Incidence of Spinal CSF Leakage on CT Myelography in Patients with Nontraumatic Intracranial Subdural Hematoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Retrospective Review of Electronic Medical Records and Imaging Findings
2.3. Statistical Analysis
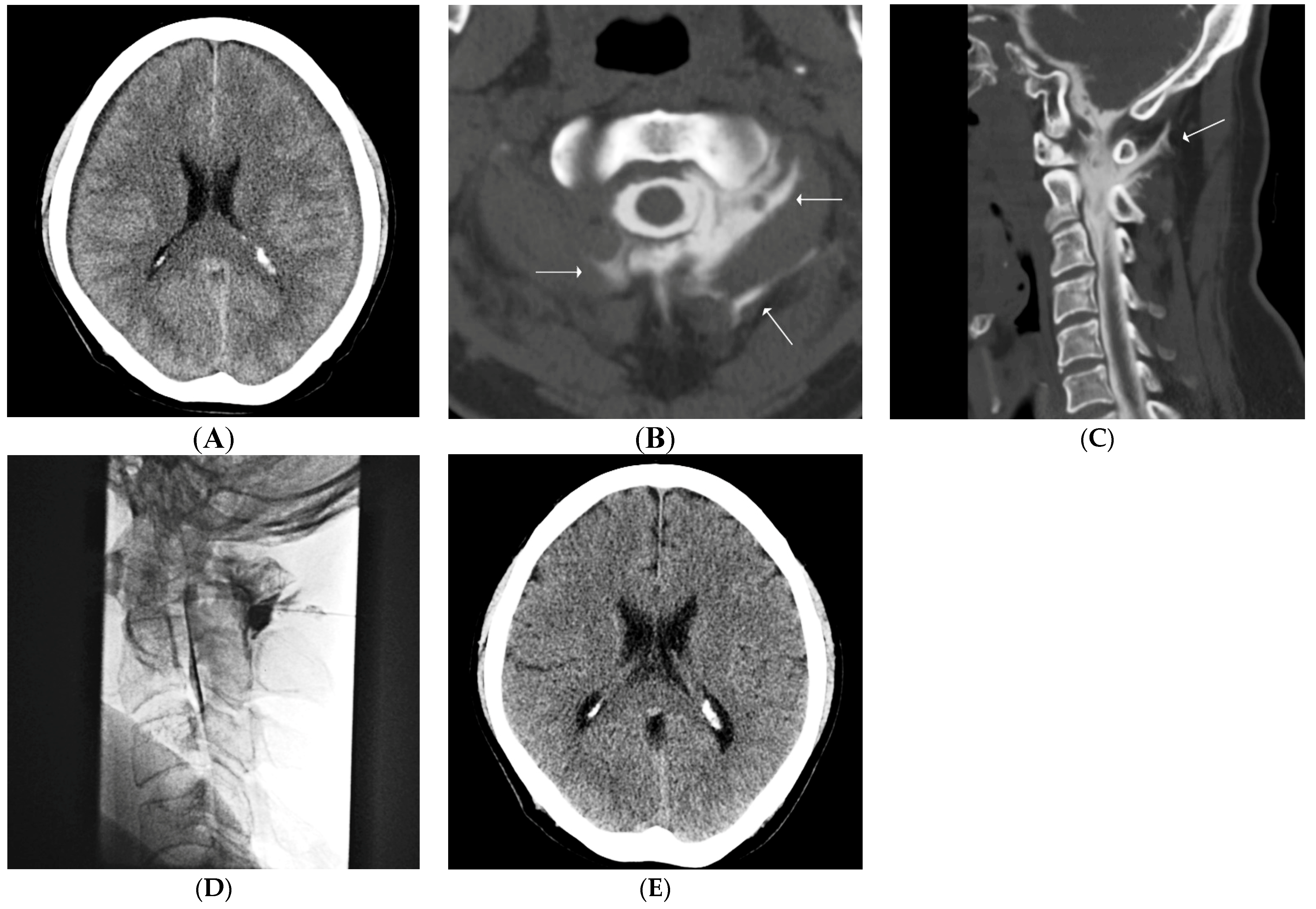
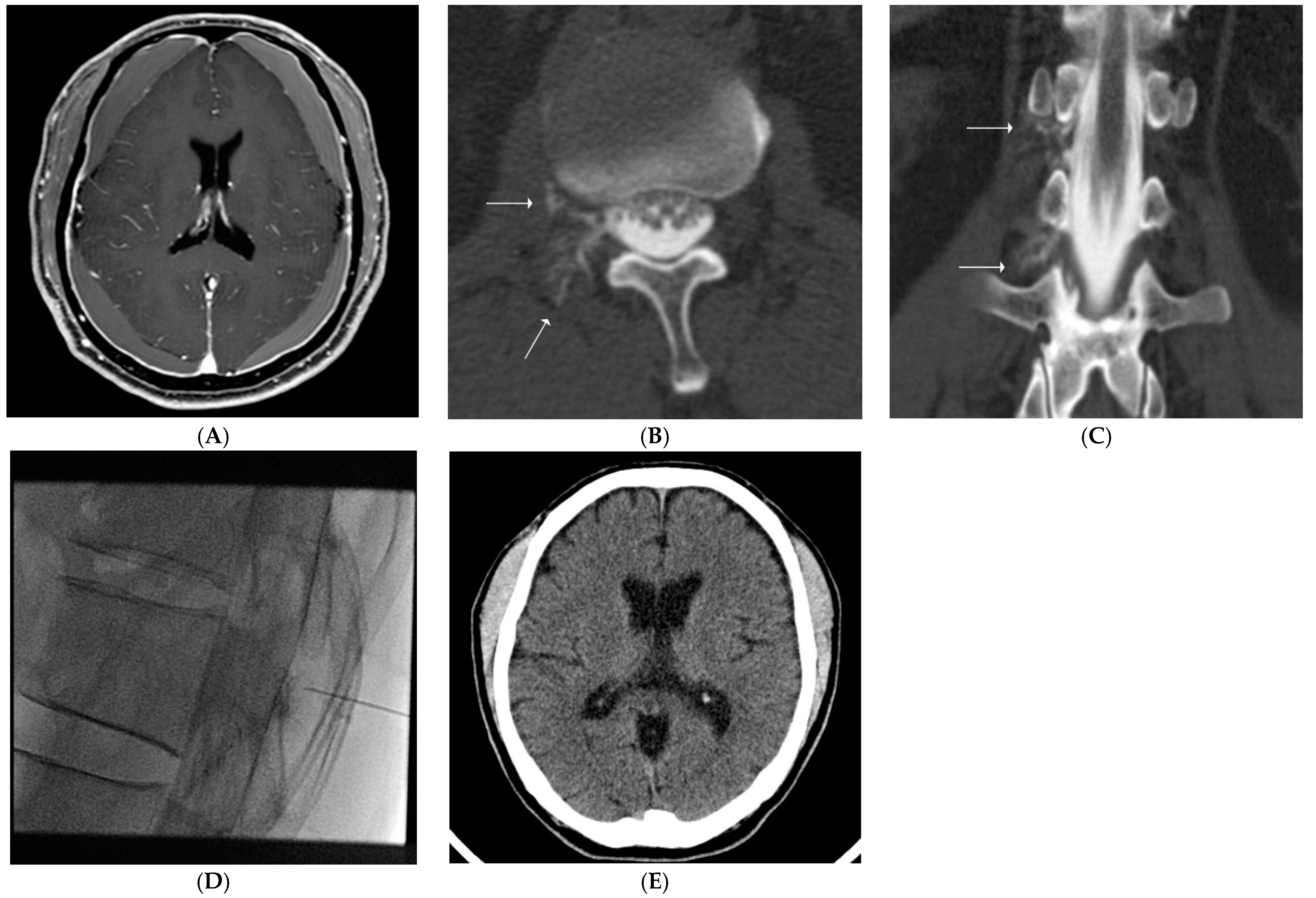
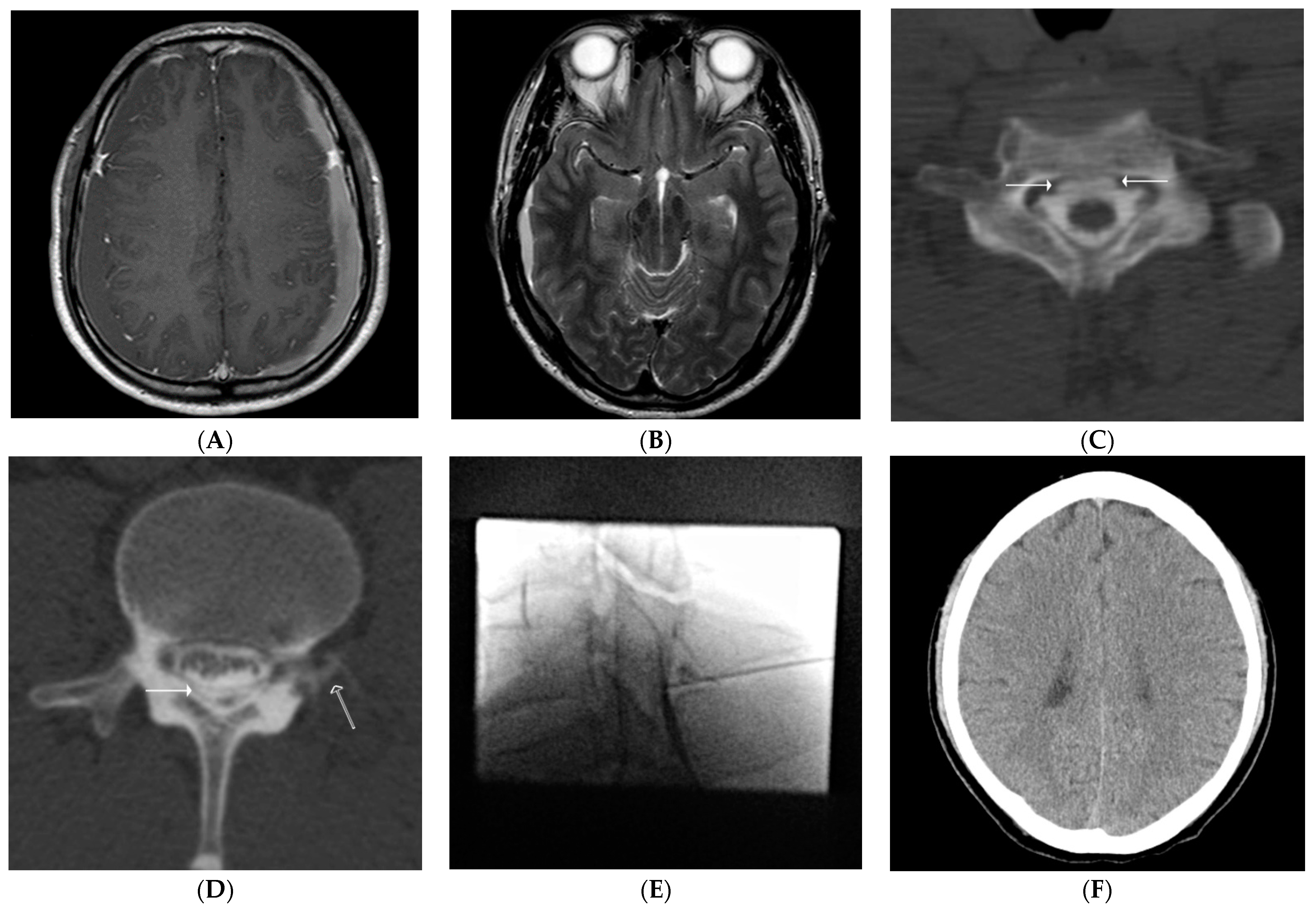
2.4. Our Routine CT Myelography Procedure & Interpretation
2.5. Our Routine EBP Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avis, S.P. Nontraumatic acute subdural hematoma: A case report and review of the literature. Am. J. Forensic Med. Pathol. 1993, 14, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Korosue, K.; Kondoh, T.; Ishikawa, Y.; Nagao, T.; Tamaki, N.; Matsumoto, S. Acute subdural hematoma associated with nontraumatic middle meningeal artery aneurysm: Case report. Neurosurgery 1988, 22, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.B.; Coombs, B.L.; Chin, E.J. Acute spontaneous subdural hematoma in a middle-aged adult: Case report and review of the literature. J. Emerg. Med. 2014, 47, e63–e68. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, A.F.; Grobelny, B.T.; Zacharia, B.; Hickman, Z.; DeRosa, P.L.; Anderson, K.; Sussman, E.; Carpenter, A.; Connolly, E.S. The surgical management of chronic subdural hematoma. Neurosurg. Rev. 2012, 35, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Nassiri, F.; Mansouri, A.; Badhiwala, J.H.; Witiw, C.D.; Shamji, M.F.; Peng, P.W.; Farb, R.I.; Bernstein, M. Spontaneous intracranial hypotension: A review and introduction of an algorithm for management. World Neurosurg. 2017, 101, 343–349. [Google Scholar] [CrossRef]
- Kranz, P.; Luetmer, P.H.; Diehn, F.E.; Amrhein, T.J.; Tanpitukpongse, T.P.; Gray, L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. Am. J. Roentgenol. 2016, 206, 8–19. [Google Scholar] [CrossRef]
- Ferrante, E.; Rubino, F.; Beretta, F.; Regna-Gladin, C.; Ferrante, M.M. Treatment and outcome of subdural hematoma in patients with spontaneous intracranial hypotension: A report of 35 cases. Acta Neurol. Belg. 2018, 118, 61–70. [Google Scholar] [CrossRef]
- Inamasu, J.; Moriya, S.; Shibata, J.; Kumai, T.; Hirose, Y. Spontaneous intracranial hypotension manifesting as a unilateral subdural hematoma with a marked midline shift. Case Rep. Neurol. 2015, 7, 71–77. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, J.; Xie, D.; Xue, Z.; Wang, Y.; Yang, S. Clinical characteristics of 15 cases of chronic subdural hematomas due to spontaneous intracranial hypotension with spinal cerebrospinal fluid leak. Acta Neurol. Belg. 2016, 116, 509–512. [Google Scholar] [CrossRef]
- Wendl, C.M.; Schambach, F.; Zimmer, C.; Foerschler, A. CT myelography for the planning and guidance of targeted epidural blood patches in patients with persistent spinal CSF leak. Am. J. Neuroradiol. 2012, 24, 1711–1714. [Google Scholar]
- Mokri, B.; Piepgras, D.G.; Miller, G.M. Syndrome of orthostatic headaches and diffuse pachymeningeal gadolinium enhancement. Mayo. Clin. Proc. 2007, 72, 400–413. [Google Scholar] [CrossRef]
- Schievink, W.I.; Deline, C.R. Headache secondary to intracranial hypotension. Curr. Pain. Headache Rep. 2014, 18, 457. [Google Scholar] [CrossRef] [PubMed]
- Kranz, P.G.; Malinzak, M.D.; Amrhein, T.J.; Gray, L. Update on the diagnosis and treatment of spontaneous intracranial hypotension. Curr. Pain Headache Rep. 2017, 21, 37. [Google Scholar] [CrossRef]
- Beck, J.; Gralla, J.; Fung, C.; Ulrich, C.T.; Schucht, P.; Fichtner, J.; Andereggen, L.; Gosau, M.; Hattingen, E.; Gutbrod, K.; et al. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J. Neurosurg. 2014, 121, 1380–1387. [Google Scholar] [CrossRef]
- Motiei-Langroudi, R.; Stippler, M.; Shi, S.; Adeeb, N.; Gupta, R.; Griessenauer, C.J.; Papavassiliou, E.; Kasper, E.M.; Arle, J.; Alterman, R.L.; et al. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. CT-guided epidural blood patching of directly observed or potential leak sites for the targeted treatment of spontaneous intracranial hypotension. J. Neurosurg. 2017, 129, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, H.; Tanishima, T.; Yoshimasu, N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J. Neurosurg. 2001, 95, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Torihashi, K.; Sadamasa, N.; Yoshida, K.; Narumi, O.; Chin, M.; Yamagata, S. Independent predictors for recurrence of chronic subdural hematoma: A review of 343 consecutive surgical cases. Neurosurgery 2008, 63, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J.E. Surgical management of acute subdural hematomas. Neurosurgery 2006, 58, S2-16–S2-24. [Google Scholar]
- Nishizaki, T.; Ikeda, N.; Nakano, S.; Sakakura, T.; Fujii, N.; Okamura, T. Clinical status of patients with cerebrospinal fluid hypovolemia treated with an epidural blood patch. Open J. Mod. Neurosurg. 2015, 5, 107–112. [Google Scholar] [CrossRef][Green Version]
- Ferrante, E.; Olgiati, E.; Sangalli, V.; Rubino, F. Early pain relief from orthostatic headache and hearing changes in spontaneous intracranial hypotension after epidural blood patch. Acta Neurol. Belg. 2016, 116, 503–508. [Google Scholar] [CrossRef]
- Staudt, M.D.; Pasternak, S.H.; Sharma, M.; Pandey, S.K.; Arango, M.F.; Pelz, D.M.; Lownie, S.P. Multilevel, ultra-large-volume epidural blood patch for the treatment of neurocognitive decline associated with spontaneous intracranial hypotension: Case report. J. Neurosurg. 2018, 129, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Monroe, B.R. Successful treatment of postdural puncture headache using epidural fibrin glue patch after persistent failure of epidural blood patches. Pain Pract. 2017, 17, 956–960. [Google Scholar] [CrossRef] [PubMed]
| Leak (+) Total N = 48 | Leak (−) Total N = 12 | p-Value | |
|---|---|---|---|
| Male sex, n (%) | 31 (64.58) | 8 (66.67) | 1.000 |
| Mean age ± standard deviation | 56.85 ± 15.50 | 65.83 ± 13.93 | 0.165 |
| Age < 69, n (%) | 34 (70.83) | 3 (25.00) | 0.006 |
| Wafarin, n (%) | 0 (0.00) | 1 (8.33) | 0.200 |
| Aspirin, n (%) | 8 (16.67) | 1 (8.33) | 0.671 |
| Clopidogrel, n (%) | 3 (6.25) | 0 (0.00) | 1.000 |
| Orthostatic headache, n (%) | 12 (25.00) | 1 (8.33) | 0.628 |
| Glasgow coma scale score of 15, n (%) | 48 (100.00) | 12 (100.00) | N/A |
| Unilateral subdural hematoma, n (%) | 9 (18.75) | 5 (41.67) | 0.13 |
| The degree of midline shift (mm ± standard deviation) | 3.34 ± 3.47 | 3.82 ± 4.46 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Lee, J.W.; Lee, E.; Kang, Y.; Ahn, J.M. Incidence of Spinal CSF Leakage on CT Myelography in Patients with Nontraumatic Intracranial Subdural Hematoma. Diagnostics 2021, 11, 2278. https://doi.org/10.3390/diagnostics11122278
Kim HJ, Lee JW, Lee E, Kang Y, Ahn JM. Incidence of Spinal CSF Leakage on CT Myelography in Patients with Nontraumatic Intracranial Subdural Hematoma. Diagnostics. 2021; 11(12):2278. https://doi.org/10.3390/diagnostics11122278
Chicago/Turabian StyleKim, Hyo Jin, Joon Woo Lee, Eugene Lee, Yusuhn Kang, and Joong Mo Ahn. 2021. "Incidence of Spinal CSF Leakage on CT Myelography in Patients with Nontraumatic Intracranial Subdural Hematoma" Diagnostics 11, no. 12: 2278. https://doi.org/10.3390/diagnostics11122278
APA StyleKim, H. J., Lee, J. W., Lee, E., Kang, Y., & Ahn, J. M. (2021). Incidence of Spinal CSF Leakage on CT Myelography in Patients with Nontraumatic Intracranial Subdural Hematoma. Diagnostics, 11(12), 2278. https://doi.org/10.3390/diagnostics11122278






