1. Introduction
Congenital diaphragmatic hernia (CDH) is a developmental defect with the incidence in Europe estimated to be 2.3 per 10.000 births [
1]. Multiple embryonic tissue sources are involved in the development of a functional diaphragm forming blood vessels, muscle cells, and connective tissue [
2]. The tissues necessary for diaphragm development are mainly supported by four embryonic components: septum transversum, pleuro-peritoneal folds (PPF), dorsal mesentery of esophagus, and muscular ingrowths from lateral body walls. During prenatal development, these structures may fail to fuse thereby resulting in formation of CDH. The contents of the abdominal cavity herniate into the thoracic cavity through the defected diaphragm because of the pressure gradient between the cavities thus causing pulmonary hypoplasia, pulmonary hypertension, and heart failure resulting in high mortality rates.
CDH is an etiologically heterogeneous developmental defect. However, the genes involved in pathogenesis are mainly unknown, Alaggio et al. in their research state that the concerted action of GATA-4, Fog2, COUP-TFII and WT-1 [
3] is required for a proper development of the diaphragm. GATA-4, COUP-TFII, and WT-1 significance in CDH development is intertwined with the retinoic acid signaling pathway.
GATA-4 activation and expression are both influenced by retinoids.
COUP-TFII is a downstream target of retinoid signaling, and
WT-1 gene is responsible for encoding a zinc-finger-containing protein which is involved in the retinoic acid signaling pathway. The retinoic acid signaling pathway is crucial for normal pre- and post-natal development being involved in the specification and formation of many organs and tissues one of which is a proper diaphragm development [
4]. The transcription factors GATA-4, COUP-TFII and Fog2 are expressed in early development of PPFs [
5].
Although the morbidity and mortality rate of the patients with CDH is significant, the pathogenesis of CDH has been studied insufficiently due to the decreased accessibility of human pathological material. Growth factors and their receptors, muscle and nerve quality, local defense factor, programmed cell death, and separate gene markers could reveal their crucial role in the development of CDH.
Basic fibroblast growth factor (bFGF) manifests broad mitogenic and cell survival activities. It plays an important role in a variety of biologic processes including embryonic development, cell growth morphogenesis, tissue repair, tumor growth, survival, proliferation, apoptosis and invasion [
6]. bFGF influences mitosis by inhibiting phosphorylation of translation initiation factor, which is crucial for cell progression [
7]. bFGF can activate fibroblast growth factor receptor (FGFR) 1c, 1b, 2c, 3c and 4 thus initiating a tyrosine kinase signaling pathway that has a fundamental role in many biologic processes including embryonic development, tissue regeneration, and angiogenesis. It also plays important roles in diverse cell functions, including proliferation, differentiation, apoptosis and migration [
6,
8]. bFGF, via activation of FGFR1, is a highly potent inducer of angiogenesis. It can even be twice as potent as vascular endothelial growth factor (VEGF). It is hypothesized that there is a crosstalk between members of the VEGF family and bFGF during angiogenesis due to their synergetic effect [
9].
Transforming growth factor-beta (TGF-β) is a pleiotropic cytokine involved in development, various cellular responses and homeostasis of most human tissues [
10]. Activation of TGF-beta receptors affects gene expression through activation of SMAD family transcription factors, thus regulating cell proliferation, differentiation and growth and it can modulate expression and activation of other growth factors [
11,
12]. For example, TGF-β1 has a significant inhibitory effect on hepatocyte growth factor (HGF) mRNA expression; thus, the decrease in the fibroblast size leads to the up-regulation of the HGF expression [
13]. The production of numerous extracellular matrix proteins and inhibition of degrading of these proteins is stimulated by TGF-β [
10]. TGF-β influences angiogenesis by various mechanisms; for instance, by alternating two signaling cascades with opposite results (ALK1 and ALK5); thereby it is involved in vessel proliferation and maturation. TGF-β can also stimulate its own expression and control the expression of other angiogenic factors, such as platelet-derived growth factor, interleukine-1, bFGF, tumor necrosis factor alpha and transforming growth factor alpha [
14].
Insulin-like growth factor 1 (IGF-1) is an insulin-like protein with a similar function and structure involved in mediating growth and development [
15]. IGF-1 is the major mediator of prenatal and post-natal growth [
16]. IGF-1 exerts multiple physiological and pathophysiological effects on the vasculature including hypertrophic, survival, vasomotor and metabolic effects via its receptor (IGF-1R), stimulating smooth muscle cell proliferation, migration and inhibiting smooth muscle cell apoptosis through both endocrine and autocrine/paracrine mechanisms [
17,
18]. The effects of IGF-1 are mediated principally through IGF-1R, but they are modulated by complex interactions with multiple IGF binding proteins that are regulated by kinase activation, proteolysis, polymerization and the initiation of intracellular signaling via the AKT signaling pathway [
18]. The signaling mechanism of IGF-1/PI3-kinase/Akt plays an important role in controlling the angiogenic phenotype through the activation of angiogenic growth factors to induce autocrine PI3-kinase/Akt signaling in endothelial cells [
19].
Hepatocyte growth factor (HGF) is a central growth factor that modulates proliferation, angiogenesis, morphogenesis, motility and regulates cell growth [
13,
20]. HGF acts as a morphogen in embryonic development and promotes cell migration. Muscle cells migrate from somites into the PPFs in the presence of HGF. It is expressed along the migratory path of migratory muscle precursors, including the PPFs. The muscle precursors will then spread to all regions of mesenchymal cells as well as endothelium and non-hepatocyte liver cells [
21]. This protein also plays a role in angiogenesis, tumorigenesis and tissue regeneration [
22].
Furthermore, skeletal muscle fibers display distinct quality markers such as myosin, dystrophin, and alpha actin. We can speculate about loss of muscle mass, muscle atrophy as well as quality of muscle tissue through these factors.
Nerve growth factor (NGF) is expressed in developing and differentiating sympathetic and sensory neurons [
23]. NGF can bind to tyrosine kinase receptors (TrkA) inhibiting apoptosis and to p75 nerve growth factor receptors (p75NGFR) in which case it stimulates sensory neuronal cell growth and differentiation, but it might also trigger apoptosis [
23]. NGFR is also located at sites that do not have access to NGF [
24]. NGFR are found along the path of the neuronal cell migration from the neural tube to the PPFs and thus may lead to the outgrowth of the phrenic nerve [
2]. No effect of NGF on motor neurons is known [
25], although proNGF (the precursor form of NGF) promotes apoptosis of motor neurons by binding to p75NGFR [
26]. Myoblast cells produce p75NGFR during myogenesis. NGF is expressed adjacently to the developing muscle fibers. Once the myotubes have formed, NGF and p75NGFR cease to be expressed [
26].
Neurofilaments (NF) are a part of the mature neuronal cytoskeleton and the quality marker of the neuronal structures. They are essential in nervous system development, neuronal regeneration and electrical impulse transmission [
27].
Human beta defensins (β-defensin) are a part of an organism’s defense mechanism; therefore, in normal conditions they are not expressed in great amount, but their expression is triggered by inflammation, microbial pathogenicity factors, tissue damage, and other host factors. They are released primary by epithelial cells as well as by leukocytes, skeletal muscle, heart and other organs [
28]. According to the study of Baroni et al. β-defensin 2 can stimulate chemotaxis of human endothelial cells to a similar degree of VEGF [
29]. β-defensin 4 exhibits anti-microbial activity, but it does not respond to inflammatory markers: interleukin 6 (IL-6), interferon γ (IFNγ), tumor necrosis factor α (TNF-α) and IL-1. It is also expressed in neutrophils and it attracts monocytes as well [
30].
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assays are used to detect DNS fragmentation during apoptosis or sometimes DNA destruction from genotoxic agents [
31]. The labeling of free 3′-OH ends in DNA is catalyzed by the TdT enzyme to prevent the cross-linking of free ends [
31]. Nitrofen-exposed rat models show opposing results: some show that CDH has formed due to decreased cell proliferation but others voice an opinion that the increased apoptosis has been the cause of CDH [
32].
Wnt-1 is a wingless class signaling protein that induces a pathway, which leads to an increased expression of genes that are important in regulation of cell death, proliferation, migration and patterning in embryonic development by inhibiting or activating the frizzled receptors [
33]. During early organogenesis, genes associated with Wnt signaling in the diaphragm were shown to be preferentially expressed, and a few Wnt pathway members have been associated with CDH [
34].
Based on the mentioned above, our aim was the detection of appearance and distribution of different growth factors and their receptors, muscle and nerve quality factors, local defense factors, programmed cell death, and separate gene expression in the different diaphragm sites of diaphragm hernia patients and comparison of the results with the control group in order to find those changes that could lead to the morphopathogenetic mechanism in the development of CDH.
2. Materials and Methods
2.1. Materials Characteristics of Subjects
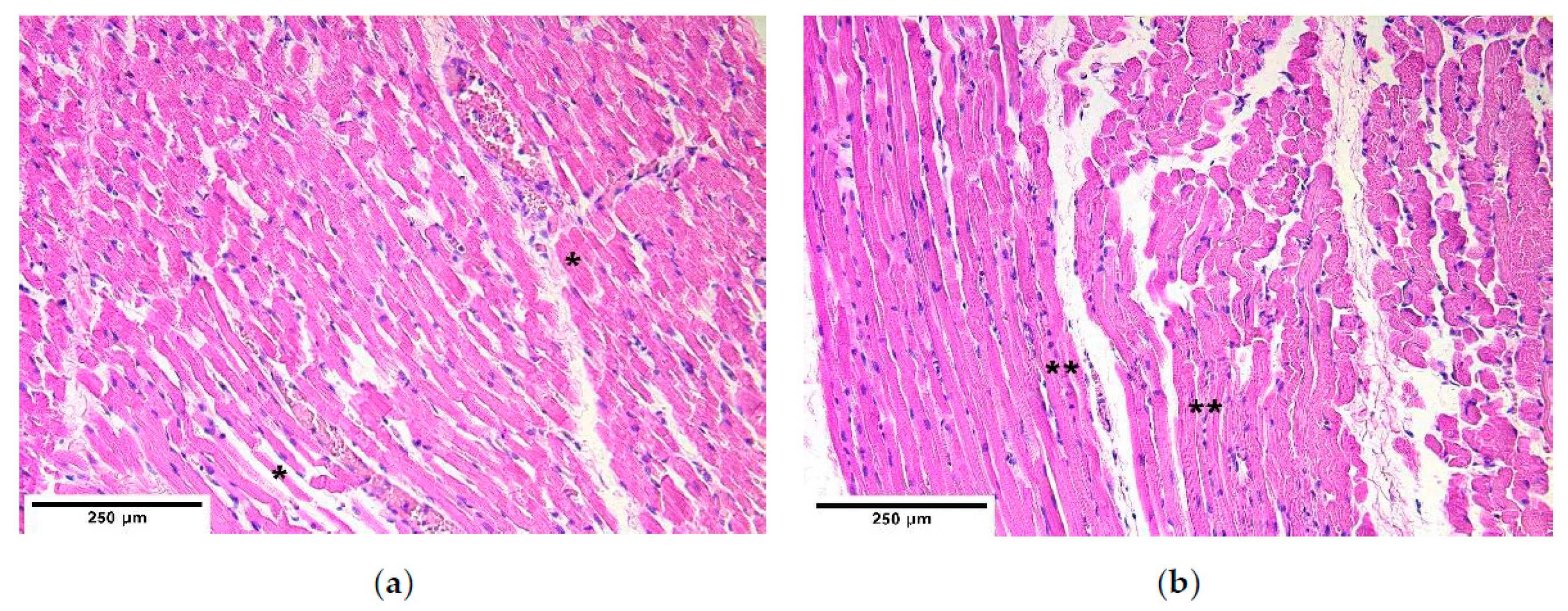
The research work was done in accordance with the Helsinki declaration. The study was approved by the Ethical Committee at Riga Stradins University, the permit was issued on 10 May 2007. The diaphragm material was obtained from 5 children (2 boys and 3 girls) age from 1 to 2 days. The patient group represented 4 children with CDH, and the control group represented the intact part of the diaphragm of a patient with CDH and one child without pathology in the diaphragm. All the examined patients’ hernias were localized posterolaterally, where the mean size of hernias was 4–6 × 2–3 cm. Four necropsies of CDH from the margin of diaphragm hernia and two control necropsies from 3 different diaphragm sites were obtained from neonates 12 h after their death at Children Clinical University Hospital. The localizations of diaphragm necropsy sites were proximal—near the longitudinal axis, distal—near the body wall, central—in between proximal and distal sites. The compiled material is the property of the Institute of Anatomy and Anthropology in Riga Stradins University.
2.2. Immunohistochemical Analysis
The samples were fixed in a mixture of 2% formaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.2) for 24 h. Then the samples were rinsed for 12 h in Tyrode buffer (content: NaCl, KCl, CaCl2 ·2H2O, MgCl2·6H2O, NaHCO3, NaH2PO4·H2O, glucose) containing 10% saccharose. Afterwards the samples were embedded into paraffin. Three micrometers thin sections had been cut; they were then stained with hematoxylin and eosin for routine morphological evaluation. For immunohistochemistry (IMH) Biotin-Streptavidin biochemical method was used to detect: TGF-β (orb77216, working dilution 1:100, Biorbyt Limited, Cambridge, UK), bFGF (ab16828, working dilution 1:200, Abcam, Cambridge, UK), FGFR1 (orb38277, working dilution 1:100, Biorbyt Limited, UK), IGF-1 (MAB291, working dilution 1:50, RD systems, McKinley Place NE, MN, USA), IGF-1R (AF-305-NA, working dilution 1:100, RD systems, USA), HGF (AF-294-NA, working dilution 1:200, RD systems, USA), NGF (ab6199, working dilution 1:500, abcam, UK), NGFR (sc-56448, working dilution 1:150, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), myosin (ab7784, working dilution 1:150, abcam, UK), dystrophin (ab15277, working dilution 1:100, abcam, UK), alpha actin (α-actin, HHF35, working dilution 1:100, Cell Marque Corporation, USA), wingless gene 1 (Wnt-1, ab15251, working dilution 1:100, abcam, UK), beta defensin 2 (ß-defensin 2, sc-20798, working dilution 1:100, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), beta defensin 4 (ß-defensin 4, ab14419, working dilution 1:150, abcam, UK), NF (2F11, working dilution 1:100, Cell Marque Corporation, USA). TUNEL, In Situ Cell Death Detection, POD (1684817, Roche, Indianapolis, IN, USA), DAB Substrate Vector Kit (SK4100, Vector Laboratories, Burlingame, CA, USA), was used to reveal apoptosis.
A semi-quantitative counting method was used for the evaluation of the tissues and structures in the stained slides. Tissue and morphological structures were graded by the appearance of positively stained cells in the visual field. The used designations were as follows: 0—no positive structures, 0/+: occasional positive structures, +: few positive structures, +/++: few to moderate number of positive structures, ++: moderate number of positive structures, ++/+++: moderate to numerous positive structures, +++: numerous positive structures, +++/++++: numerous to abundant structures, ++++: abundance of positive structures in the visual field [
35].
The stained slides were evaluated using Leica DC 300F digital camera and image processing and analysis software Image Pro Plus (Media Cybernetics, Inc., Rockville, MD, USA).
2.3. Statistical Analysis
The data processing was conducted using Statistical Package for the Social Sciences (SPSS) program version 20.0. The results from semi-quantitative evaluated tissue and structures were transformed into numerical form as follows: 0: equals to 0, 0/+: equals to 0.5, +: equals to 1, +/++: equals to 1.5, ++: equals to 2, ++/+++: equals to 2.5, +++: equals to 3, +++/++++: equals to 3.5, ++++: equals to 4. The test of normal distribution showed that data was not distributed normally, thus nonparametric statistics was used. Nonparametric Mann–Whitney U test was used to determine statistically significant differences between the patient and the control groups and Spearman’s rank correlation coefficient was calculated to detect correlations between the factors in the patient group, where r = 0–0.19 was assumed as a very weak correlation, r =0.2–0.39—weak correlation, r = 0.4–0.59—moderate correlation, r = 0.6–0.8—strong correlation and r = 0.8–1.0—a very strong correlation. For all the tests p-value was chosen 5% (p-value < 0.05) as statistically significant.
4. Discussion
Immunoreactive expression was compared between the patient and the control groups in different diaphragm sites of diaphragm hernia to determine the significance of different growth factors and their receptors, muscle and nerve quality factors, local defense factors, programmed cell death, and separate gene expression in the development of CDH. Histological structures and correlations between immunoreactives were also made.
Although the data showed slight alterations between different sites of diaphragm hernia, no significant changes were determined between these sites, showing either that the immunoreactives are distributed equally in different sites of the diaphragm or these changes are not possible to detect with the number of patients we had in our research.
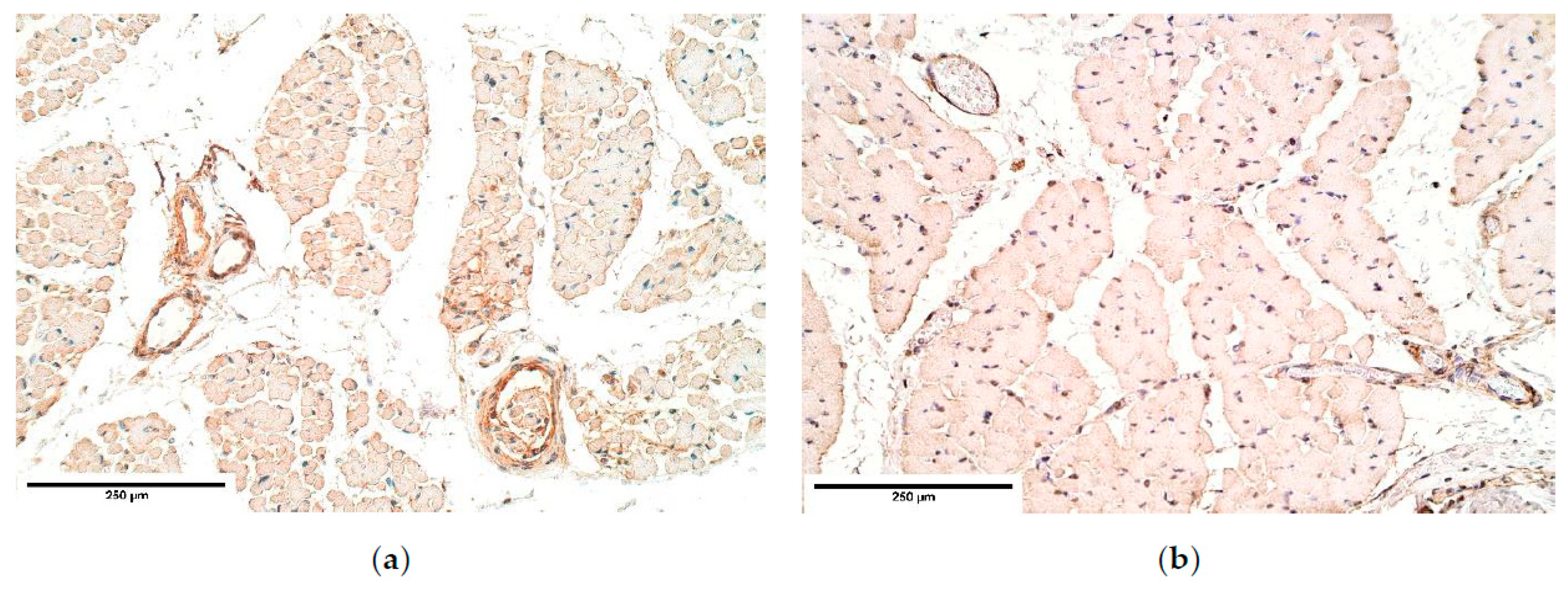
CDH patient muscle fibers showed variable expression of bFGF and IGF-1 growth factors, IGF-1 had the most notable fluctuations. FGFR1 also presented a variable expression in muscle fibers. Statistically significant changes in bFGF and FGFR expression were noted between the patient and the control group muscle fibers. These growth factors presented strong correlations between IGF-1 and bFGF, FGFR1 and IGF-1, bFGF and IGF-1R, showing that either these growth factors interact with each other or there is a pathway that makes alternations in their expression. For instance, one of such pathways is a retinoid acid pathway and alterations in this pathway are determined as a significant factor for the development of CDH [
36]. Furthermore, bFGF and IGF-1 expression can be affected by a retinoid acid pathway [
37,
38], thus we speculate that immunoreactive alternations in muscle fibers detected in our patients can be caused by alternations in the retinoid acid pathway.
Remarkably, bFGF had no expression in the control group diaphragm tissue whereas the CDH patient muscle fibers showed an unstable expression of bFGF. We assume this to be a complete novelty for this anomaly. A strong correlation between bFGF and FGFR was detected. It was found that with a higher expression of bFGF in the patient tissue, FGFR expression was lower compared to the control group, where bFGF was not expressed at all.
Muscle quality markers showed compelling data. Myosin and α-actin expression in the diaphragm muscle fibers had a stable expression; however, dystrophin presented a variable expression in both the patient and the control group diaphragm muscle fibers. We assume these relative changes in dystrophin expression could be due to the diaphragm development processes involving apoptosis in the muscle mass [
39].
Immunoreactive expression in blood vessels had numerous inconsistencies, showing significant changes in TGF-β, FGFR1, IGF-1R, NGF, β-defensin 2 and apoptosis between the patient and the control groups. We suppose these factors could play an important role in the pathogenesis of CDH through the development of blood vessels.
It should be noted that TGF-β signaling pathway is considered important for the proper diaphragm formation [
40]. TGF-β expression was slightly higher and had variations in CDH patients comparing the patient and the control group tissue; meanwhile the control group presented a stable expression, suggesting CDH patients could have TGF-β pathway alterations in blood vessels. Significance of the alternations in TGF-β pathway has also been proved by other scientists that studied the development of CDH [
36].
Strong intercorrelations in blood vessels were found between FGFR1, IGF-1, IGF-1R and Wnt-1 as well as significant changes in FGFR1, IGF-1R expression between the patient and the control group. As the activation of FGFR1 and IGF-1R receptors can lead to activation of PI3K/Akt pathway [
41,
42,
43], these strong intercorrelations with wnt-1 suggest the presence of synergism between the PI3K/Akt pathway and the wnt/β-catenin pathway. Such synergy was found in Skurk et al. research suggesting that VEGF/PI3-kinase/Akt signaling is a downstream of β-catenin, and that it contributes to the pro-angiogenic actions of β-catenin on endothelial cells [
43]. Therefore, fluctuations in Wnt-1 gene expression could cause the significant changes in FGFR1 and IGF-1R expression between the groups.
IGF-1R had a relatively high and stable expression in the diaphragm tissue, with alternations between the patient and the control groups only in blood vessels. This suggests that IGF-1R is not affected in other tissues in the presence of CDH. IGF-1R could be involved in CDH pathogenesis through angiogenetic processes as well as previously mentioned IGF-1R, FGFR1, Wnt pathway interactions.
The evaluation of β-defensin 2 expression revealed that it was higher in the control group than in the CDH patients. According to Baroni et al., research β-defensin 2 has properties to promote endothelial cell proliferation [
29]. Although the exact mechanism through which β-defensin 2 exerts its pro-angiogenetic properties is still unclear, it is clearly seen that the various cytokines involved in pro-angiogenetic processes are less expressed in CDH affected blood vessels than in the control group tissue. These findings further accent that there is a dysregulation in angiogenetic processes of the formation of CDH. Angiogenetic processes might have a crucial role in the pathogenesis of CDH.
However, the data shows various cytokines responsible for angiogenetic processes being affected, a more detailed research on the interactions between the above-mentioned pathways and growth factors should be carried out to confirm this theory.
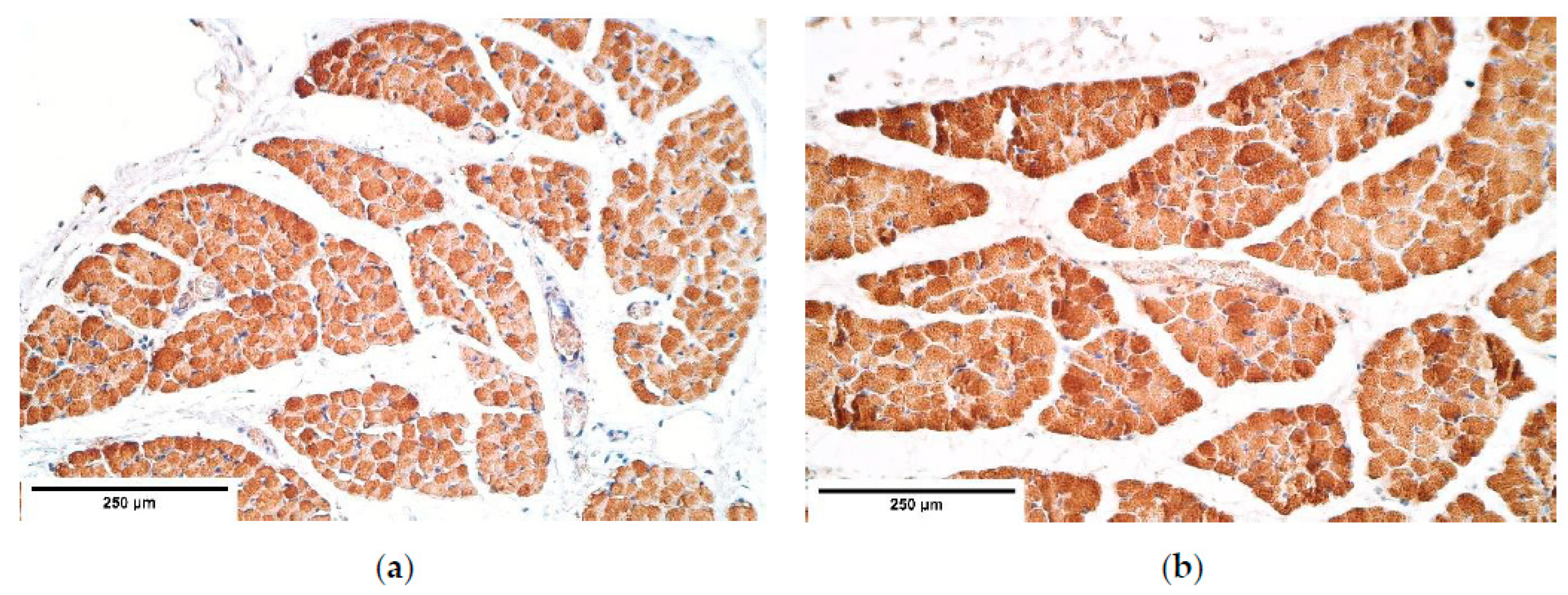
The immunoreactive expression was stable in connective tissue with an alteration between the patient and the control groups in HGF expression with a higher expression in the patient tissue. Overall HGF expression was stable and had a relatively high expression comparing it to other immunoreactives. The studies reveal that muscle cells migrate from somites into the PPFs [
21] in the presence of HGF. This shows that HGF should have a significant impact on CDH development; however, no statistically significant changes were found in other diaphragm tissues. We presume alternations in other tissues may be seen in embryonal development. As for an increased HGF expression in the connective tissue, it could be a compensatory mechanism after the development of CDH to increase the number of myocytes in areas where the hernia has not developed and to increase the overall strength of a diaphragm.
Mesothelium had very few alternations in the immunoreactive expression, showing that mesothelium is either slightly or not affected by CDH at all.
β-defensin 4 showed an absence of positive muscle fibers in the proximal and distal parts of the diaphragm and its expression fluctuated from absent to few positive structures in the central part. β-defensin 4 is an anti-microbial agent and its expression increases in response to inflammatory signals. It has been reported that the gene of the human β-defensin 4, called hBD-4, is limited to only a few tissues and the diaphragm is not one of them [
30]. The β-defensin levels coincide with the controls and the herniated diaphragms. We can see no bacterial infection therefore we can conclude that in normal condition β-defensin 4 is probably not expressed in diaphragm tissue. β-defensin 2 was expressed in both the patient group and their controls without significant differences. It provided fluctuating results in the CDH patient tissues. As β-defensin genes are close to chromosome 8p23.1, which is a hotspot with genes responsible for CDH formation [
44,
45], some deletions might include the defensin gene clusters, where variants in the non-deleted allele or total deletion of defensin coding gene might further influence defensin expression in tissues.
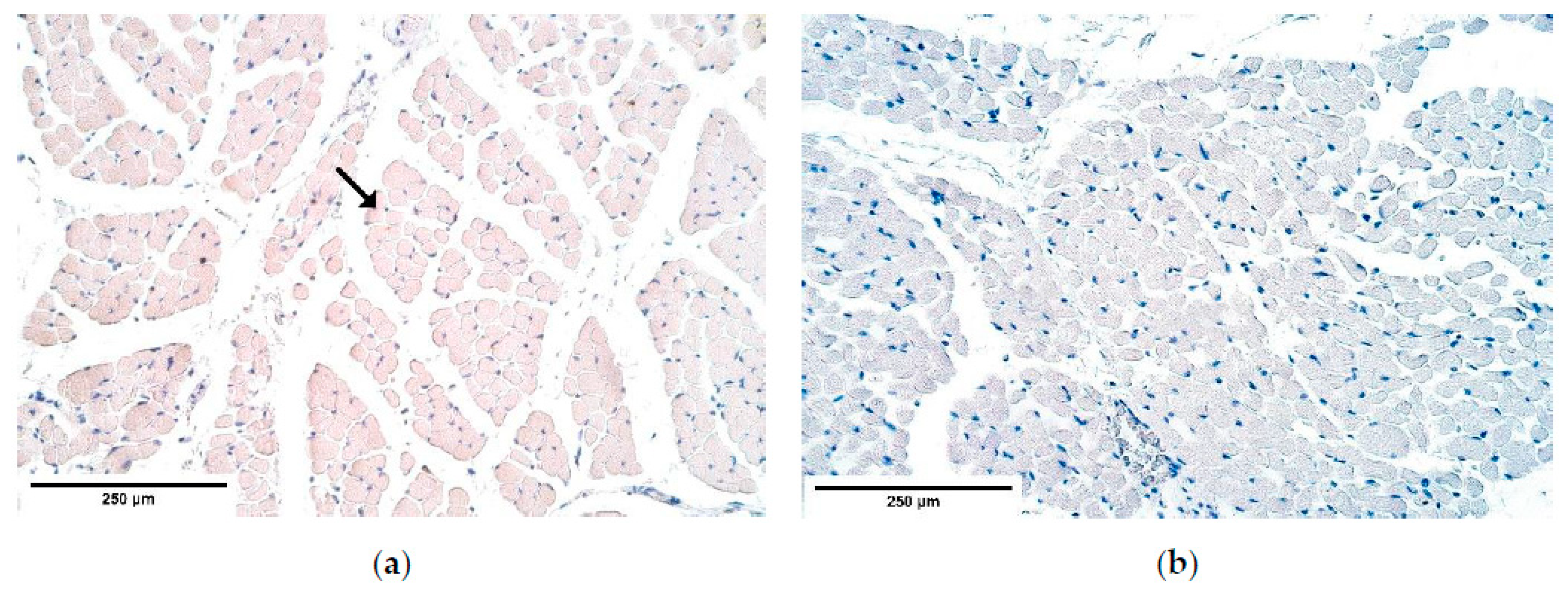
There are two existing hypotheses of how CDH occurs. Some investigators voice an opinion of an increased apoptosis [
46,
47], others—of a decreased cell proliferation [
32,
48] to be at fault of CDH development. Our findings contradict the increased cell death theory because the number of apoptotic cells in the patient tissue was the same or slightly decreased compared to the number of apoptotic cells in the control group. One of the herniated samples showed a few solitary apoptotic cells in the connective tissue, while all surrounding structures appeared negative showing that there was a high variability of cells affected by apoptosis in the patient tissue. Therefore, we can conclude that CDH can develop without elevated programmed cell death numbers. We can hypothesize that there are different pathways how CDH can be formed, through a high variability of apoptotic cell numbers in CDH patient tissue for instance.
The number of NGF and NGFR positive nerve fibers varied from one nerve bundle to another within each sample. It is notable that NGF and its receptor did not correlate with each other. Still, NGF remained higher in all samples. High NGF numbers have been reported to induce increased levels of matrix metalloproteases (MMPs). MMPs enhance skeletal muscle fiber regeneration by promoting satellite muscle cell migration and differentiation [
49]. In this context, MMP-1,-2,-7,-9,-13 have been emphasized [
49]. Consequently, NGF expression needs to be elevated in case of the diaphragm injury, but we see that it is not so. In addition, a theory has been proposed that NGF, in fact, inhibits the function of MMPs [
50]. Therefore, the decreased number of NGF positive structures could imply to either over-expression or under-expression of MMPs.
NGF is expressed in higher numbers than NGFR, which may suggest that a part of NGF attaches to the TrkA NGFR whose expression was not measured in this study. Other studies say that MMP-2 is activated exactly through TrkA NGFR stimulation, which promotes angiogenesis [
51]. In addition, VEGF levels have been found to respond to elevated NGF expression with an increase in numbers, further giving rise to neovascularization [
23,
52]. We noticed a decrease of NGF in blood vessel walls, which indicates that blood vessel formation in CDH might be impaired.
NGFR guides phrenic nerve growth from neural tube to PPFs. At the same time, p75NGFR when binding to NGF is capable of inducing apoptosis in fibroblasts and myofibroblasts [
53]. The marker’s expression is decreased in the nerve bundles and the blood vessel walls of the herniated diaphragm samples, which may have led to an increased fibroblast lifespan that decreased healing abilities of the diaphragmatic defects. This coincides with the reduced number of apoptotic cells visualized with TUNEL.
Wnt-1 demonstrated high expression variability in blood vessels and mesothelium. Its levels of signaling did not correlate with the cell death marker, which might be because Wnt-1 protects cells from apoptosis only when expressed in exceedingly high numbers as in cancer cells [
54]. Wnt-1 stabilizes β-catenin, whose loss of function in mouse models leads to diaphragm development defect [
34]. We, however, observed that Wnt-1 was up-regulated in most CDH patient endotheliocytes, mesotheliocytes, and muscle fibers, which might be a compensatory readjustment. One hypothesis is that elevated Wnt-1 numbers might be at fault of underdeveloped diaphragm tissue architecture. This explains why Wnt-1 levels in muscle fibers correlate with myosin, which is expressed only in differentiated muscle cells. However, this rise in Wnt-1 numbers alone most likely could not be responsible for CDH development. In Wilms Tumor 1 (Wt-1) knock-out mice models the diaphragms develop without CDH when β-catenin and, probably, Wnt-1 levels are kept elevated [
34]. The loss of Twist-related protein 1 (Twist1) positive cells is assumed to be a common factor why CDH develops in reduced Wt-1 and β-catenin pathway models [
34]. Both Wt-1 and Wnt-1 influence β-catenin signaling and Twist1 expression [
34,
55].
5. Conclusions
Different tissue factor appearance and distribution seem to correlate with the specific morphopathogenetic mechanism but do not depend on the specific site of affected diaphragm.
With a notable increase of bFGF, FGFR1 expression decreased in patient tissue (supported by a significant intercorrelation between these factors). This reveals a new pathogenetic phenotype for CDH, suggesting the prominent stimulation of growth in mesenchymal origin tissue.
The prominent and stable expression of IGF-1R and HGF and moderate expression of TGF-β show these factors as essential growth stimulators in CDH affected tissue. Intercorrelations between these factors and local defense factors (β-defensin 2, but not 4) and apoptosis indicate the leading role of tissue growth, anti-microbial defense, and programmed cell death in the morphopathogenesis of CDH.
Variable Wnt-1 expression in patient blood vessels, while being stable in controls and having strong intercorrelations with IGF-1, FGFR1, and IGF-1R suggests dysregulation in angiogenetic processes in herniated diaphragms.
The minor changes of muscle quality markers myosin and α-actin expression between the patient and the control groups are less likely to be involved in CDH pathogenesis, while the variable dystrophin expression in both the CDH patient and the controls seems to manifest itself more due to the diaphragm development processes involving apoptosis in muscle mass.
Only mesothelium presents insignificant alternations in different tissue factor expressions throughout the herniated diaphragm tissue, thus suggesting that CDH has almost no effect on mesotheliocytes.
Decreased NGF, NGFR and NF expression in CDH diaphragm indicates the possible decrease of neuronal structure quality in the pathogenesis of this anomaly.