Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): Technical Updates and Pathological Yield
Abstract
1. Introduction
2. Procedure-Related Technical Factors and Their Effects on Diagnostic Yield
2.1. Choice of Sedation
2.2. Needle Size
2.3. Use of Suction and Stylet
2.4. Fanning
2.5. Core Needle
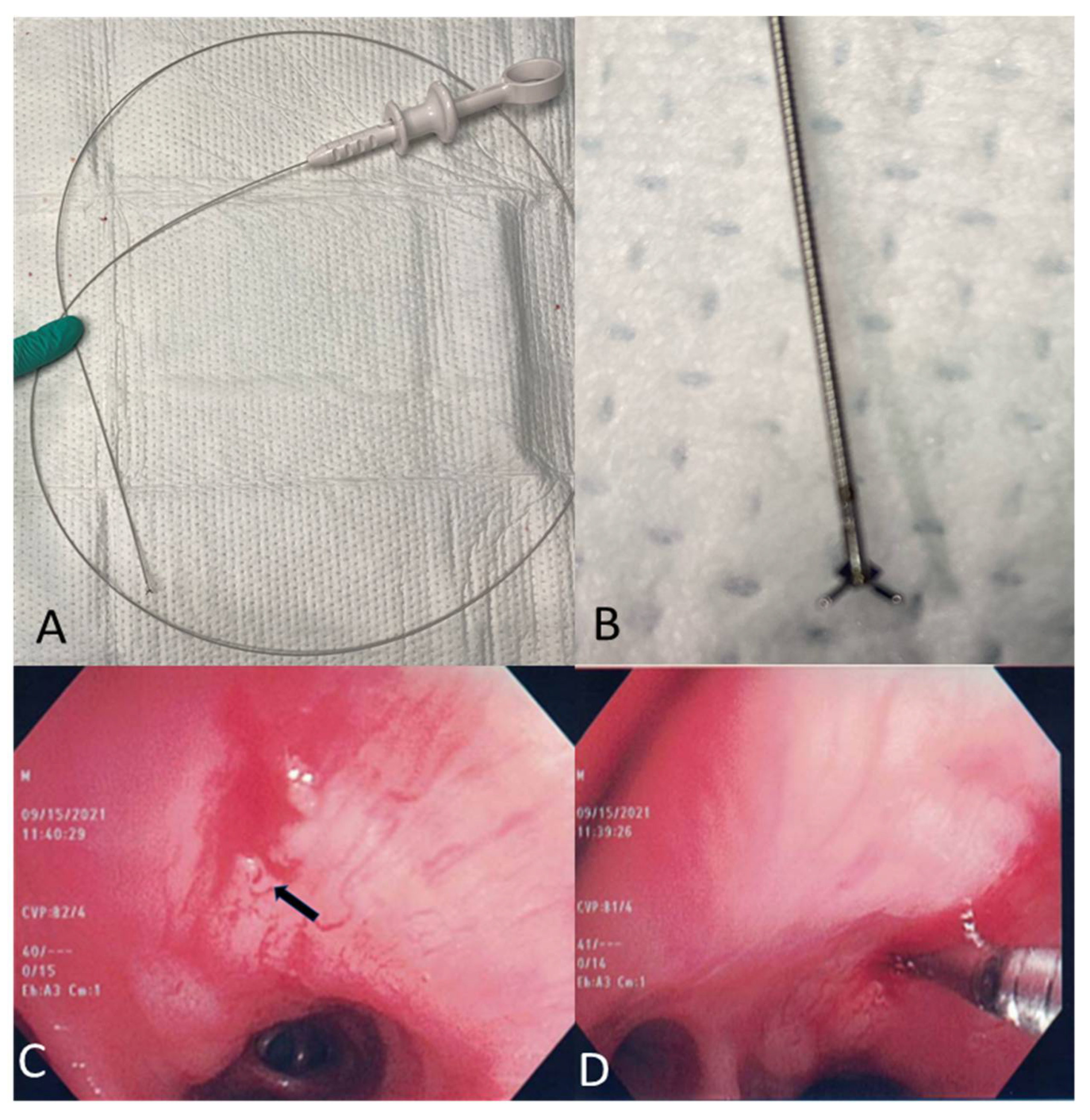
2.6. Mini-Forceps Biopsy
2.7. Lymph Node Cryobiopsy under EBUS Guidance
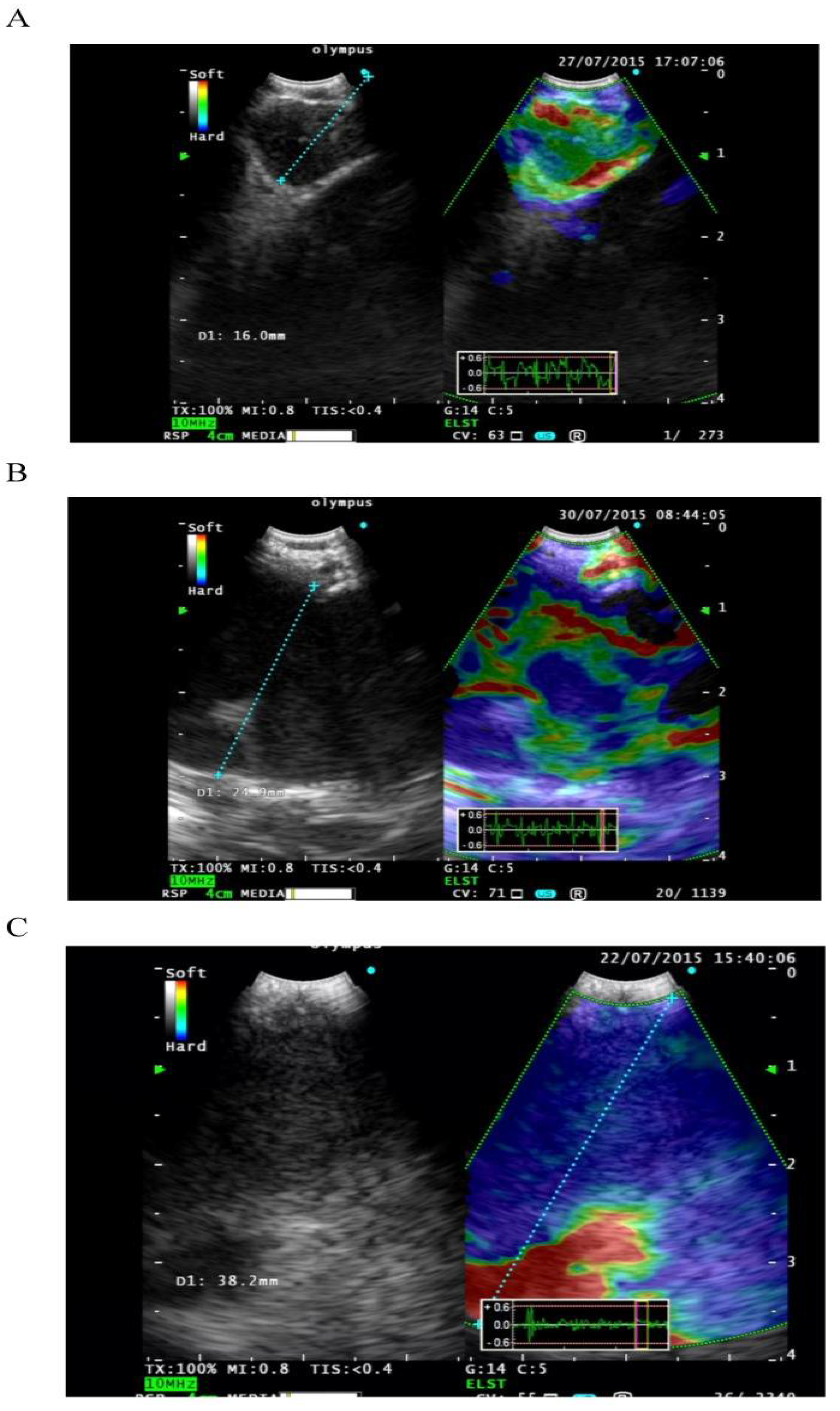
2.8. EBUS Elastography
3. Evaluation and Processing of Cytopathological Material Obtained by EBUS
3.1. Rapid On-Site Evaluation
3.2. Adequacy of Samples Obtained during EBUS
3.3. Cell Block and Molecular Testing
4. Optimizing the Diagnostic Yield of EBUS in Lymphoma and Sarcoidosis
4.1. Lymphoma
4.2. Sarcoidosis
5. Conclusions
| Intervention | Type of Study and Number of Patients/LN (n) | Overall Findings |
|---|---|---|
| Needle size21 vs. 22 G |
| |
| Needle size22 vs. 25 G |
| |
| Needle size21 vs. 25 G |
|
|
| Needle size22 vs. 19 G |
| |
| Needle size21 vs. 19 G |
|
|
| Needle size19 vs. 21 vs. 22 G |
|
|
| Stylet use and suction |
|
|
| Fanning vs. no fanning |
|
|
| Core needle versus 22 G |
|
|
| Number of passes |
|
|
| ROSE vs. no ROSE |
| |
| Cell block |
|
|
| Mini-forceps vs. TBNA |
| |
| Lymph node cryobiopsy vs. TBNA |
|
|
| Elastography |
|
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hürter, T.; Hanrath, P. Endobronchial sonography: Feasibility and preliminary results. Thorax 1992, 47, 565–567. [Google Scholar] [CrossRef]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef] [PubMed]
- Canneto, B.; Ferraroli, G.; Falezza, G.; Infante, M.V. Ideal conditions to perform EBUS-TBNA. J. Thorac. Dis. 2017, 9, S414–S417. [Google Scholar] [CrossRef][Green Version]
- Casal, R.F.; Lazarus, D.R.; Kuhl, K.; Nogueras-González, G.; Perusich, S.; Green, L.K.; Ost, D.E.; Sarkiss, M.; Jimenez, C.A.; Eapen, G.A.; et al. Randomized Trial of Endobronchial Ultrasound–guided Transbronchial Needle Aspiration under General Anesthesia versus Moderate Sedation. Am. J. Respir. Crit. Care Med. 2015, 191, 796–803. [Google Scholar] [CrossRef]
- Yarmus, L.B.; Akulian, J.; Lechtzin, N.; Yasin, F.; Kamdar, B.; Ernst, A.; Ost, D.E.; Ray, C.; Greenhill, S.R.; Jimenez, C.A.; et al. Comparison of 21-Gauge and 22-Gauge Aspiration Needle in Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: Results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013, 143, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Udagawa, H.; Kirita, K.; Nomura, S.; Itotani, R.; Tamiya, Y.; Sugimoto, A.; Ota, T.; Naito, T.; Izumi, H.; et al. Comparison of the efficiency of endobronchial ultrasound-guided transbronchial needle aspiration using a 22G needle versus 25G needle for the diagnosis of lymph node metastasis in patients with lung cancer: A prospective randomized, crossover study. Transl. Lung Cancer Res. 2021, 10, 3745–3758. [Google Scholar] [CrossRef]
- Sakai, T.; Udagawa, H.; Kirita, K.; Nomura, S.; Sugimoto, A.; Itotani, R.; Tamiya, Y.; Izumi, H.; Nosaki, K.; Ikeda, T.; et al. P02.03 Comparison of the Efficiency of 22G Versus 25G Needle in EBUS-TBNA for Diagnosis of Lung Cancer; A Prospective Randomized, Crossover Study. J. Thorac. Oncol. 2021, 16, S247. [Google Scholar] [CrossRef]
- Di Felice, C.; Young, B.; Matta, M. Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration. J. Thorac. Dis. 2019, 11, 3643–3649. [Google Scholar] [CrossRef]
- Sood, R.; Alape, D.; Thakkar, D.; Shadchehr, S.; Acash, G.; Tronic, B.J.; Lamb, C.R. Comparison of Sample Adequacy and Diagnostic Yield of the 21-G and 25-G EBUS TBNA Needles. J. Bronchol. Interv. Pulmonol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chaddha, U.; Ronaghi, R.; Elatre, W.; Chang, C.-F.; Mahdavi, R. Comparison of sample adequacy and diagnostic yield of 19-and 22-G EBUS-TBNA needles. J. Bronchol. Interv. Pulmonol. 2018, 25, 264–268. [Google Scholar] [CrossRef]
- Dooms, C.; Vander Borght, S.; Yserbyt, J.; Testelmans, D.; Wauters, E.; Nackaerts, K.; Vansteenkiste, J.; Verbeken, E.; Weynand, B. A Randomized Clinical Trial of Flex 19G Needles versus 22G Needles for Endobronchial Ultrasonography in Suspected Lung Cancer. Respir. Int. Rev. Thorac. Dis. 2018, 96, 275–282. [Google Scholar] [CrossRef]
- Pickering, E.M.; Holden, V.K.; Heath, J.E.; Verceles, A.C.; Kalchiem-Dekel, O.; Sachdeva, A. Tissue acquisition during EBUS-TBNA. J. Bronchol. Interv. Pulmonol. 2019, 26, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wolters, C.; Darwiche, K.; Franzen, D.; Hager, T.; Bode-Lesnievska, B.; Kneuertz, P.J.; He, K.; Koenig, M.; Freitag, L.; Wei, L.; et al. A Prospective, Randomized Trial for the Comparison of 19-G and 22-G Endobronchial Ultrasound-Guided Transbronchial Aspiration Needles; Introducing a Novel End Point of Sample Weight Corrected for Blood Content. Clin Lung Cancer 2019, 20, e265–e273. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.; Bhatt, N.; Medford, A.R.L. The effect of 19-gauge endobronchial ultrasound-guided transbronchial needle aspiration biopsies on characterisation of malignant and benign disease. The Bristol experience. Monaldi Arch. Chest Dis. 2018, 88, 915. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Iyer, H.; Madan, K.; Hadda, V.; Mittal, S.; Tiwari, P.; Jain, D.; Pandey, R.; Garg, A.; Guleria, R. A Randomized Comparison of Sample Adequacy and Diagnostic Yield of EBUS-TBNA using various Suction Pressures. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Harris, K.; Maroun, R.; Attwood, K.; Chalhoub, M. Comparison of cytologic accuracy of endobronchial ultrasound transbronchial needle aspiration using needle suction versus no suction. Endosc. Ultrasound 2015, 4, 115–119. [Google Scholar] [CrossRef]
- Casal, R.F.; Staerkel, G.A.; Ost, D.; Almeida, F.A.; Uzbeck, M.H.; Eapen, G.A.; Jimenez, C.A.; Nogueras-Gonzalez, G.M.; Sarkiss, M.; Morice, R.C. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest 2012, 142, 568–573. [Google Scholar] [CrossRef]
- Lin, X.; Ye, M.; Li, Y.; Ren, J.; Lou, Q.; Li, Y.; Jin, X.; Wang, K.-P.; Chen, C. Randomized controlled trial to evaluate the utility of suction and inner-stylet of EBUS-TBNA for mediastinal and hilar lymphadenopathy. BMC Pulm. Med. 2018, 18, 192. [Google Scholar] [CrossRef]
- Bang, J.Y.; Magee, S.H.; Ramesh, J.; Trevino, J.M.; Varadarajulu, S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013, 45, 445–450. [Google Scholar] [CrossRef]
- Parthiban, S.; Sczaniecka, A.; Dillard, D.; Gonzalez, X. Comparison of Fanning and No-Fanning Sampling Techniques for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in an Ex Vivo Tissue Model. Chest 2017, 152, A964. [Google Scholar] [CrossRef]
- El, H.I.; Wu, H.; Reuss, S.; Randolph, M.; Harris, A.; Gromski, M.A.; Al-Haddad, M. Prospective Assessment of the Performance of a New Fine Needle Biopsy Device for EUS-Guided Sampling of Solid Lesions. Clin. Endosc. 2018, 51, 576–583. [Google Scholar] [CrossRef]
- Balwan, A.; Bixby, B.; Grotepas, C.; Witt, B.L.; Iravani, A.; Ansari, S.; Reddy, C.B. Core needle biopsy with endobronchial ultrasonography: Single center experience with 100 cases. J. Am. Soc. Cytopathol. 2020, 9, 249–253. [Google Scholar] [CrossRef]
- Yang, L.; Gu, Y.; Wang, H.; Yu, D.; Zhang, H.; Wang, H. Novel ProCore 25-gauge needle for endobronchial ultrasound-guided transbronchial needle aspiration reduces the puncture time and frequency, with comparable diagnostic rate for mediastinal and hilar lymphadenopathy. Thorac. Cancer 2020, 11, 748–753. [Google Scholar] [CrossRef]
- McCracken, D.J.; Bailey, M.; McDermott, M.-T.; McManus, T.E. A retrospective analysis comparing the use of ProCore® with standard fine needle aspiration in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Ir. J. Med. Sci. 2019, 188, 85–88. [Google Scholar] [CrossRef]
- Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Muthu, V.; Gupta, N.; Bal, A.; Ram, B.; Aggarwal, A.N.; Agarwal, R. Diagnostic yield and safety of the ProCore versus the standard EBUS-TBNA needle in subjects with suspected sarcoidosis. Expert Rev. Med. Devices 2021, 18, 211–216. [Google Scholar] [CrossRef]
- Herth, F.J.; Morgan, R.K.; Eberhardt, R.; Ernst, A. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann. Thorac. Surg. 2008, 85, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Chrissian, A.; Misselhorn, D.; Chen, A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann. Thorac. Surg. 2011, 92, 284–288. [Google Scholar] [CrossRef]
- Shiari, A.; Aljundi, L.; Boshara, P.; Zein, R.; Zalt, M. Miniforceps EBUS-guided lymph node biopsy: Impact on diagnostic yield. Adv. Respir. Med. 2021, 89, 37–42. [Google Scholar] [CrossRef]
- Agrawal, A.; Ghori, U.; Chaddha, U.; Murgu, S. Combined EBUS-IFB and EBUS-TBNA vs EBUS-TBNA alone for intrathoracic adenopathy: A Meta-analysis. Ann. Thorac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Mahajan, A.; Oh, S.; Benzaquen, S.; Chen, A. Endobronchial ultrasound-guided intranodal forceps biopsy (EBUS-IFB)-technical review. J. Thorac. Dis. 2019, 11, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Gonuguntla, H.K.; Shah, M.; Gupta, N.; Agrawal, S.; Poletti, V.; Nacheli, G.C. Endobronchial ultrasound-guided transbronchial cryo-nodal biopsy: A novel approach for mediastinal lymph node sampling. Respirol. Case Rep. 2021, 9, e00808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, J.-R.; Huang, Z.-S.; Fu, W.-L.; Wu, X.-L.; Wu, N.; Kuebler, W.M.; Herth, F.J.F.; Fan, Y. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: A randomised trial. Eur. Respir. J. 2021. [Google Scholar] [CrossRef]
- Korrungruang, P.; Boonsarngsuk, V. Diagnostic value of endobronchial ultrasound elastography for the differentiation of benign and malignant intrathoracic lymph nodes. Respirology 2017, 22, 972–977. [Google Scholar] [CrossRef]
- Saftoiu, A.; Vilman, P. Endoscopic ultrasound elastography—A new imaging technique for the visualization of tissue elasticity distribution. J. Gastrointestin Liver Dis. 2006, 15, 161–165. [Google Scholar]
- Izumo, T.; Sasada, S.; Chavez, C.; Matsumoto, Y.; Tsuchida, T. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn. J. Clin. Oncol. 2014, 44, 956–962. [Google Scholar] [CrossRef]
- Huang, H.; Huang, Z.; Wang, Q.; Wang, X.; Dong, Y.; Zhang, W.; Zarogoulidis, P.; Man, Y.-G.; Schmidt, W.H.; Bai, C. Effectiveness of the Benign and Malignant Diagnosis of Mediastinal and Hilar Lymph Nodes by Endobronchial Ultrasound Elastography. J. Cancer 2017, 8, 1843–1848. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Wang, Y.; Ge, L.; Jin, Y.; Wang, Z. Diagnostic value of endobronchial ultrasound elastography for differentiating benign and malignant hilar and mediastinal lymph nodes: A systematic review and meta-analysis. Med. Ultrason 2021. [Google Scholar] [CrossRef]
- Griffin, A.C.; Schwartz, L.E.; Baloch, Z.W. Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal 2011, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Mallya, V.; Kumar, S.P.; Meganathan, P.; Shivkumar, S.; Mehta, R. The utility of ROSE (rapid on-site evaluation) in endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA): Is the picture rosy? J. Cytol. 2015, 32, 230–233. [Google Scholar] [CrossRef]
- Şimon, M.; Pop, B.; Toma, I.L.; Vallasek, A.K.; Şimon, I. The use of EBUS-TBNA and ROSE in the diagnosis of lung cancer. Rom J. Morphol. Embryol. 2017, 58, 79–87. [Google Scholar]
- Haranguş, A.; Berindan-Neagoe, I.; Toma, L.; Şimon, I.; Pop, O.; Şimon, M. EBUS in optimizing non-small cell lung cancer diagnosis and treatment. Med. Pharm. Rep. 2021, 94, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, S.; Guo, J.; Li, B.; Li, W.; Lu, Z.; Sun, J.; Zhang, B.; Yu, J. Rapid on-site evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of hilar and mediastinal lymphadenopathy in patients with lung cancer. Cancer Lett. 2016, 371, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Saka, H.; Kitagawa, C.; Kogure, Y.; Murata, N.; Adachi, T.; Ando, M. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: A randomized study. Respir. Int. Rev. Thorac. Dis. 2013, 85, 486–492. [Google Scholar] [CrossRef]
- Jain, D.; Allen, T.C.; Aisner, D.L.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Hariri, L.P.; Lantuejoul, S.; Miller, R.; Mino-Kenudson, M.; et al. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2018, 142, 253–262. [Google Scholar] [CrossRef]
- Nayak, A.; Sugrue, C.; Koenig, S.; Wasserman, P.G.; Hoda, S.; Morgenstern, N.J. Endobronchial ultrasound-guided transbronchial needle aspirate (EBUS-TBNA): A proposal for on-site adequacy criteria. Diagn. Cytopathol. 2012, 40, 128–137. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, A.R.; Choe, J.Y.; Nam, S.J.; Chung, D.H.; Lee, J.; Lee, C.H.; Lee, S.M.; Yim, J.J.; Yoo, C.G.; et al. Adequacy Criteria of Rapid On-Site Evaluation for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Simple Algorithm to Assess the Adequacy of ROSE. Ann. Thorac. Surg. 2016, 101, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, L.; Kholová, I. Cell Block in Cytological Diagnostics: Review of Preparatory Techniques. Acta Cytol. 2018, 62, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Santos, J.; Serra, P.; Andreo, F.; Llatjós, M.; Castellà, E.; Monsó, E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer 2012, 12, 34. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, G.K.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Kim, H.Y.; Nam, B.H.; Zo, J.I.; Hwangbo, B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest 2008, 134, 368–374. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Reck, M.; Rabe, K.F. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Yarmus, L.; Akulian, J.; Gilbert, C.; Feller-Kopman, D.; Lee, H.J.; Zarogoulidis, P.; Lechtzin, N.; Ali, S.Z.; Sathiyamoorthy, V. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann. Am. Thorac. Soc. 2013, 10, 636–643. [Google Scholar] [CrossRef]
- Cho, M.; Ahn, S.; Hong, M.; Bang, H.; van Vrancken, M.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; et al. Tissue recommendations for precision cancer therapy using next generation sequencing: A comprehensive single cancer center’s experiences. Oncotarget 2017, 8, 42478–42486. [Google Scholar] [CrossRef] [PubMed]
- Righi, L.; Franzi, F.; Montarolo, F.; Gatti, G.; Bongiovanni, M.; Sessa, F.; La Rosa, S. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)-from morphology to molecular testing. J. Thorac. Dis. 2017, 9, S395–S404. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; de Biase, D.; Valentini, I.; Casadei, G.; Paioli, D.; Ferrari, F.; Gordini, G.; Patelli, M.; et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration with and without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015, 148, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Martin-Deleon, R.; Teixido, C.; Lucena, C.M.; Martinez, D.; Fontana, A.; Reyes, R.; García, M.; Viñolas, N.; Vollmer, I.; Sanchez, M. EBUS-TBNA Cytological Samples for Comprehensive Molecular Testing in Non–Small Cell Lung Cancer. Cancers 2021, 13, 2084. [Google Scholar] [CrossRef]
- Perrotta, F.; Nankivell, M.; Adizie, J.B.; Maqsood, U.; Elshafi, M.; Jafri, S.; Lerner, A.D.; Woolhouse, I.; Munavvar, M.; Evison, M.; et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for PD-L1 Testing in Non-small Cell Lung Cancer. Chest 2020, 158, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Temiz, D.; İn, E.; Kuluöztürk, M.; Kırkıl, G.; Artaş, G.; Turgut, T.; Deveci, F. The role of endobronchial ultrasound-guided transbronchial needle aspiration in the differential diagnosis of isolated mediastinal and/or hilar lymphadenopathy. Diagn. Cytopathol. 2021, 49, 1012–1021. [Google Scholar] [CrossRef]
- Grosu, H.B.; Iliesiu, M.; Caraway, N.P.; Medeiros, L.J.; Lei, X.; Jimenez, C.A.; Morice, R.C.; Casal, R.F.; Ost, D.; Eapen, G.A. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis and Subtyping of Lymphoma. Ann. Am. Thorac. Soc. 2015, 12, 1336–1344. [Google Scholar] [CrossRef]
- Erer, O.F.; Erol, S.; Anar, C.; Aydoğdu, Z.; Özkan, S.A. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc. Ultrasound 2017, 6, 317–322. [Google Scholar] [CrossRef]
- Moonim, M.T.; Breen, R.; Fields, P.A.; Santis, G. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am. J. Respir. Crit. Care. Med. 2013, 188, 1216–1223. [Google Scholar] [CrossRef]
- Dayan, G.; Soder, S.; Godin, A.; Maietta, A.; Stephenson, P.; Lemieux, B.; Liberman, M. Endosonography-Guided Biopsy as a First Test in the Diagnosis of Lymphoma. Semin. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Barroca, H.; Marques, C. A Basic Approach to Lymph Node and Flow Cytometry Fine-Needle Cytology. Acta Cytol. 2016, 60, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Grosu, H.B. EBUS-TBNA for the Diagnosis of Lymphoma: Time to Give In? J. Bronchol. Interv. Pulmonol. 2018, 25, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, R.; Baughman, R.P.; Spagnolo, P.; Culver, D.A. Endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis: Beyond the diagnostic yield. Respirol. Carlton Vic. 2019, 24, 531–542. [Google Scholar] [CrossRef]
- Pedro, C.; Melo, N.; Novais, E.B.H.; Magalhães, A.; Fernandes, G.; Martins, N.; Morais, A.; Caetano Mota, P. Role of Bronchoscopic Techniques in the Diagnosis of Thoracic Sarcoidosis. J. Clin. Med. 2019, 8, 1327. [Google Scholar] [CrossRef]
- Goyal, A.; Gupta, D.; Agarwal, R.; Bal, A.; Nijhawan, R.; Aggarwal, A.N. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis: A prospective study of 151 patients. J. Bronchol. Interv. Pulmonol. 2014, 21, 220–226. [Google Scholar] [CrossRef]
- Dziedzic, D.A.; Peryt, A.; Orlowski, T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin. Respir. J. 2017, 11, 58–63. [Google Scholar] [CrossRef]
- Kasper, L.; Andrychiewicz, A.; Gross-Sondej, I.; Soja, J.; Kasper, M.; Tomaszewska, R.; Urbanczyk, K.; Sladek, K. Combination of endosonography-guided fine-needle aspiration and conventional endoscopic techniques in sarcoidosis diagnosis. Optimal strategy to achieve high diagnostic yield. Clin. Respir. J. 2021, 15, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chandra, S. A “ROSE” in every “EBUS” keeps transbronchial lung biopsy away. Chest 2014, 146, e97. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Saka, H.; Ando, M.; Nakashima, H.; Shiraki, A.; Murakami, Y.; Kogure, Y.; Kitagawa, C.; Kato, T. How Many Passes Are Needed for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Sarcoidosis? A Prospective Multicenter Study. Respir. Int. Rev. Thorac. Dis. 2018, 95, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, H.; Teng, J.; Zhang, J.; Zhao, H.; Garfield, D.H.; Han, B. Determining factors in diagnosing pulmonary sarcoidosis by endobronchial ultrasound-guided transbronchial needle aspiration. Ann. Thorac. Surg. 2015, 99, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Salman, R.; Sutherland, T. Does Endobronchial Ultrasound-guided transbronchial needle aspiration (EBUS) alter the diagnosis in suspected Sarcoidosis? In Proceedings of the ERS International Congress. Madrid, Spain, 28 September–2 October; 2019. [Google Scholar]
- Ayub, I.; Mohan, A.; Madan, K.; Hadda, V.; Jain, D.; Khilnani, G.C.; Guleria, R. Identification of specific EBUS sonographic characteristics for predicting benign mediastinal lymph nodes. Clin. Respir. J. 2018, 12, 681–690. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Teng, J.; Zhong, R.; Han, B.; Sun, J. Sonographic Features of Endobronchial Ultrasound in Differentiation of Benign Lymph Nodes. Ultrasound Med. Biol. 2016, 42, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Muthu, V.; Gupta, N.; Dhooria, S.; Sehgal, I.S.; Bal, A.; Aggarwal, A.N.; Behera, D.; Agarwal, R. A Prospective, Randomized, Double-Blind Trial Comparing the Diagnostic Yield of 21- and 22-Gauge Aspiration Needles for Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Sarcoidosis. Chest 2016, 149, 1111–1113. [Google Scholar] [CrossRef]
- Dhooria, S.; Sehgal, I.S.; Gupta, N.; Bal, A.; Prasad, K.T.; Aggarwal, A.N.; Ram, B.; Agarwal, R. A Randomized Trial Evaluating the Effect of 10 versus 20 Revolutions Inside the Lymph Node on the Diagnostic Yield of EBUS-TBNA in Subjects with Sarcoidosis. Respir. Int. Rev. Thorac. Dis. 2018, 96, 464–471. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaliawala, H.A.; Farooqui, S.M.; Harris, K.; Abdo, T.; Keddissi, J.I.; Youness, H.A. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): Technical Updates and Pathological Yield. Diagnostics 2021, 11, 2331. https://doi.org/10.3390/diagnostics11122331
Jaliawala HA, Farooqui SM, Harris K, Abdo T, Keddissi JI, Youness HA. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): Technical Updates and Pathological Yield. Diagnostics. 2021; 11(12):2331. https://doi.org/10.3390/diagnostics11122331
Chicago/Turabian StyleJaliawala, Huzaifa A., Samid M. Farooqui, Kassem Harris, Tony Abdo, Jean I. Keddissi, and Houssein A. Youness. 2021. "Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): Technical Updates and Pathological Yield" Diagnostics 11, no. 12: 2331. https://doi.org/10.3390/diagnostics11122331
APA StyleJaliawala, H. A., Farooqui, S. M., Harris, K., Abdo, T., Keddissi, J. I., & Youness, H. A. (2021). Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): Technical Updates and Pathological Yield. Diagnostics, 11(12), 2331. https://doi.org/10.3390/diagnostics11122331






