1) | 5.8 (5.4–7.4) | 5.9 (5.3–7.7) | 0.185 |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 0.9 (0.8–1.1) | 0.8 (0.8–1.2) | 0.9 (0.8–1.4) | 1.1 (0.9–1.5) a | 0.002 |
| Total cholesterol, mg/dL | 186 (51) | 164 (38) | 172 (50) | 153 (50) | 173 (49) | 0.329 |
| LDL-cholesterol, mg/dL | 99 (77–141) | 95.0 (71–120) | 106 (83–120) | 65 (56–132) | 117 (82–142) | 0.297 |
| HDL-cholesterol, mg/dL | 51 (44–58) | 35 (31–41) a | 35 (31–45) a | 40 (27–44) a | 40 (32–42) a | <0.001 |
| Triglycerides, mg/dL | 106 (67–144) | 142 (98–206) | 115 (83–204) | 100 (62–177) | 117 (95–171) | 0.423 |
| C-reactive protein, mg/L | 4.1 (2.0) | 3.7 (1.4) | 3.8 (1.1) | 4.1 (2.3) | 3.3 (2.0) | 0.151 |
| Coronary Artery Disease | ||||||
| Nr. of vessels with obstructive disease * | 0 (0–0) | 3 (2–3) a | 3 (2–4) a | 3 (3–3) a | 3 (2–4) a | <0.001 |
| Nr. of lesions | 0 (0–0) | 4 (2–5) a | 4 (3–5) a | 4 (3–5) a | 4 (3–5) a | <0.001 |
| SYNTAX score | 0 (0–0) | 23.3 (8.4) a | 29.6 (9.9) a | 25.3 (7.2) a | 28.1 (10.6) a | <0.001 |
| Prior CABG, n (%) | 0 (0.0) | 7 (35.0) a | 7 (38.9) a | 6 (50.0) a | 3 (16.7) a | 0.002 |
| LE Arterial Disease | ||||||
| Bilateral disease, n (%) | 0 (0.0) | 0 (0.0) | 10 (55.5) a,b,d,e | 0 (0.0) | 15 (83.3) a–d | <0.001 |
| Nr. of segments with obstructive disease | 0 (0–0) | 0 (0–0) | 2 (1–4) a,b,d,e | 0 (0–0) | 4 (3–5) a–d | <0.001 |
| Prior bypass surgery, n (%) | 0 (0.0) | 0 (0.0) | 2 (11.1) a,b,d | 0 (0.0) | 6 (33.3) a,b,d | <0.001 |
| Carotid Artery Disease | ||||||
| Bilateral disease, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) a–c | 8 (44.4) a–c | <0.001 |
| Study Group | Control | Group 1 | Group 2 | Group 3 | Group 4 | p-Value |
|---|---|---|---|---|---|---|
| Territories of atherosclerosis | None | Coronary | Coronary + LE | Coronary + Carotid | Coronary + LE + Carotid | |
| miRNAs | ||||||
| miR-21 | 14.73 (4.63) | 15.60 (4.22) | 14.14 (4.41) | 13.83 (5.06) | 18.22 (4.01) | 0.064 |
| miR-27b | 17.82 (3.43) | 19.39 (4.06) | 19.03 (4.37) | 18.71 (5.45) | 22.34 (3.93) a | 0.041 |
| miR-29a | 20.90 (3.52) | 21.09 (2.02) | 20.67 (2.90) | 20.99 (5.06) | 23.50 (2.79) | 0.121 |
| miR-126 | 17.38 (15.12–21.89) | 17.79 (14.99–22.88) | 16.79 (15.57–22.74) | 14.85 (13.36–21.34) | 23.32 (17.88–24.86) | 0.102 |
| miR-146 | 18.06 (3.00) | 19.63 (3.34) | 19.43 (4.39) | 18.76 (4.21) | 22.20 (3.47) a | 0.048 |
| miR-218 | 22.63 (10.01–23.51) | 23.15 (16.18–26.12) | 22.63 (10.87–25.71) | 22.67 (9.19–23.36) | 24.70 (23.88–25.68) | 0.744 |
| β | 95% CI | p-Value | |
|---|---|---|---|
| ΔCt miR-27b | |||
| Coexistence of coronary, LE, and carotid atherosclerosis | 3.41 | 0.55–6.27 | 0.020 |
| SYNTAX score | 0.072 | 0.001–0.143 | 0.049 |
| ΔCt miR-146 | |||
| Coexistence of coronary, LE, and carotid atherosclerosis | 3.10 | 0.73–5.46 | 0.011 |
| Number of coronary artery lesions | 0.373 | 0.015–0.730 | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-da-Silva, T.; Napoleão, P.; Costa, M.C.; Gabriel, A.F.; Selas, M.; Silva, F.; Enguita, F.J.; Ferreira, R.C.; Carmo, M.M. Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146. Diagnostics 2021, 11, 318. https://doi.org/10.3390/diagnostics11020318
Pereira-da-Silva T, Napoleão P, Costa MC, Gabriel AF, Selas M, Silva F, Enguita FJ, Ferreira RC, Carmo MM. Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146. Diagnostics. 2021; 11(2):318. https://doi.org/10.3390/diagnostics11020318
Chicago/Turabian StylePereira-da-Silva, Tiago, Patrícia Napoleão, Marina C. Costa, André F. Gabriel, Mafalda Selas, Filipa Silva, Francisco J. Enguita, Rui Cruz Ferreira, and Miguel Mota Carmo. 2021. "Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146" Diagnostics 11, no. 2: 318. https://doi.org/10.3390/diagnostics11020318
APA StylePereira-da-Silva, T., Napoleão, P., Costa, M. C., Gabriel, A. F., Selas, M., Silva, F., Enguita, F. J., Ferreira, R. C., & Carmo, M. M. (2021). Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146. Diagnostics, 11(2), 318. https://doi.org/10.3390/diagnostics11020318









