Evaluation of Eye-Pain Severity between Dry-Eye Subtypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DE Subtype Classification
2.3. DE-Related Symptoms Evaluated by VAS-Based Questionnaires (DSQ-VAS)
2.4. DE-Related Eye Pain Symptoms Evaluated by SF-MPQ-2
2.5. Quantitative Evaluation of Eye Pain Using PainVision®
2.6. Ocular Surface Examinations
2.7. Statistical Analysis
3. Results
3.1. Patient Background for Each DE Subtype
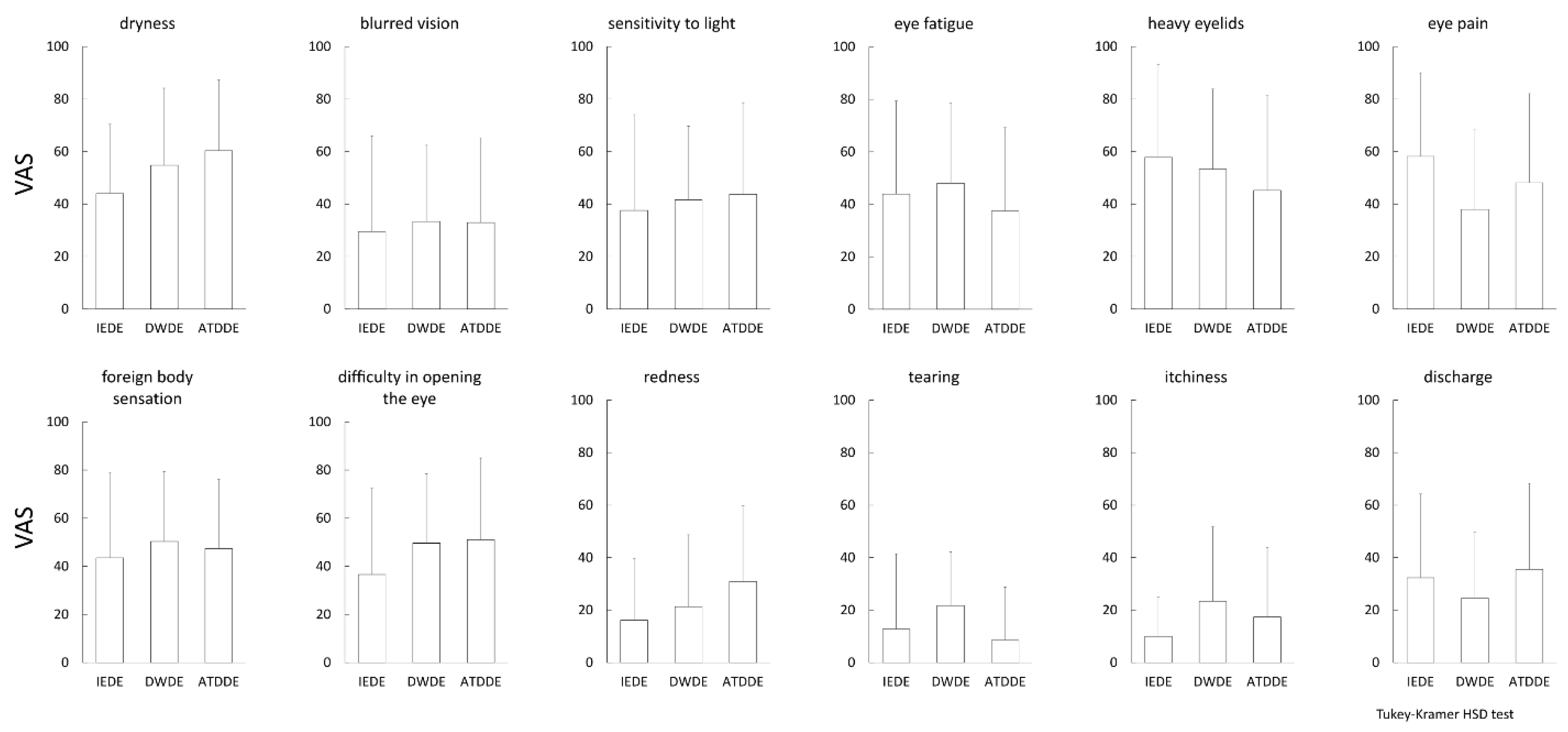
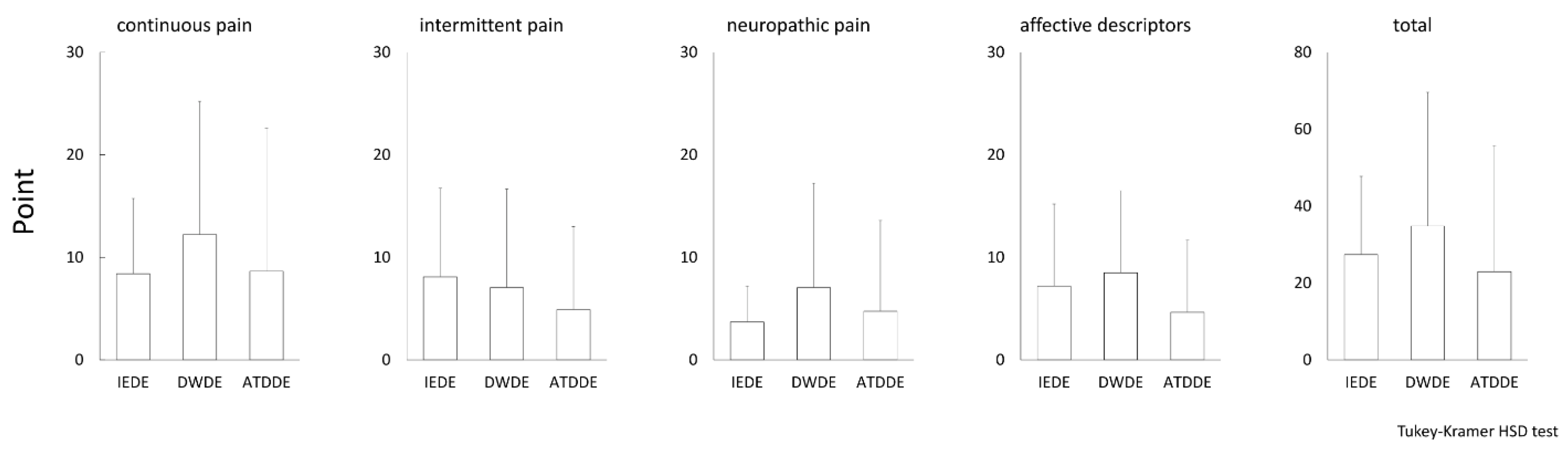
3.2. Pain Subscale of DE-Related Symptoms Evaluated by DSQ-VAS and SF-MPQ-2
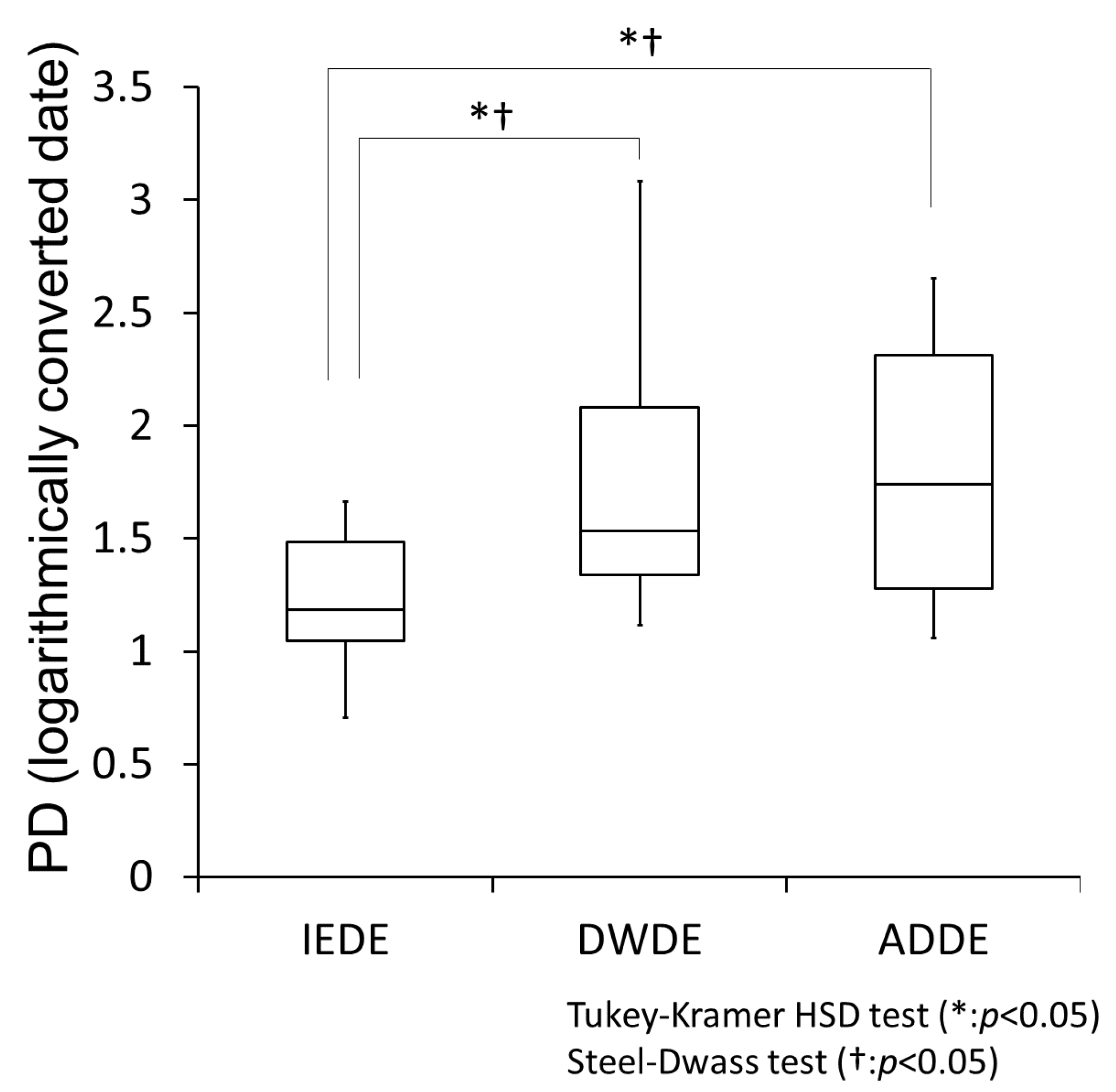
3.3. Comparison of PD Evaluated by PainVision® among the DE Subtype Groups
3.4. Relationship between PD and Objective Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 2016, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of fluorescein breakup patterns a novel method of differential diagnosis for dry eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear film-oriented diagnosis and tear film-oriented therapy for dry eye based on tear film dynamics. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES13–DES22. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. A new perspective on dry eye classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; Mangoni, L.; Gentile, P.; Braghiroli, M.; d’Aloja, E.; Fossarello, M. Fourier-domain OCT imaging of the ocular surface and tear film dynamics: A review of the state of the art and an integrative model of the tear behavior during the inter-blink period and visual fixation. J. Clin. Med. 2020, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. The bull’s eye pattern of the tear film in humans during visual fixation on en-face optical coherence tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Kalangara, J.P.; Galor, A.; Levitt, R.C.; Covington, D.B.; McManus, K.T.; Sarantopoulos, C.D.; Felix, E.R. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye Contact Lens 2017, 43, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Venincasa, V.D.; Perez, V.L. Ocular surface parameters in older male veterans. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1426–1433. [Google Scholar] [CrossRef]
- Martinez, J.D.; Galor, A.; Ramos-Betancourt, N.; Lisker-Cervantes, A.; Beltrán, F.; Ozorno-Zárate, J.; Sánchez-Huerta, V.; Torres-Vera, M.A.; Hernández-Quintela, E. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin. Ophthalmol. 2016, 10, 1335–1342. [Google Scholar] [CrossRef]
- Yokoi, N.; Uchino, M.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Tsubota, K.; Kinoshita, S. Importance of tear film instability in dry eye disease in office workers using visual display terminals: The Osaka study. Am. J. Ophthalmol. 2015, 159, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Fidilio, A.; Fresta, C.G.; Lazzara, F.; Platania, C.B.M.; Cantarella, G.; Di Benedetto, G.; Burgaletto, C.; Bernardini, R.; Piazza, C.; et al. Ocular pharmacological profile of hydrocortisone in dry eye disease. Front. Pharmacol. 2019, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H. Studies of pain in normal man. In Textbook of Pain, 3rd ed.; Wall, P.D., Melzack, R., Eds.; Churchill Livingstone: London, UK, 1994; pp. 315–336. [Google Scholar]
- Ikeno, S.; Kawamata, M. PainVison. Masui 2009, 58, 1367–1372. (In Japanese) [Google Scholar] [PubMed]
- Kim, J.; Lee, K.S.; Kong, S.W.; Kim, T.; Kim, M.J.; Park, S.B.; Lee, K.H. Correlations between electrically quantified pain degree, subjectively assessed visual analogue scale, and the McGill Pain Questionnaire: A pilot study. Ann. Rehabil. Med. 2014, 38, 665–672. [Google Scholar] [CrossRef]
- Hiraki, M.; Takemasa, I.; Uemura, M.; Haraguchi, N.; Nishimura, J.; Hata, T.; Mizushima, T.; Yamamoto, H.; Doki, Y.; Mori, M. Evaluation of invasiveness in single-site laparoscopic colectomy, using “the PainVision™ system” for quantitative analysis of pain sensation. Surg. Endosc. 2014, 28, 3216–3223. [Google Scholar] [CrossRef]
- Yoshida, Y.; Satoh, A.; Yamada, T.; Aisu, N.; Matsuoka, T.; Koganemaru, T.; Kajitani, R.; Munechika, T.; Matsumoto, Y.; Nagano, H.; et al. The relationship between evaluation methods for chemotherapy-induced peripheral neuropathy. Sci. Rep. 2019, 9, 20361. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yoshida, Y.; Aisu, N.; Yamada, T.; Mogi, A.; Komono, A.; Sakamoto, R.; Kojima, D.; Yoshimatsu, G.; Kiyomi, F.; et al. Evaluation of vascular pain in patients with colorectal cancer receiving peripheral venous chemotherapy with or without oxaliplatin. Sci. Rep. 2019, 9, 1819. [Google Scholar] [CrossRef]
- Watanabe, S.N.; Imai, K.; Kimura, T.; Saito, Y.; Takashima, S.; Matsuzaki, I.; Kurihara, N.; Atari, M.; Matsuo, T.; Iwai, H.; et al. Effect of lidocaine cream analgesia for chest drain tube removal after video-assisted thoracoscopic surgery for lung cancer: A randomized clinical trial. Reg. Anesth. Pain Med. 2019, 45, 16–21. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, K.; Han, S.L.; Yu, L. PainVision® apparatus for assessment of efficacy of pulsed radiofrequency combined with pharmacological therapy in the treatment of postherpetic neuralgia and correlations with measurements. Biomed Res. Int. 2017, 2017, 5670219. [Google Scholar] [CrossRef]
- Amano, S.; Arita, R.; Kinosihta, S.; Yokoi, N.; Sotozono, C.; Komuro, A.; Suzuki, T.; Shimazaki, J.; Den, S.; Maeda, N.; et al. Definition and diagnostic criteria for meibomian gland dysfunction. Atarashii Ganka (J. Eye) 2010, 27, 627–631. (In Japanese) [Google Scholar]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Contribution of mucins towards the physical properties of the tear film a modern update. Int. J. Mol. Sci. 2019, 20, 6132. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, T.I.; Turk, D.C.; Morasco, B.J. Evaluation of the Psychometric Properties of the Revised Short-Form McGill Pain Questionnaire. J. Pain 2012, 13, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Watanabe, H.; Hosohata, J.; Hori, Y.; Hibino, S.; Nishida, K.; Maeda, N.; Tano, Y. Diagnosing dry eye using a blue-free barrier filter. Am. J. Ophthalmol. 2003, 136, 513–519. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar] [PubMed]
- Miyata, K.; Amano, S.; Sawa, M.; Nishida, T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch. Ophthalmol. 2003, 121, 1537–1539. [Google Scholar] [CrossRef]
- Van Bijsterveld, O.P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 1969, 82, 10–14. [Google Scholar] [CrossRef]
- Fechner, G.T. Outline of a New Principle of Mathematical Psychology (1851). By Gustav Theodor Fechner (Translation). Psychol. Res. 1987, 49, 203–207. [Google Scholar] [CrossRef]
- Rao, S.B.S. Psychophysical and Clinical Investigations of Ocular Discomfort. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Merskey, H. Pain terms: A list with definitions and notes on usage. Recommended by the IASP subcommittee on taxonomy. Pain 1979, 6, 249–252. [Google Scholar]
- Takeji, Y.; Urashima, H.; Aoki, A.; Shinohara, H. Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. J. Ocul. Pharmacol. Ther. 2012, 28, 259–263. [Google Scholar] [CrossRef]
- Uchino, Y.; Woodward, A.M.; Argüeso, P. Differential effect of rebamipide on transmembrane mucin biosynthesis in stratified ocular surface epithelial cells. Exp. Eye Res. 2016, 153, 1–7. [Google Scholar] [CrossRef]
- Toda, I.; Fujishima, H.; Tsubota, K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol. 1993, 71, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Saatchi, M.; Yekta, A.; Ali, B.; Ostadimoghaddam, H.; Nabovati, P.; Aghamirsalim, M.; Khabazkhoob, M. High prevalence of asthenopia among a population of university students. J. Ophthalmic Vis. Res. 2019, 14, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic corneal pain: Approaches for management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef] [PubMed]
| IEDE (n = 10) | DWDE (n = 22) | ADDE (n = 20) | p (IEDE vs. DWDE) | p (IEDE vs. ADDE) | p (DWDE vs. ADDE) | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, years, mean (SD) | 65.9 (15.9) | 64.9 (12.2) | 62.7 (13.4) | one way ANOVA p = 0.78 | ||
| Female, n (%) | 7 (70.0%) | 19 (86.4%) | 17 (85.0%) | one way ANOVA p = 0.51 | ||
| Ocular Surface Evaluations, mean (SD) | ||||||
| FBUT, s $ | 5.2 (2.5) | 1.9 (1.7) | 2.0 (1.7) | 0.004 | 0.004 | 1.0 |
| Schirmer 1 test, mm $ | 20.0 (12.1) | 19.7 (11.3) | 5.3 (5.8) | 1.0 | 0.002 | <0.0001 |
| Corneal staining score (0–15) # | 1.0 (1.2) | 1.0 (1.4) | 8.0 (3.3) | 1.0 | <0.0001 | <0.0001 |
| Conjunctival staining score (0–6) # | 0.1 (0.3) | 0.5 (1.0) | 3.5 (2.2) | 0.81 | <0.0001 | <0.0001 |
| SF-MPQ-2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Continuous Pain | Intermittent Pain | Neuropathic Pain | Affective Descriptors | Total Pain | ||||||
| VAS | R | p | R | p | R | p | R | p | R | p |
| Dryness | 0.15 | 0.30 | 0.09 | 0.50 | 0.09 | 0.51 | 0.16 | 0.27 | 0.13 | 0.35 |
| Blurred vision | 0.34 | 0.01 | 0.17 | 0.21 | 0.30 | 0.03 | 0.18 | 0.20 | 0.30 | 0.03 |
| Sensitivity to light | 0.22 | 0.11 | 0.32 | 0.02 | 0.19 | 0.17 | 0.14 | 0.32 | 0.26 | 0.06 |
| Eye fatigue | 0.47 | 0.0004 | 0.27 | 0.05 | 0.16 | 0.26 | 0.47 | 0.0004 | 0.49 | 0.0002 |
| Heavy eyelids | 0.56 | <0.0001 | 0.33 | 0.01 | 0.19 | 0.17 | 0.51 | 0.0001 | 0.55 | <0.0001 |
| Eye pain | 0.50 | 0.0001 | 0.49 | 0.0002 | 0.47 | 0.0003 | 0.25 | 0.07 | 0.56 | <0.0001 |
| Foreign body sensation | 0.36 | 0.008 | 0.35 | 0.01 | 0.30 | 0.03 | 0.15 | 0.30 | 0.37 | 0.007 |
| Difficulty in opening the eye | 0.24 | 0.08 | 0.07 | 0.62 | 0.11 | 0.42 | 0.06 | 0.66 | 0.18 | 0.20 |
| Redness | 0.06 | 0.65 | 0.22 | 0.12 | 0.17 | 0.24 | −0.05 | 0.70 | 0.11 | 0.44 |
| Tearing | 0.28 | 0.04 | 0.21 | 0.12 | 0.16 | 0.25 | 0.08 | 0.56 | 0.23 | 0.10 |
| Itchiness | 0.24 | 0.08 | −0.01 | 0.94 | 0.13 | 0.35 | 0.10 | 0.47 | 0.14 | 0.32 |
| Discharge | 0.08 | 0.56 | 0.22 | 0.12 | 0.16 | 0.24 | 0.04 | 0.75 | 0.14 | 0.32 |
| PD | FBUT | Schirmer 1 Test | Corneal Staining Score | Conjunctival Staining Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ocular Surface Evaluations | R | p | R | p | R | p | R | p | R | p |
| PD | −0.24 | 0.089 | −0.04 | 0.80 | 0.14 | 0.33 | 0.13 | 0.35 | ||
| FBUT | −0.24 | 0.089 | 0.26 | 0.065 | −0.44 | 0.001 | −0.17 | 0.24 | ||
| Schirmer 1 test | −0.04 | 0.80 | 0.26 | 0.065 | −0.61 | <0.0001 | −0.58 | <0.0001 | ||
| Corneal staining score | 0.14 | 0.33 | −0.44 | 0.001 | −0.61 | <0.0001 | 0.67 | <0.0001 | ||
| Conjunctival staining score | 0.13 | 0.35 | −0.17 | 0.24 | −0.58 | <0.0001 | 0.67 | <0.0001 | ||
| Multivariate Analysis 1 | Multivariate Analysis 2 | Multivariate Analysis 3 | Multivariate Analysis 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Logarithmic Value | p Value | Logarithmic Value | p Value | Logarithmic Value | p Value | Logarithmic Value | p Value |
| Subtype classification of DE | 1.534 | 0.029 | 1.635 | 0.023 | 1.467 | 0.034 | 1.340 | 0.046 |
| Age | 0.253 | 0.56 | 0.179 | 0.66 | 0.131 | 0.74 | 0.094 | 0.80 |
| Sex | 1.270 | 0.053 | 1.293 | 0.051 | 1.067 | 0.086 | 1.146 | 0.071 |
| FBUT | 0.608 | 0.25 | 0.485 | 0.33 | ||||
| Schirmer 1 test | 0.083 | 0.83 | 0.108 | 0.78 | ||||
| Corneal staining score | 0.323 | 0.48 | 0.016 | 0.96 | ||||
| Conjunctival staining score | 0.132 | 0.74 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, Y.; Yokoi, N.; Kato, H.; Sakai, R.; Komuro, A.; Sonomura, Y.; Ikeda, T.; Sotozono, C. Evaluation of Eye-Pain Severity between Dry-Eye Subtypes. Diagnostics 2021, 11, 166. https://doi.org/10.3390/diagnostics11020166
Yoshikawa Y, Yokoi N, Kato H, Sakai R, Komuro A, Sonomura Y, Ikeda T, Sotozono C. Evaluation of Eye-Pain Severity between Dry-Eye Subtypes. Diagnostics. 2021; 11(2):166. https://doi.org/10.3390/diagnostics11020166
Chicago/Turabian StyleYoshikawa, Yamato, Norihiko Yokoi, Hiroaki Kato, Rieko Sakai, Aoi Komuro, Yukiko Sonomura, Tsunehiko Ikeda, and Chie Sotozono. 2021. "Evaluation of Eye-Pain Severity between Dry-Eye Subtypes" Diagnostics 11, no. 2: 166. https://doi.org/10.3390/diagnostics11020166
APA StyleYoshikawa, Y., Yokoi, N., Kato, H., Sakai, R., Komuro, A., Sonomura, Y., Ikeda, T., & Sotozono, C. (2021). Evaluation of Eye-Pain Severity between Dry-Eye Subtypes. Diagnostics, 11(2), 166. https://doi.org/10.3390/diagnostics11020166







