Doppler Ultrasound Monitoring of Echogenicity in Asymptomatic Subcritical Carotid Stenosis and Assessment of Response to Oral Supplementation of Vitamin K2 (PLAK2 Randomized Controlled Trial)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Endpoint
2.4. Exclusion Criteria
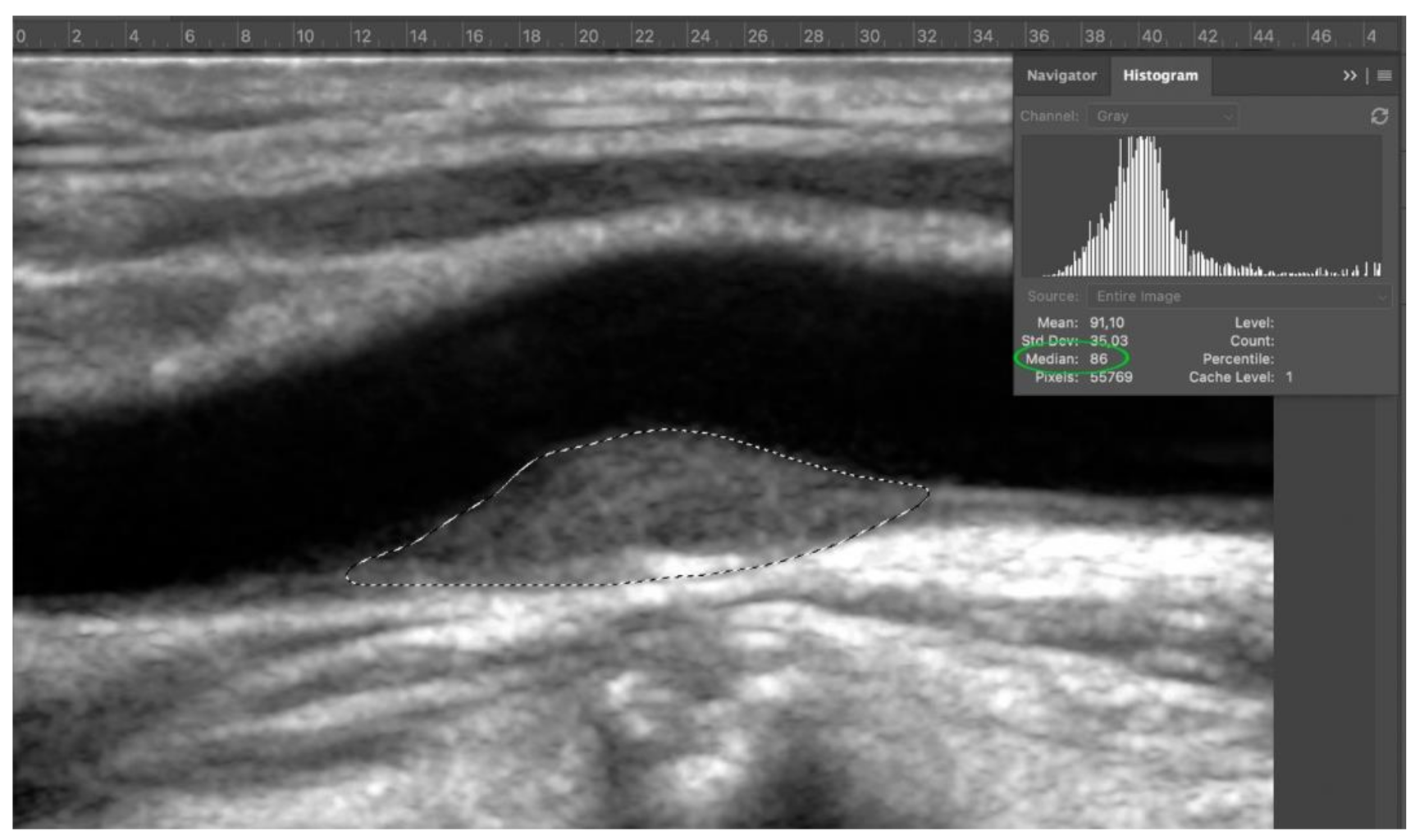
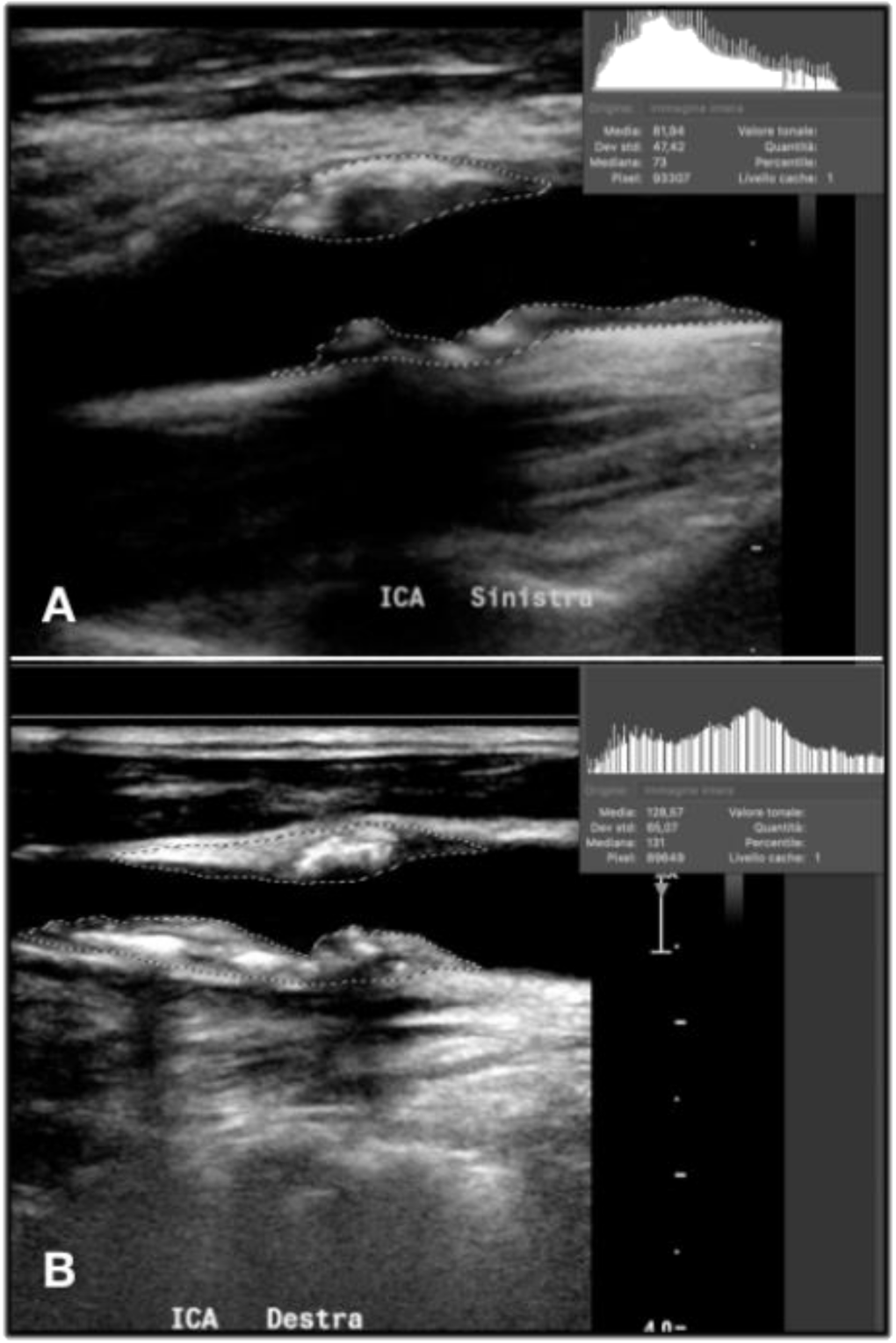
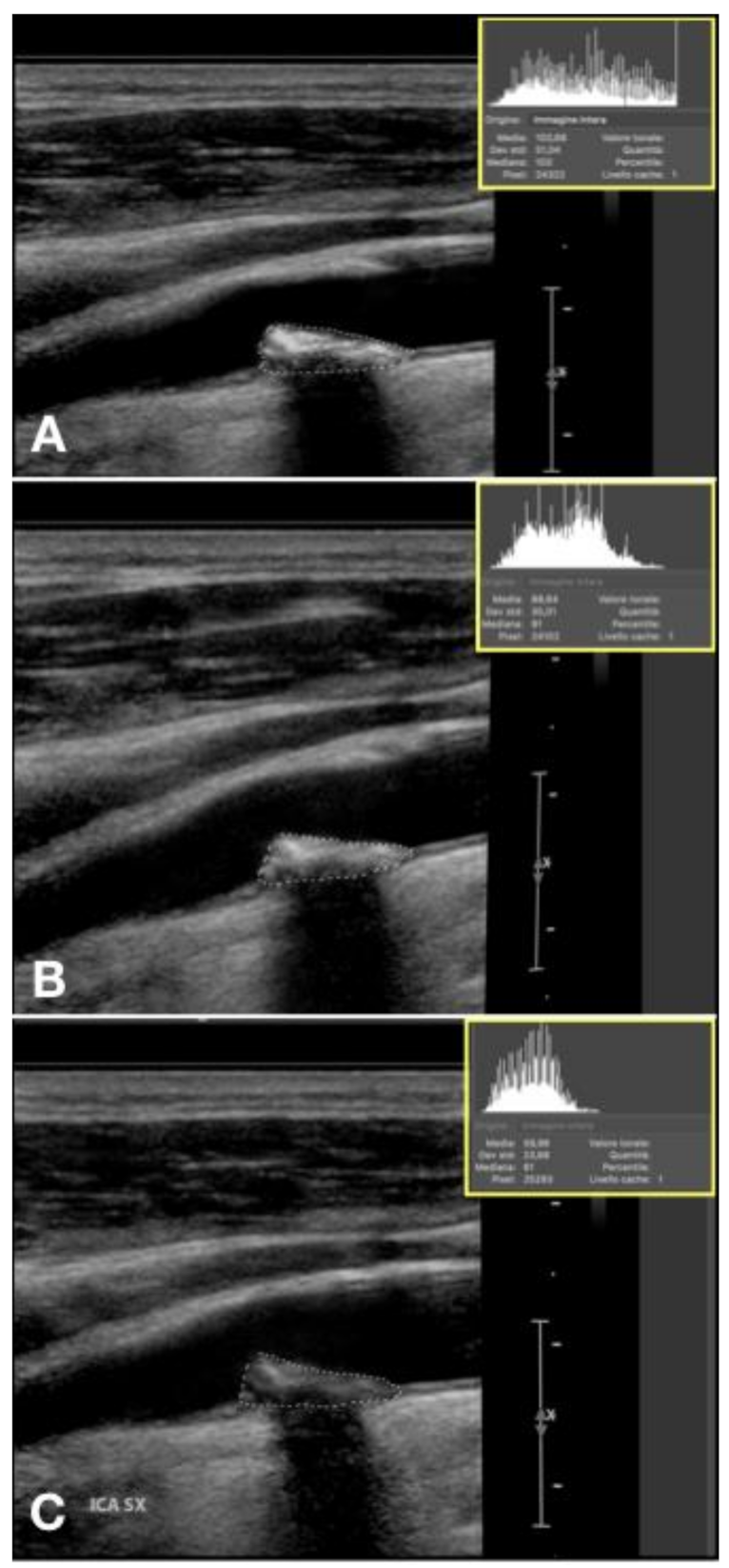
2.5. GSM Value
2.6. Doppler US Setting
2.7. Image Acquisition
2.7.1. Image Exportation and Analysis
2.7.2. Statistical Analyses
2.7.3. Case Report Form (CRF)
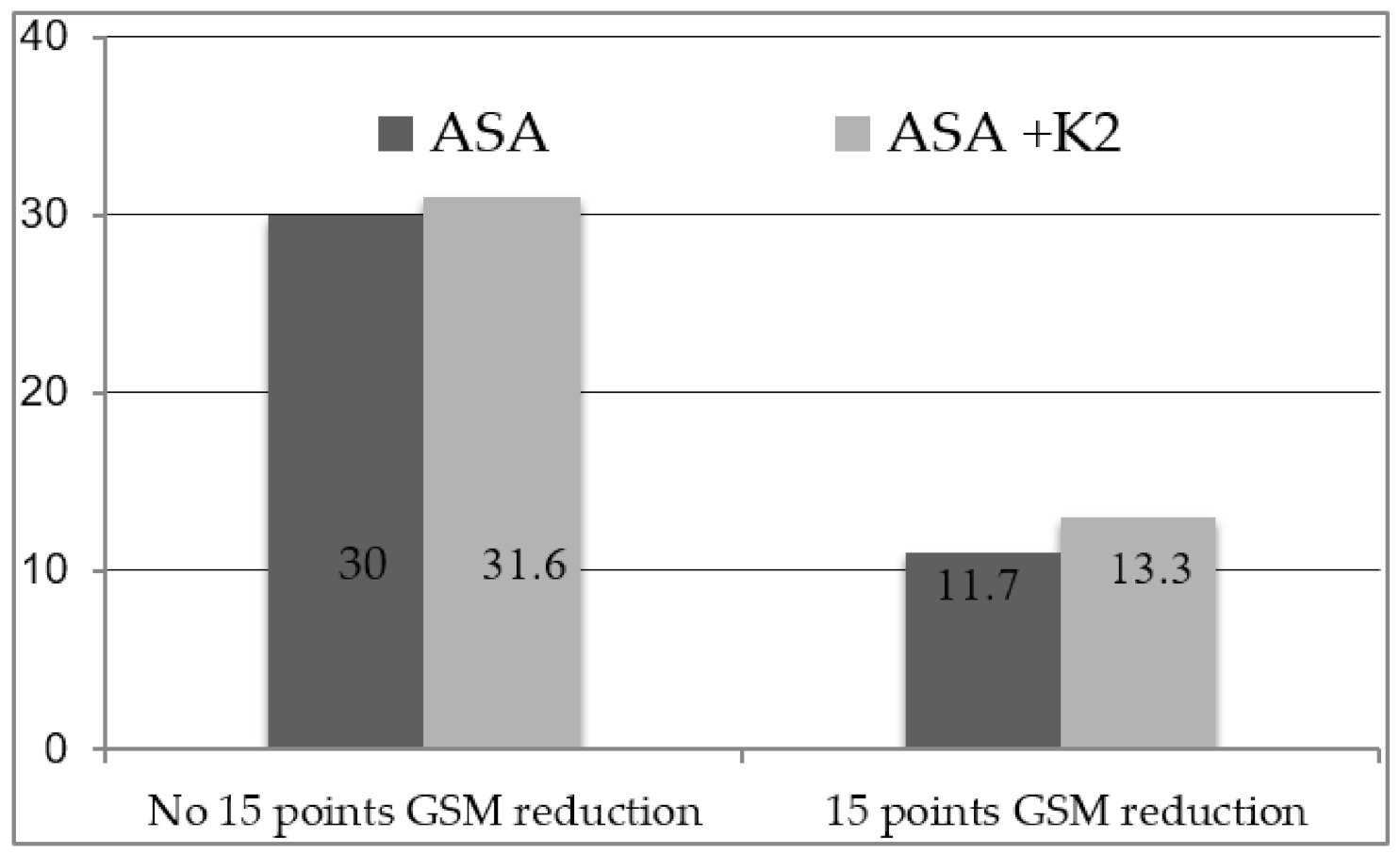
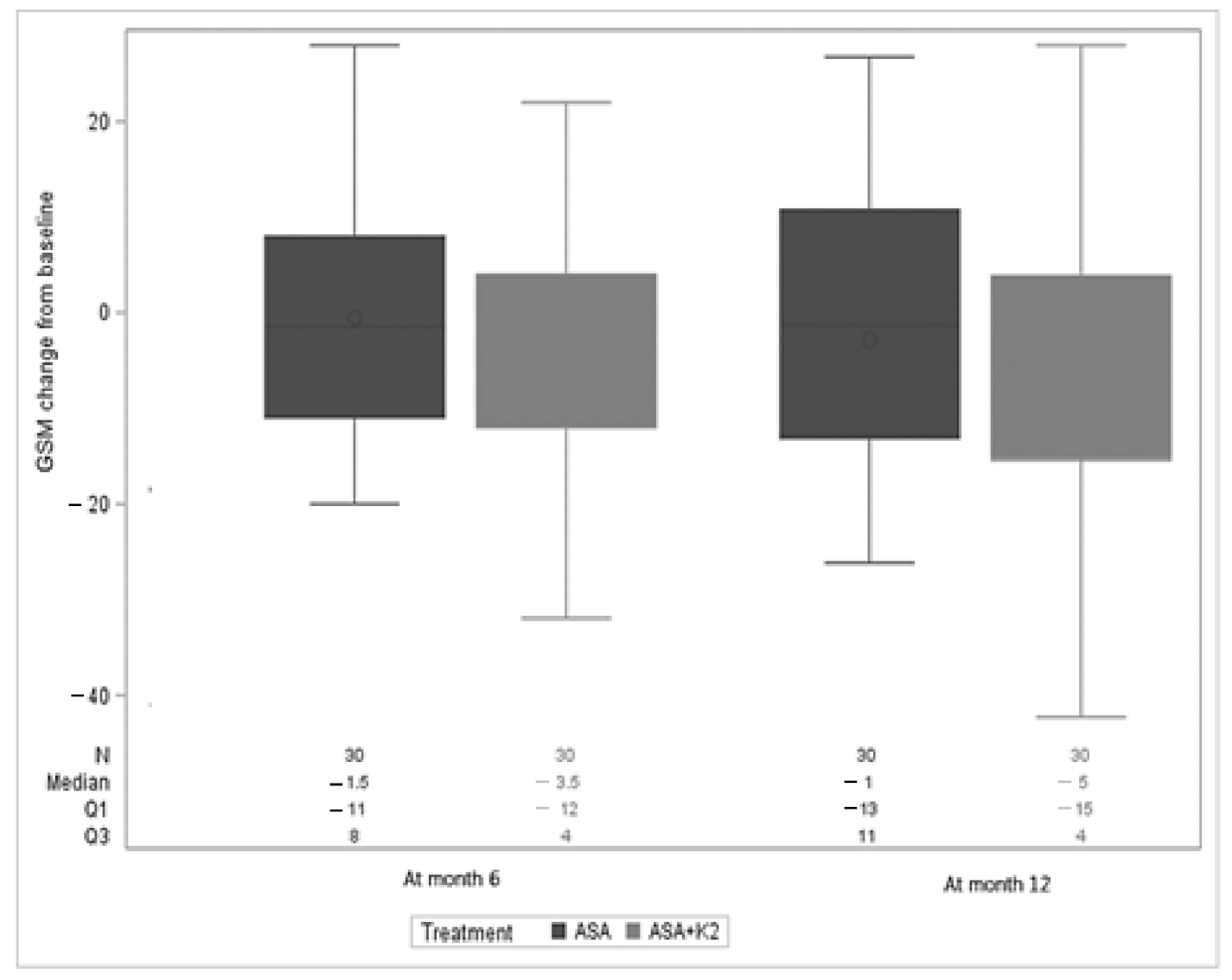
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shrikhande, G.; McKinsey, J. Choosing the appropriate intervention for symptomatic and asymptomatic carotid disease in the era of multiple therapies: Integration of risk profile and technical data. Semin. Vasc. Surg. 2011, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J. Vasc. Surg. 2010, 51, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Greenhalgh, R.; Davies, A. The symptomatic carotid plaque. Stroke 2000, 31, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Tzorbatzoglou, I.; Lazari, P.; Maras, D.; Giannoukas, A. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J. Vasc. Surg. 2018, 68, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.; Shaalan, W.; Cheng, H.; Gewertz, B.; Desai, T.; Schwarze, G.; Glagov, S.; Lozanski, L.; Griffin, A.; Castilla, M.; et al. B-mode ultrasonographic characterization of carotid atherosclerotic plaques in symptomatic and asymptomatic patients. J. Vasc. Surg. 2005, 42, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Sztajzel, R.; Momjian, S.; Momjian-Mayor, I.; Murith, N.; Djebaili, K.; Boissard, G.; Comelli, M.; Pizolatto, G. Stratified gray-scale median analysis and color mapping of the carotid plaque. Stroke 2005, 36, 741–745. [Google Scholar] [CrossRef]
- Salem, M.; Bown, M.; Sayers, R.; West, K.; Moore, D.; Nicolaides, A.; Robinson, T.; Naylor, A. Identification of patients with a histologically unstable carotid plaque using ultrasonic plaque image analysis. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 118–125. [Google Scholar] [CrossRef]
- Ibrahimi, P.; Jashari, F.; Bajraktari, G.; Wester, P.; Henein, M. Ultrasound assessment of carotid plaque echogenicity response to statin therapy: A systematic review and meta-analysis. Int. J. Mol. Sci. 2015, 16, 10734–10747. [Google Scholar] [CrossRef]
- Pini, R.; Faggioli, G.; Fittipaldi, S.; Vasuri, F.; Longhi, M.; Gallitto, E.; Pasquinelli, G.; Gargiulo, M.; Stella, A. Relationship between calcification and vulnerability of the carotid plaques. Ann. Vasc. Surg. 2017, 44, 336–342. [Google Scholar] [CrossRef]
- Chu, Z.; Cheng, L.; Tong, Q. Carotid artery calcification score and its association with cognitive impairment. Clin. Interv. Aging 2019, 14, 167–177. [Google Scholar] [CrossRef]
- Bos, D.; Portegies, M.; van der Lugt, A.; Bos, M.; Koudstaal, P.; Hofman, A.; Krestin, G.; Franco, O.; Vernooij, M.; Ikram, M. Intracranial carotid artery atherosclerosis and the risk of stroke in whites. JAMA Neurol. 2014, 71, 405. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.; Leening, M.; Kavousi, M.; Hofman, A.; Franco, O.; Lugt, A.; Vernooij, M.; Ikram, M. Comparison of atherosclerotic calcification in major vessel beds on the risk of all-cause and cause-specific mortality. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.; Reid, M. Timed appearance of a calcium-binding protein containing γ-carboxyglutamic acid in developing chick bone. Dev. Biol. 1978, 65, 426–434. [Google Scholar] [CrossRef]
- Cranenburg, E.; Schurgers, L.; Vermeer, C. Vitamin K: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.; Vermeer, C.; Schurgers, L. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar] [CrossRef]
- McCabe, K.; Booth, S.; Fu, X.; Shobeiri, N.; Pang, J.; Adams, M.; Holden, R. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013, 83, 835–844. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Intern. Med. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- Vissers, L.; Dalmeijer, G.; Boer, J.; Verschuren, W.; van der Schouw, Y.; Beulens, J. The relationship between vitamin K and peripheral arterial disease. Atherosclerosis 2016, 252, 15–20. [Google Scholar] [CrossRef]
- Oikonomaki, T.; Papasotiriou, M.; Ntrinias, T.; Kalogeropoulou, C.; Zabakis, P.; Kalavrizioti, D.; Papadakis, I.; Goumenos, D.; Papachristou, E. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int. Urol. Nephrol. 2019, 51, 2037–2044. [Google Scholar] [CrossRef]
- Nohara, R.; Daida, H.; Hata, M.; Kaku, K.; Kawamori, R.; Kishimoto, J.; Kurabayashi, M.; Masuda, I.; Sakuma, I.; Yamazaki, T.; et al. Effect of long-term intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness. Circ. J. 2013, 77, 1526–1533. [Google Scholar] [CrossRef]
- Nicolaides, A.N.; Kakkos, S.K.; Kyriacou, E.; Griffin, M.; Sabetai, M.; Thomas, D.J.; Tegos, T.; Geroulakos, G.; Labropoulos, N.; Doré, C.J.; et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J. Vasc. Surg. 2010, 52, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Biasi, G.M.; Froio, A.; Diethrich, E.B.; Deleo, G.; Galimberti, S.; Mingazzini, P.; Nicolaides, A.N.; Griffin, M.; Raithel, D.; Reid, D.B.; et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: The Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004, 110, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Biasi, G.M.; Sampaolo, A.; Mingazzini, P.; De Amicis, P.; El-Barghouty, N.; Nicolaides, A.N. Computer analysis of ultrasonic plaque echolucency in identifying high risk carotid bifurcation lesions. Neurol. Res. 2011, 33, 991–994. [Google Scholar] [CrossRef][Green Version]
- Sabetai, M.; Tegos, T.; Nicolaides, A.; Dhanjil, S.; Pare, G.; Stevens, J. Reproducibility of computer-quantified carotid plaque echogenicity. Stroke 2000, 31, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Ibrahimi, P.; Jashari, F.; Johansson, E.; Gronlund, C.; Bajraktari, G.; Wester, P.; Henein, M.Y. Vulnerable plaques in the contralateral carotid arteries in symptomatic patients: A detailed ultrasound analysis. Atherosclerosis 2014, 235, 526–531. [Google Scholar] [CrossRef]
- Denzel, C.; Lell, M.; Maak, M.; Höckl, M.; Balzer, K.; Müller, K.; Fellner, C.; Fellner, F.; Lang, W. Carotid artery calcium: Accuracy of a calcium score by computed tomography—An in vitro study with comparison to sonography and histology. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 214–220. [Google Scholar] [CrossRef]
- Kadoglou, N.; Sailer, N.; Moumtzouoglou, A.; Kapelouzou, A.; Fotiadis, G.; Vitta, I.; Kakisis, I.; Avgerinos, E.; Gerasimidis, T.; Karayannacos, P.L. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. J. Vasc. Surg. 2009, 49, S4–S5. [Google Scholar] [CrossRef]
- Della-Morte, D.; Moussa, I.; Elkind, M.; Sacco, R.; Rundek, T. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: A pilot study. Neurol. Res. 2011, 33, 991–994. [Google Scholar] [CrossRef]
- Kadoglou, N.; Gerasimidis, T.; Moumtzouoglou, A.; Kapelouzou, A.; Sailer, N.; Fotiadis, G.; Vitta, I.; Katinios, A.; Kougias, P.; Bandios, S.; et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 661–668. [Google Scholar] [CrossRef]
- Stenudd, I.; Sjödin, E.; Nyman, E.; Wester, P.; Johansson, E.; Grönlund, C. Ultrasound risk marker variability in symptomatic carotid plaque: Impact on risk reclassification and association with temporal variation pattern. Int. J. Cardiovasc. Imaging 2020, 36, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Smit, E.; Vermeer, C. The role of vitamin K in soft-tissue calcification. Adv. Nutr. 2012, 3, 166–173. [Google Scholar] [CrossRef] [PubMed]
- London, G. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.; Giachelli, C. Regulation of cardiovascular calcification. Cardiovasc. Pathol. 2004, 13, 63–70. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef]
- Sage, A.; Tintut, Y.; Demer, L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Sturek, M.; Alavi, A.; Gerke, O. Atherosclerosis imaging with 18F-sodium fluoride PET: State-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1538–1551. [Google Scholar] [CrossRef]
- Nagano, K.; Yamagami, H.; Tsukamoto, Y.; Nagatsuka, K.; Yasaka, M.; Nagata, I. Quantitative evaluation of carotid plaque echogenicity by integrated backscatter analysis: Correlation with symptomatic history and histologic findings. Cerebrovasc. Dis. 2008, 26, 578–583. [Google Scholar] [CrossRef]

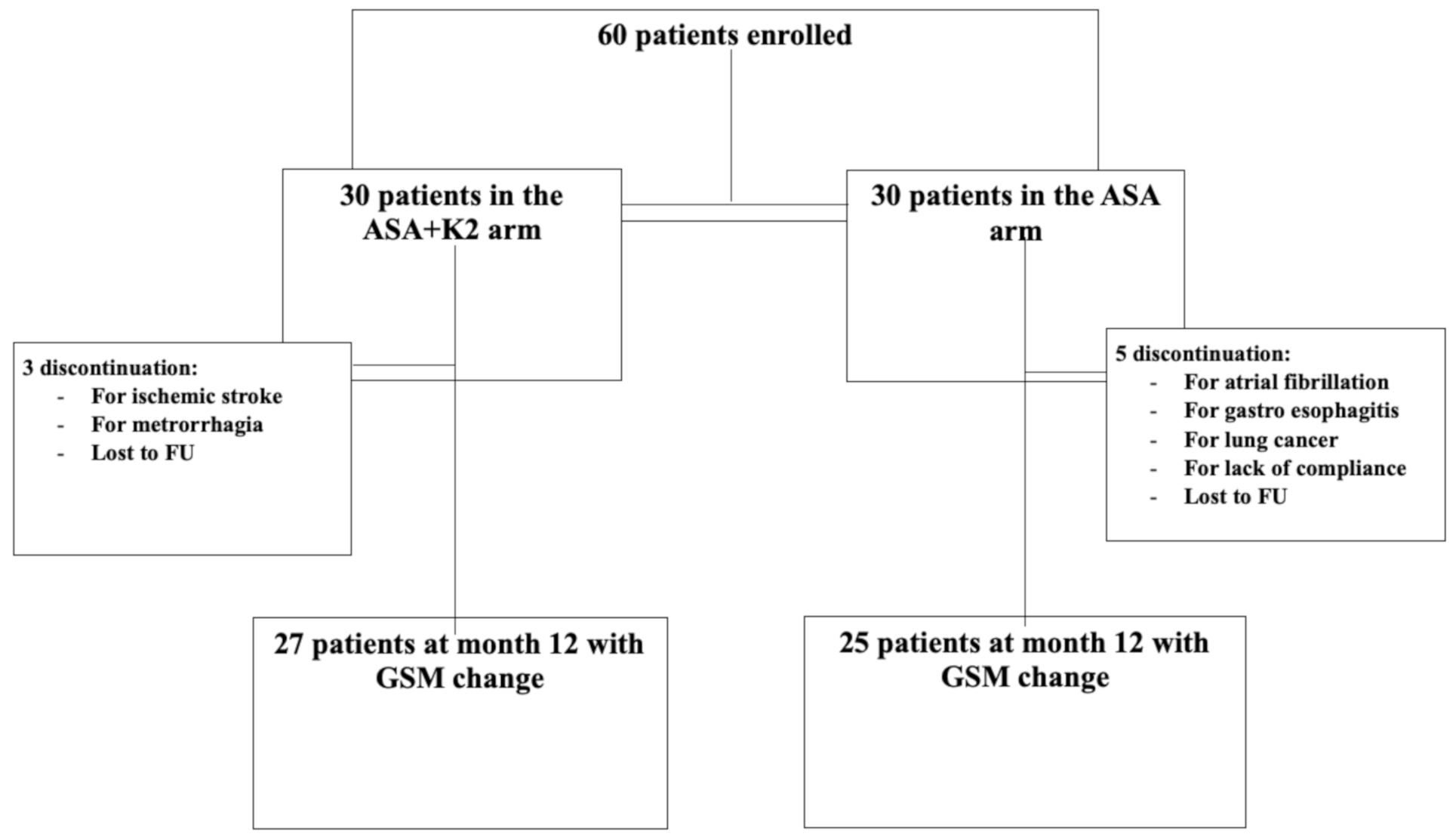
| Events | Arm | Time |
|---|---|---|
| Gastro-esophagitis | ASA | 2 weeks |
| Atrial fibrillation | ASA | 1 month |
| Ischemic stroke | ASA + K2 | 1 month |
| Carotid endarterectomy | ASA | 2 months |
| Lung cancer | ASA | 2 months |
| Metrorrhagia | ASA + K2 | 4 months |
| Percutaneous transluminal Coronary angioplasty | ASA + K2 | 9 months |
| Characteristics | Overall (n = 60) | ASA + K2 Arm (n = 30) | ASA Arm (n = 30) | p-Value |
|---|---|---|---|---|
| Age (years) | 62.5 (55–71) | 60 (53–68) | 65 (57–73) | 0.155 |
| Gender | 0.382 | |||
| Female | 16 (26.7%) | 6 (20%) | 10 (33.3%) | |
| Male | 44 (73.3%) | 24 (80%) | 20 (66.7%) | |
| Weight (Kg) | 74 (66–82.5) | 74 (66–82) | 76.5 (66–83) | 0.739 |
| Height (cm) | 172 (166–178) | 172 (167–176) | 172 (165–180) | 0.888 |
| Body Mass Index (Kg/m2) | 25.06 (23.24–27.44) | 25.11 (23.1–27.4) | 25.06 (23.44–28.34) | 0.802 |
| Smoke | 0.751 | |||
| Yes | 15 (25%) | 7 (23.4%) | 8 (26.7%) | |
| Former | 22 (36.7%) | 13 (43.3%) | 9 (30%) | |
| No | 23 (38.3%) | 10 (33.3%) | 13 (43.3%) | |
| Arterial Hypertension | 0.938 | |||
| Yes | 33 (55%) | 16 (53.4%) | 17 (56.7%) | |
| No | 27 (45%) | 14 (46.7%) | 13 (43.3%) | |
| Dyslipidemia | 0.999 | |||
| No | 29 (48.3%) | 15 (50%) | 14 (46.7%) | |
| Yes | 31 (51.7%) | 15 (50%) | 16 (53.3%) | |
| Use of dyslipidemia drugs | 0.389 | |||
| Statin | 54 (90%) | 28 (93.4%) | 26 (86.7%) | |
| Statin plus ezetimibe or omega3 fatty acid | 6 (10%) | 2 (6.6%) | 4 (13.3) | |
| Diabetes | 0.357 | |||
| Yes | 6 (1.7%) | 2 (6.7%) | 4 (13.3%) | |
| No | 54 (91.5%) | 28 (93.3%) | 26 (86.7%) | |
| Peripheral/coronary artery disease | 0.999 | |||
| No | 58 (96.7%) | 29 (96.7%) | 29 (96.7%) | |
| Yes | 2 (3.3%) | 1 (3.3%) | 1 (3.3%) | |
| GSM | 73.5 (62.5–100.5) | 71.5 (64–93) | 89 (55–113) | 0.315 |
| Stenosis (%) | 40 (40–50) | 40 (40–50) | 40 (40–50) | 0.628 |
| Characteristics | Crude Mean Change per Month in GSM from BL (95%CI) | p-Value | Adjusted Mean Change per Month in GSM from BL (95%CI) | p-Value |
|---|---|---|---|---|
| ASA + K2 arm | −0.46 (−0.99;0.07) p = 0.096 | 0.570 | −0.55 (−1.10; −0.01) p = 0.048 | 0.617 |
| ASA arm | −0.26 (−0.82;0.30) p = 0.362 | −0.18 (−0.75;0.39) p = 0.529 | ||
| Age (per year older) | 0.03 (−0.08;0.14) | 0.598 | 0.06 (−0.06;0.17) | 0.356 |
| BL GSM (per point higher) | 0.01 (−0.03;0.05) | 0.665 | 0.03 (−0.02;0.08) | 0.184 |
| Gender (Male versus Female) | −0.46 (−3.01;2.09) | 0.716 | −0.89 (−3.40;1.62) | 0.480 |
| Stenosis (per point increase) | 0.03 (−0.11;0.16) | 0.691 | 0.05 (−0.08;0.17) | 0.460 |
| Smoke (yes versus no) | 0.25 (−2.03;2.54) | 0.825 | 0.59 (−1.66;2.83) | 0.602 |
| BMI (per Kg/m2 increase) | 0.05 (−0.29;0.38) | 0.778 | 0.13 (−0.18;0.44) | 0.403 |
| Peripheral/coronary artery disease (yes versus no) | −4.89 (−10.66;0.88) | 0.095 | −7.2 (−12.91; −1.51) | 0.014 |
| BL plaque type (hyperechoic versus other) | 0.31 (−1.92;2.53) | 0.787 | −0.55 (−2.88;1.79) | 0.642 |
| Variable | GSM Reduction of 15 Points (n = 15) | No GSM Reduction of 15 Points (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 71 (53–76) | 61 (55–68) | 0.075 |
| Gender | 0.999 | ||
| Female | 4 (26.7%) | 12 (26.7%) | |
| Male | 11 (73.3%) | 33 (73.3%) | |
| Weight (Kg) | 73 (66–81) | 77 (67–83) | 0.417 |
| Height (cm) | 170 (166–180) | 172 (166–176) | 0.791 |
| Body Mass Index (Kg/m2) | 24.19 (23.38–25.3) | 25.4 (23.1–28.34) | 0.140 |
| GSM at baseline | 94 (82–113) | 68 (53–96) | 0.013 |
| Stenosis (%) | 40 (40–50) | 40 (40–50) | 0.787 |
| Characteristics | Crude 12 Month Mean Change in GSM from BL (95%CI) | p-Value | Adjusted 12 Month Mean Change in GSM from BL (95%CI) | p-Value |
|---|---|---|---|---|
| Arm (ASA + K2 versus ASA) | −2.77 (−11.20;5.66) | 0.514 | −7.56 (−16.63;1.51) | 0.100 |
| Age (per year older) | −0.23 (−0.65;0.20) | 0.295 | −0.36 (−0.85;0.13) | 0.142 |
| BL GSM (per point increase) | −0.19 (−0.34;−0.04) | 0.012 | −0.27 (−0.47;−0.06) | 0.011 |
| Gender (male versus female) | 1.81 (7.75;11.36) | 0.706 | 1.59 (−8.65;11.83) | 0.756 |
| Stenosis (per point increase) | −0.34 (−0.84;0.16) | 0.174 | −0.35 (−0.86;0.17) | 0.183 |
| Smoke (yes versus no) | −0.36 (−9.06;0.36) | 0.934 | 1.01 (−8.58;10.60) | 0.834 |
| Body Mass Index (per Kg/m2 increase) | 0.52 (−0.76;1.78) | 0.422 | −0.22 (−1.58;1.13) | 0.741 |
| Use of antidiabetic drugs (yes versus no) | 2.67 (−12.61;17.96) | 0.728 | −9.80 (−27.51;7.91) | 0.271 |
| Use of antihypertensive drugs (yes versus no) | 2.60 (−5.87;11.08) | 0.541 | 10.61 (0.72;20.49) | 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshomba, Y.; Baccellieri, D.; Carta, N.; Cilli, G.; Ardita, V.; Apruzzi, L.; Loschi, D.; Kahlberg, A.; Bertoglio, L.; Castellano, R.; et al. Doppler Ultrasound Monitoring of Echogenicity in Asymptomatic Subcritical Carotid Stenosis and Assessment of Response to Oral Supplementation of Vitamin K2 (PLAK2 Randomized Controlled Trial). Diagnostics 2021, 11, 229. https://doi.org/10.3390/diagnostics11020229
Tshomba Y, Baccellieri D, Carta N, Cilli G, Ardita V, Apruzzi L, Loschi D, Kahlberg A, Bertoglio L, Castellano R, et al. Doppler Ultrasound Monitoring of Echogenicity in Asymptomatic Subcritical Carotid Stenosis and Assessment of Response to Oral Supplementation of Vitamin K2 (PLAK2 Randomized Controlled Trial). Diagnostics. 2021; 11(2):229. https://doi.org/10.3390/diagnostics11020229
Chicago/Turabian StyleTshomba, Yamume, Domenico Baccellieri, Niccolò Carta, Giuseppe Cilli, Vincenzo Ardita, Luca Apruzzi, Diletta Loschi, Andrea Kahlberg, Luca Bertoglio, Renata Castellano, and et al. 2021. "Doppler Ultrasound Monitoring of Echogenicity in Asymptomatic Subcritical Carotid Stenosis and Assessment of Response to Oral Supplementation of Vitamin K2 (PLAK2 Randomized Controlled Trial)" Diagnostics 11, no. 2: 229. https://doi.org/10.3390/diagnostics11020229
APA StyleTshomba, Y., Baccellieri, D., Carta, N., Cilli, G., Ardita, V., Apruzzi, L., Loschi, D., Kahlberg, A., Bertoglio, L., Castellano, R., Simonini, E., Andreotti, F., & Chiesa, R. (2021). Doppler Ultrasound Monitoring of Echogenicity in Asymptomatic Subcritical Carotid Stenosis and Assessment of Response to Oral Supplementation of Vitamin K2 (PLAK2 Randomized Controlled Trial). Diagnostics, 11(2), 229. https://doi.org/10.3390/diagnostics11020229








