Prenatal Diagnosis of Clubfoot: Where Are We Now? Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Search
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias
2.8. Statistical Analysis
3. Results
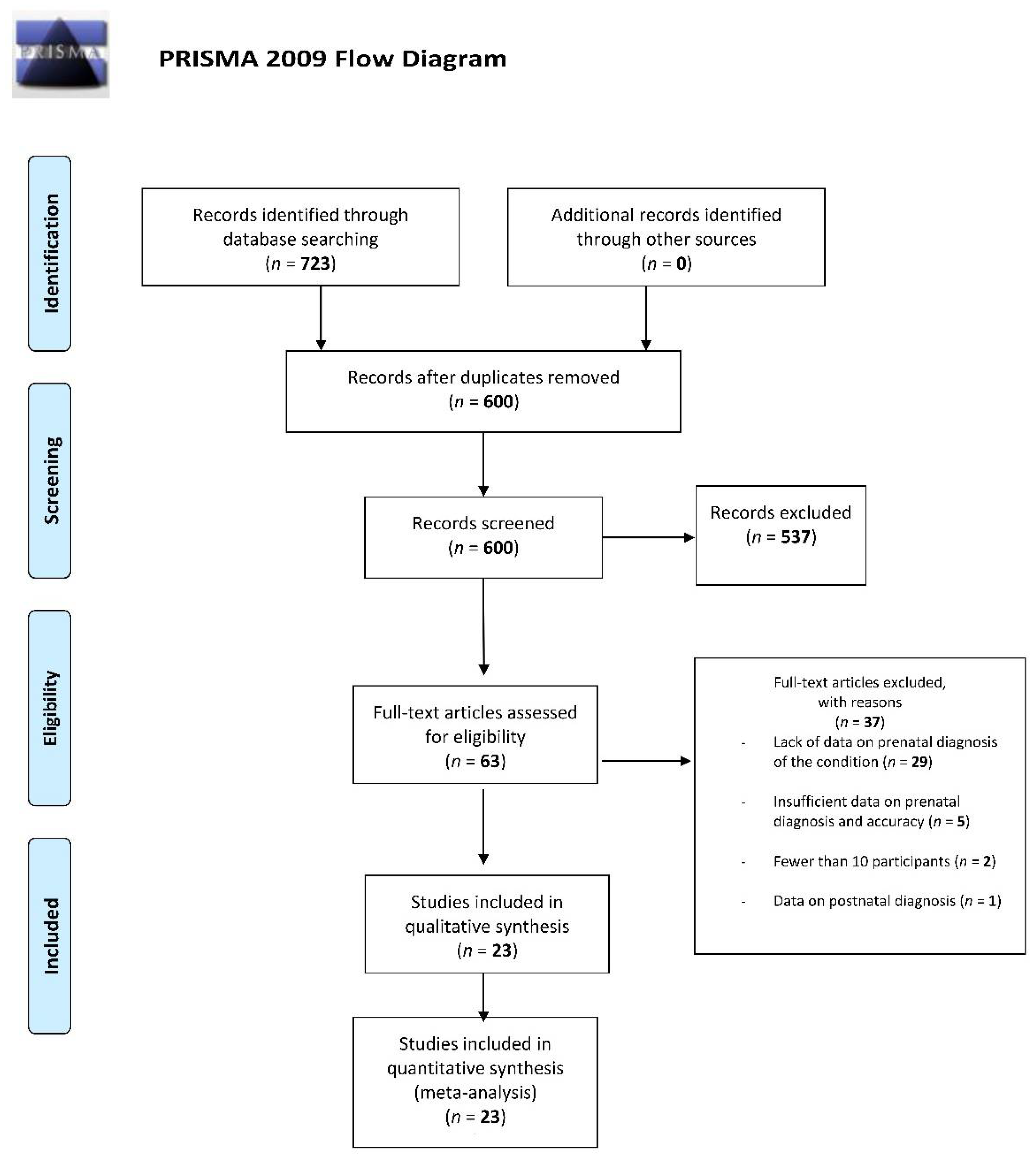
3.1. Study Selection
3.2. Study Characteristics
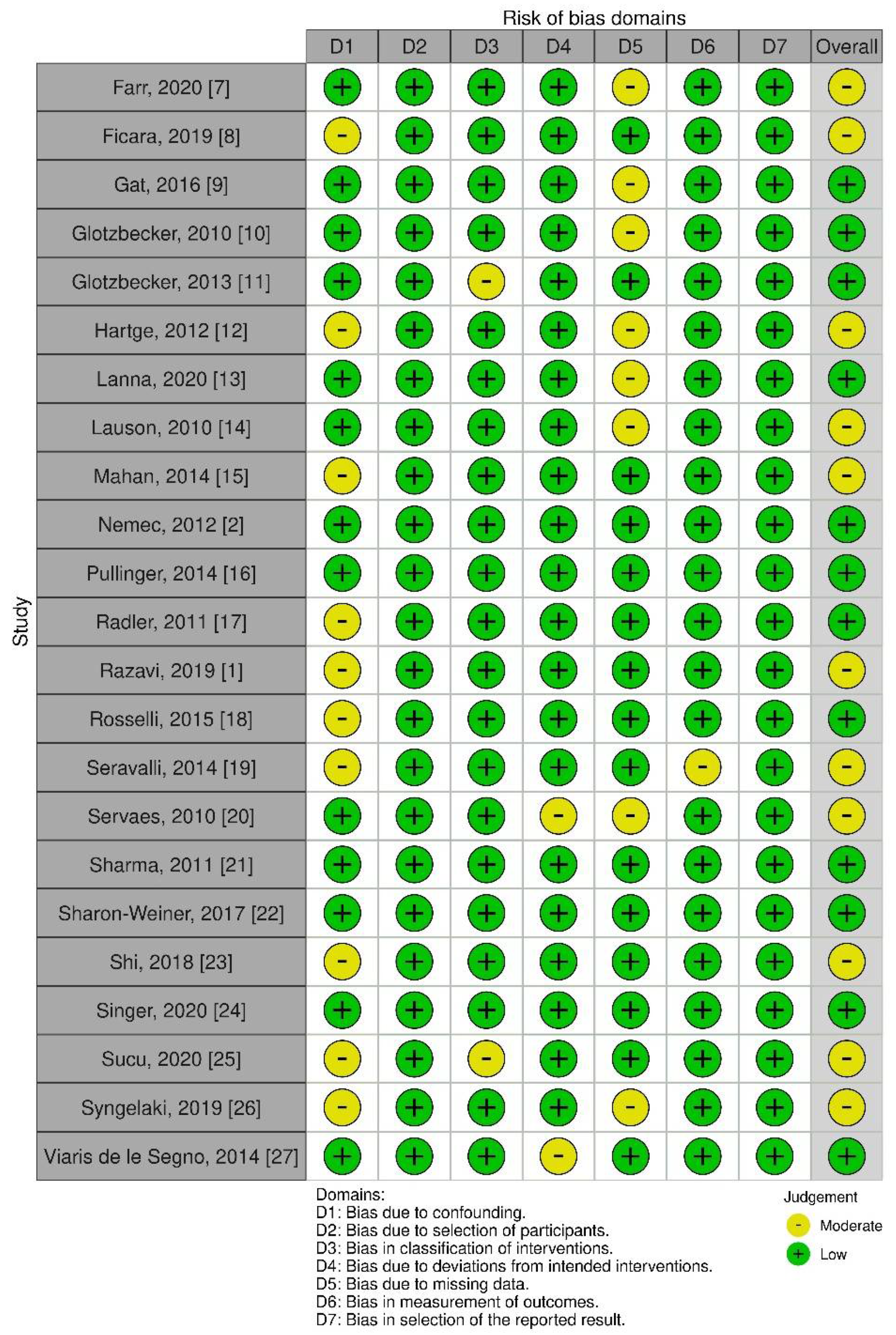
3.3. Quality of Evidence
3.4. Associated Pathologies
3.5. Diagnostic Procedure
3.6. Timing of Diagnosis
3.7. Ultrasound
3.8. Magnetic Resonance Imaging
3.9. Karyotyping
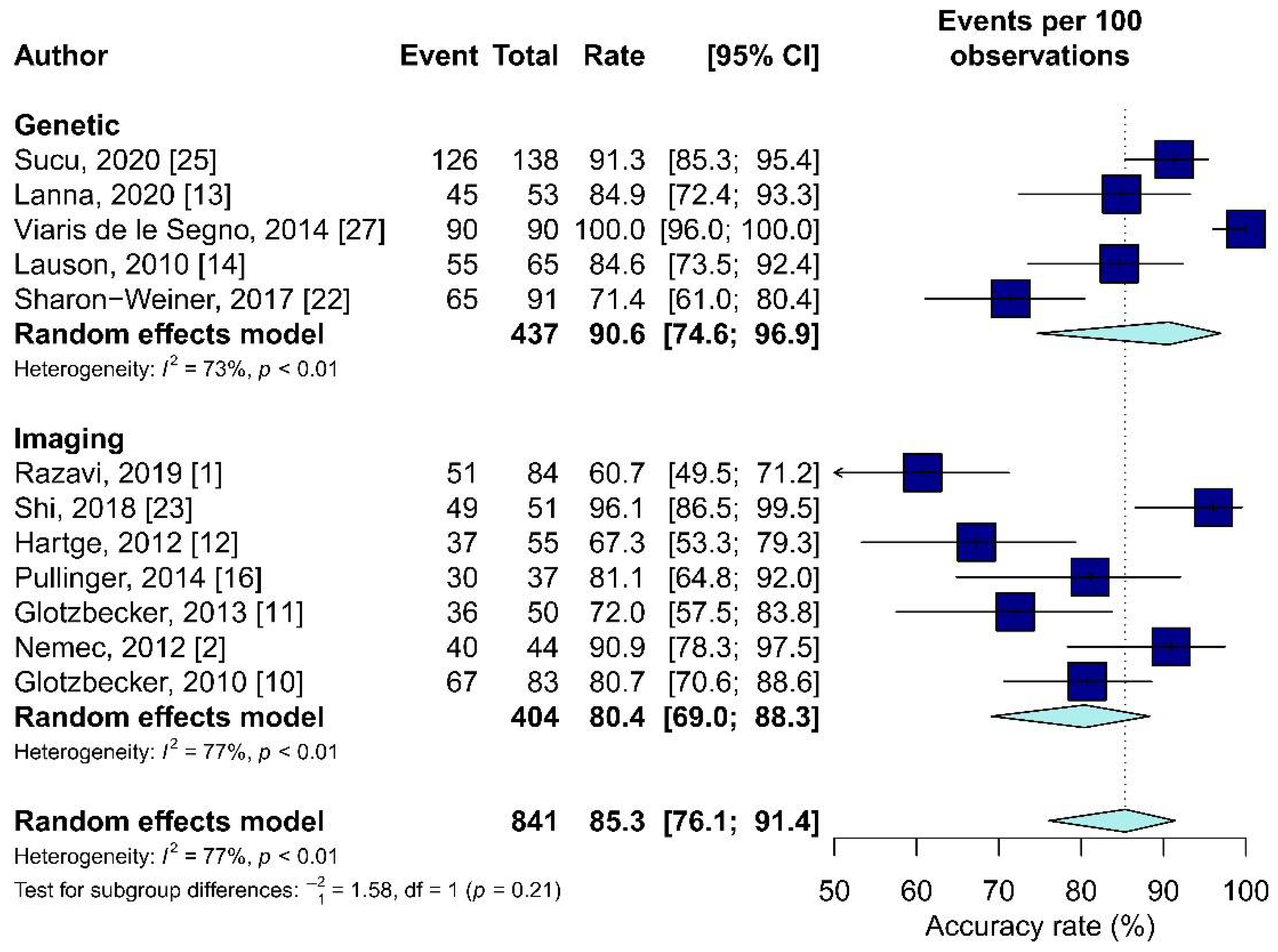
3.10. Meta-Analysis Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razavi, A.S.; Chasen, S.T.; Coombs, S.; Kalish, R.B. Diagnostic accuracy of isolated clubfoot in twin compared to singleton gestations. J. Perinat. Med. 2019, 47, 564–567. [Google Scholar] [CrossRef]
- Nemec, U.; Nemec, S.F.; Kasprian, G.; Brugger, P.C.; Bettelheim, D.; Wadhawan, I.; Kolb, A.; Graham, J.M.; Rimoin, D.L.; Prayer, D. Clubfeet and associated abnormalities on fetal magnetic resonance imaging. Prenat. Diagn. 2012, 32, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Smythe, T.; Kuper, H.; Macleod, D.; Foster, A.; Lavy, C. Birth prevalence of congenital talipes equinovarus in low- and middle-income countries: A systematic review and meta-analysis. Trop Med. Int. Health 2017, 22, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Ebrashy, A.; Kurjak, A.; Adra, A.; Aliyu, L.D.; Wataganara, T.; de Sá, R.A.; Pooh, R.; Sen, C.; Stanojevic, M. Controversial ultrasound findings in mid trimester pregnancy. Evidence based approach. J. Perinat. Med. 2016, 44, 131–137. [Google Scholar] [CrossRef]
- Brasseur-Daudruy, M.; Abu Amara, S.; Ickowicz-Onnient, V.; Touleimat, S.; Verspyck, E. Clubfoot Versus Positional Foot Deformities on Prenatal Ultrasound Imaging. J. Ultrasound Med. 2020, 39, 615–623. [Google Scholar] [CrossRef]
- Faldini, C.; Traina, F.; Nanni, M.; Sanzarello, I.; Borghi, R.; Perna, F. Congenital idiopathic talipes equinovarus before and after walking age: Observations and strategy of treatment from a series of 88 cases. J. Orthop. Traumatol. 2016, 17, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Farr, A.; Wachutka, E.; Bettelheim, D.; Windsperger, K.; Farr, S. Perinatal outcomes of infants with congenital limb malformations: An observational study from a tertiary referral center in Central Europe. BMC Pregnancy Childbirth 2020, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Ficara, A.; Syngelaki, A.; Hammami, A.; Akolekar, R.; Nicolaides, K.H. Value of routine ultrasound examination at 35–37 weeks’ gestation in diagnosis of fetal abnormalities. Ultrasound Obstet. Gynecol. 2020, 55, 75–80. [Google Scholar] [CrossRef]
- Gat, I.; Bar Yosef, O.; Hoffmann, C.; Lebovitz, O.; Shashar, D.; Gilboa, Y.; Yagel, I.; Achiron, R.; Katorza, E. Prenatal Brain Imaging in Isolated vs. Complicated Club Foot: A Cohort Study. Ultraschall Med. 2016, 37, 591–597. [Google Scholar] [CrossRef]
- Glotzbecker, M.P.; Estroff, J.A.; Spencer, S.A.; Bosley, J.C.; Parad, R.B.; Kasser, J.R.; Mahan, S.T. Prenatally diagnosed clubfeet: Comparing ultrasonographic severity with objective clinical outcomes. J. Pediatr. Orthop. 2010, 30, 606–611. [Google Scholar] [CrossRef]
- Glotzbecker, M.P.; Estroff, J.A.; Curtis, T.A.; Kalish, L.A.; Spencer, S.A.; Parad, R.B.; Kasser, J.R.; Mahan, S.T. Prospective evaluation of a prenatal sonographic clubfoot classification system. Fetal. Diagn. Ther. 2013, 34, 236–240. [Google Scholar] [CrossRef]
- Hartge, D.R.; Gaertner, S.; Weichert, J. Prenatal detection and postnatal outcome of congenital talipes equinovarus in 106 fetuses. Arch. Gynecol. Obstet. 2012, 286, 831–842. [Google Scholar] [CrossRef]
- Lanna, M.; Casati, D.; Torre, C.; Monforte, S.; Andreacchio, A.; Faiola, S.; Cetin, I.; Rustico, M. Congenital isolated clubfoot: Correlation between prenatal assessment and postnatal degree of severity. Prenat. Diagn 2020, 40, 1547–1552. [Google Scholar] [CrossRef]
- Lauson, S.; Alvarez, C.; Patel, M.S.; Langlois, S. Outcome of prenatally diagnosed isolated clubfoot. Ultrasound Obstet. Gynecol. 2010, 35, 708–714. [Google Scholar] [CrossRef]
- Mahan, S.T.; Yazdy, M.M.; Kasser, J.R.; Werler, M.M. Prenatal screening for clubfoot: What factors predict prenatal detection? Prenat. Diagn. 2014, 34, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Pullinger, M.; Southorn, T.; Easton, V.; Hutchinson, R.; Smith, R.P.; Sanghrajka, A.P. An evaluation of prenatal ultrasound screening for CTEV: Accuracy data from a single NHS University Teaching Hospital. Bone Jt. J. 2014, 96, 984–988. [Google Scholar] [CrossRef]
- Radler, C.; Myers, A.K.; Burghardt, R.D.; Arrabal, P.P.; Herzenberg, J.E.; Grill, F. Maternal attitudes towards prenatal diagnosis of idiopathic clubfoot. Ultrasound Obstet. Gynecol. 2011, 37, 658–662. [Google Scholar] [CrossRef]
- Rosselli, P.; Nossa, S.; Huérfano, E.; Betancur, G.; Guzmán, Y.; Castellanos, C.; Morcuende, J. Prenatal Ultrasound Diagnosis of Congenital Talipes Equinovarus in Bogota (Colombia) Between 2003 and 2012. Iowa Orthop. J. 2015, 35, 156–159. [Google Scholar]
- Seravalli, V.; Pierini, A.; Bianchi, F.; Giglio, S.; Vellucci, F.L.; Cariati, E. Prevalence and prenatal ultrasound detection of clubfoot in a non-selected population: An analysis of 549, 931 births in Tuscany. J. Matern. Fetal Neonatal. Med. 2015, 28, 2066–2069. [Google Scholar] [CrossRef] [Green Version]
- Servaes, S.; Hernandez, A.; Gonzalez, L.; Victoria, T.; Johnson, M.; Jaramillo, D.; Christopher Edgar, J.; Johnson, A. Fetal MRI of clubfoot associated with myelomeningocele. Pediatr. Radiol. 2010, 40, 1874–1879. [Google Scholar] [CrossRef]
- Sharma, R.; Stone, S.; Alzouebi, A.; Hamoda, H.; Kumar, S. Perinatal outcome of prenatally diagnosed congenital talipes equinovarus. Prenat. Diagn. 2011, 31, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Sharon-Weiner, M.; Sukenik-Halevy, R.; Tepper, R.; Fishman, A.; Biron-Shental, T.; Markovitch, O. Diagnostic accuracy, work-up, and outcomes of pregnancies with clubfoot detected by prenatal sonography. Prenat. Diagn. 2017, 37, 754–763. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B.; Kong, F.; Li, X. Prenatal limb defects: Epidemiologic characteristics and an epidemiologic analysis of risk factors. Medicine 2018, 97, e11471. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Maya, I.; Banne, E.; Baris Feldman, H.; Vinkler, C.; Ben Shachar, S.; Sagi-Dain, L. Prenatal clubfoot increases the risk for clinically significant chromosomal microarray results-Analysis of 269 singleton pregnancies. Early Hum. Dev. 2020, 145, 105047. [Google Scholar] [CrossRef]
- Sucu, M.; Demir, S.C. The relationship between isolated pes equinovarus and aneuploidies and perinatal outcomes: Results of a tertiary center. Turk. J. Obstet. Gynecol. 2020, 17, 270–277. [Google Scholar] [CrossRef]
- Syngelaki, A.; Hammami, A.; Bower, S.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11-13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2019, 54, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Viaris de le Segno, B.; Gruchy, N.; Bronfen, C.; Dolley, P.; Leporrier, N.; Creveuil, C.; Benoist, G. Prenatal diagnosis of clubfoot: Chromosomal abnormalities associated with fetal defects and outcome in a tertiary center. J. Clin. Ultrasound 2016, 44, 100–105. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Piergentili, I.; Oggiano, L.; Costici, P.F.; Denaro, V. Developmental Hip Dysplasia: An Epidemiological Nationwide Study in Italy from 2001 to 2016. Int. J. Environ. Res. Public Health 2021, 18, 6589. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Piergentili, I.; Oggiano, L.; Costici, P.F.; Denaro, V. Trends in hospitalisation of Subtalar Joint Arthroereisis in Italy from 2009 to 2016. Foot Ankle Surg. 2021. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Candela, V.; Piergentili, I.; Oggiano, L.; Costici, P.F.; Denaro, V. Slipped capital femoral epiphysis: An epidemiological Nationwide study in Italy from 2001 to 2015. BMC Musculoskelet. Disord. 2021, 22, 570. [Google Scholar] [CrossRef] [PubMed]
- Bogers, H.; Rifouna, M.S.; Cohen-Overbeek, T.E.; Koning, A.H.J.; Willemsen, S.P.; van der Spek, P.J.; Steegers-Theunissen, R.P.M.; Exalto, N.; Steegers, E.A.P. First trimester physiological development of the fetal foot position using three-dimensional ultrasound in virtual reality. J. Obstet. Gynaecol. Res. 2019, 45, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Offerdal, K.; Jebens, N.; Blaas, H.G.; Eik-Nes, S.H. Prenatal ultrasound detection of talipes equinovarus in a non-selected population of 49 314 deliveries in Norway. Ultrasound Obstet. Gynecol. 2007, 30, 838–844. [Google Scholar] [CrossRef]
| Author, Year | Country | Type of Study, Level of Evidence | Sample Size | Diagnosis | Accuracy (%) | Sex (F/M) | Diagnosis | Timing | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging | Genetic | ||||||||||
| MRI (n) | US (n) | A (n) | Other (n) | ||||||||
| Razavi, 2019 [1] | Germany | Retrospective Comparative Study, III | * | 84 | 51/84 60.7% | 29/55 (Sample size) | - | x | - | - | - |
| Nemec, 2012 [2] | Austria, USA | Retrospective Study, III | - | 44 | - | - | X $ | x | - | - | - |
| Farr, 2020 [7] | Austria | Retrospective Cohort Study, III | 104 | 56 | - | - | - | x | - | - | Mean: Week 20.5 ± 5.4 |
| Ficara, 2019 [8] | UK | Prospective Comparative Study, II | 52,400 | 61 | - | - | - | x | - | - | T1 and T2 (60) T3 (1) |
| Gat, 2016 [9] | Israel | Retrospective Study, III | 28 | 12 | - | - | x (14) | x | - | - | - |
| Glotzbecker, 2010 [10] | USA | Retrospective Study, III | - | 107 of which: 83 survived | 67/83 80.7% | - | - | x | - | - | - |
| Glotzbecker, 2013 [11] | USA | Prospective Study, I | - | 50 | 36/50 72.0% | - | - | x | - | - | - |
| Hartge, 2012 [12] | Germany | Retrospective Study, III | 106, survived: 55 | 55 | 37/55 67.0% | - | - | x | - | - | - |
| Lanna, 2020 [13] | Italy | Retrospective Cohort Study, III | 64 | 53 | 45/53 84.9% | - | - | x | X | - | T2 + T3 Follow-up or T3 only |
| Lauson, 2010 [14] | Canada | Retrospective Study, III | - | 65 | 55/65 84.6% | - | - | x | x (41) | - | - |
| Mahan, 2014 [15] | USA | Retrospective Study, III | - | 421 | - | - | x | - | - | - | |
| Pullinger, 2014 [16] | UK | Retrospective Comparative Study, III | - | 74 of which: 37 found suitable for study | 30/37 81.0% | - | - | x | - | - | Between Weeks 18 and 20 |
| Radler, 2011 [17] | Austria, USA | Retrospective Study, III | - | 92 | - | - | - | x | - | - | - |
| Rosselli, 2015 [18] | Colombia | Descriptive, Retrospective Study, III | - | 61 | - | - | - | x | x (13) | - | T1 (8), T2 (38) T3 (14) |
| Seravalli, 2014 [19] | Italy | Descriptive Analysis | 168 | - | - | - | - | x | - | - | - |
| Servaes, 2010 [20] | USA | Prospective Study, I | 13 | - | - | - | X $ | x | - | - | Weeks 19–28 |
| Sharma, 2011 [21] | UK | Retrospective Observational Study, III | 174 | - | - | - | - | x | - | - | Week 21 |
| Sharon-Weiner, 2017 [22] | Israel | Retrospective Study, III | 109 (51 bilateral; 58 unilateral) of which: 91 survived | 91 | 65/91 71.4% | - | - | x | x | CVS | Weeks 14–16 or 21–24 |
| Shi, 2018 [23] | China | Retrospective Study, III | 4080 | 51 | 49/51 96.1% | - | - | x | - | - | Weeks 12–14 |
| Singer, 2020 [24] | Israel | Retrospective Cohort Study, III | 5750 | 269 | - | - | - | x | x (Karyotyping and CMA, 229) | - | Mean: Week 22.6 ± 5.5 |
| Sucu, 2020 [25] | Turkey | Retrospective Cohort Study, III | 7680 | 138 | 126/138 91.3% (3FP in T1, 9FP in T2) | 43/83 (Diagnosis) | - | x | x (83) | - | T1 (10) and T2 (128) |
| Syngelaki, 2019 [26] | UK | Retrospective Cohort Study, III | 101,793 | 89 | - | - | - | x | - | - | T1 (2), T2 (82), T3 (5) |
| Viaris de le Segno, 2014 [27] | France | Retrospective Study, III | 90 | 90/90 100% | - | - | x | x (78) | - | Median: Week 23 | |
| Author, Year | Associated Pathology |
|---|---|
| Lauson, 2010 [14] | Developmental Delay, Symptomatic Epilepsy, Thin corpus callosum, Visual inattentiveness, Peroneal Nerve Palsy, Low weight gain, Mild facial asymmetry, Delayed bone age, Cleft Palate, Finger Camptodactyly, Unusual facies, Cerebral Palsy, Rett Syndrome, Hypotonia, Coarse facial features, Torticollis. |
| Sharma, 2011 [21] | Brain, Heart, and Skeletal structural abnormalities, Hydramnios, Spina Bifida. |
| Singer, 2020 [24] | Chromosomal Aberrations, Submicroscopic CNVs, Trisomies: 18, 21, Sex Chromosome Abdnormalities. |
| Sucu, 2020 [25] | Trisomies: 13, 18, 21, Neural Tube Defect, Skeletal Dysplasia, Cardiac anomalies. |
| Viaris de le Segno, 2014 [27] | 47, XY 1 18 (n = 4) 47, XX118 (n = 1) 46, XX der(8) t(8;11) (n = 1) Triploidy (n = 2) 46, XY inv(4) (n = 1) 47, XYY (n = 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzzini, L.; De Salvatore, S.; Longo, U.G.; Marino, M.; Greco, A.; Piergentili, I.; Costici, P.F.; Denaro, V. Prenatal Diagnosis of Clubfoot: Where Are We Now? Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 2235. https://doi.org/10.3390/diagnostics11122235
Ruzzini L, De Salvatore S, Longo UG, Marino M, Greco A, Piergentili I, Costici PF, Denaro V. Prenatal Diagnosis of Clubfoot: Where Are We Now? Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(12):2235. https://doi.org/10.3390/diagnostics11122235
Chicago/Turabian StyleRuzzini, Laura, Sergio De Salvatore, Umile Giuseppe Longo, Martina Marino, Alessandra Greco, Ilaria Piergentili, Pier Francesco Costici, and Vincenzo Denaro. 2021. "Prenatal Diagnosis of Clubfoot: Where Are We Now? Systematic Review and Meta-Analysis" Diagnostics 11, no. 12: 2235. https://doi.org/10.3390/diagnostics11122235
APA StyleRuzzini, L., De Salvatore, S., Longo, U. G., Marino, M., Greco, A., Piergentili, I., Costici, P. F., & Denaro, V. (2021). Prenatal Diagnosis of Clubfoot: Where Are We Now? Systematic Review and Meta-Analysis. Diagnostics, 11(12), 2235. https://doi.org/10.3390/diagnostics11122235










