Focal Cortical Dysplasia Type Ⅲ Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome
Abstract
:1. Introduction
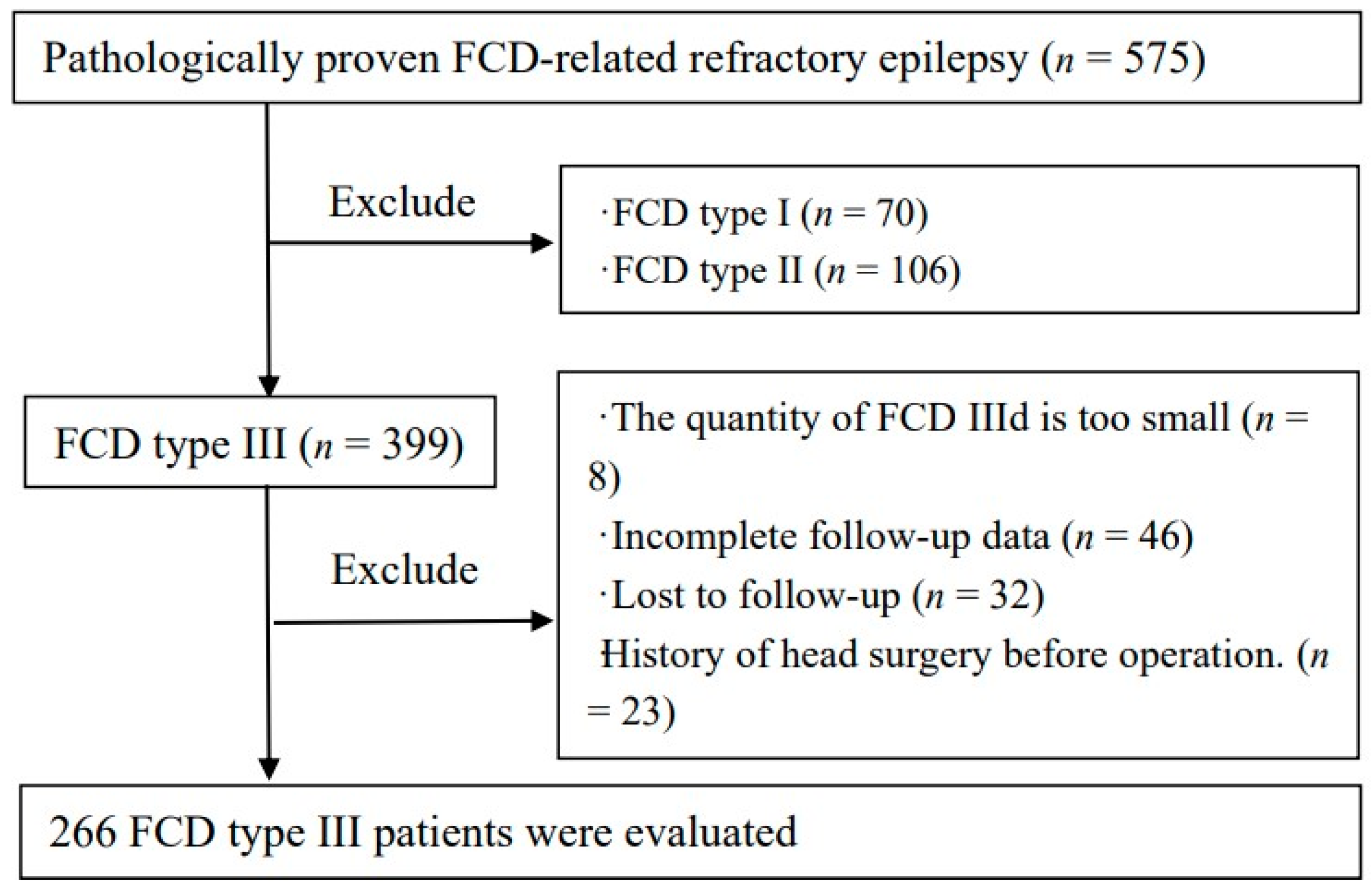
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. Patients Evaluation
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
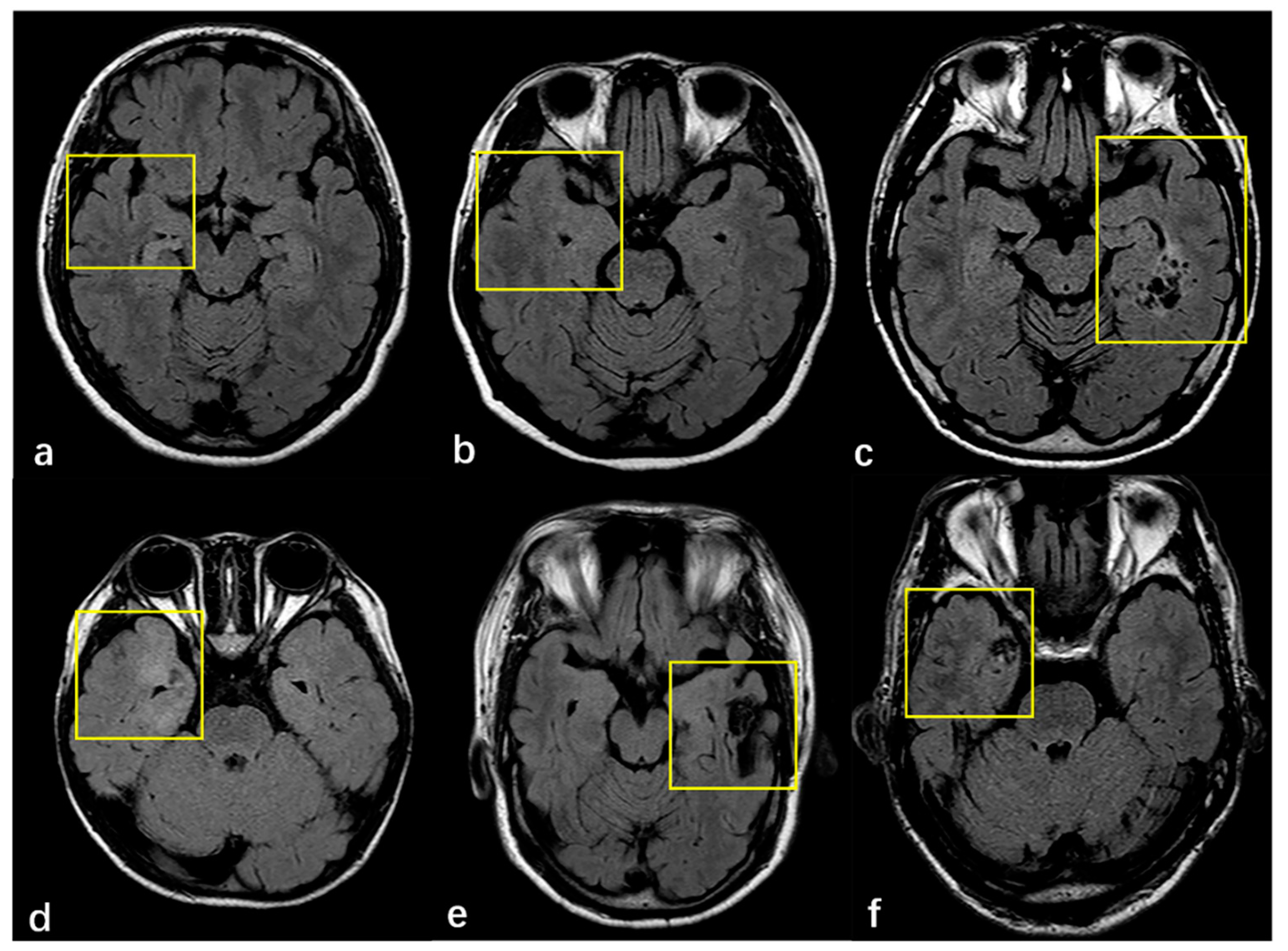
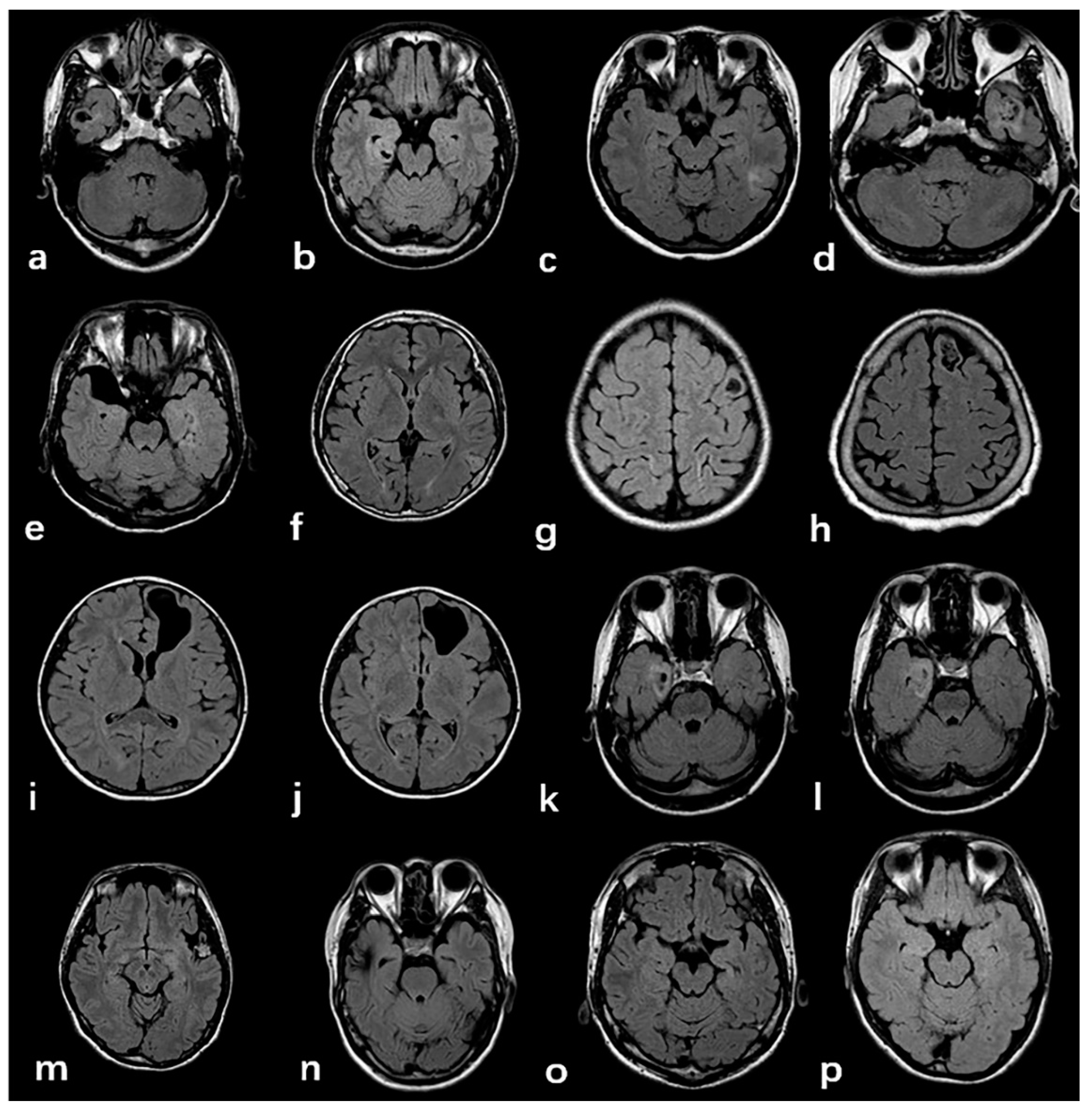
3.2. Radiologic and Pathology Findings
3.3. Predictive Variables for Postoperative Seizure-Freedom
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FCD | Focal Cortical Dysplasia |
| HS | Hippocampal Sclerosis |
| LDEATs | Low-grade Developmental Epilepsy Associated Tumors |
| ILAE | International League Against Epilepsy |
| T2W FLAIR | T2 Weight Fluid-attenuated Inversion Recovery Imaging |
| vEEG | video Electroencephalogram |
| FS | Febrile Seizures |
| SBC | Surface-based classification |
| VBM | Voxel-based morphometry |
| MAP | Morphometry analysis program |
| MSI | Magnetic Source Imaging |
| SWE | Shear-Wave Elastography |
| iMRI | Intraoperative MRI |
References
- Blumcke, I.; Spreafico, R. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumcke, I.; Thom, M. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011, 52, 158–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Choi, J. Pathological Classification of Focal Cortical Dysplasia (FCD): Personal Comments for Well Understanding FCD Classification. J. Korean Neurosurg. Soc. 2019, 62, 288–295. [Google Scholar] [CrossRef]
- Kun, Y.; Zejun, D. Surgical histopathologic findings of 232 Chinese children cases with drug-resistant seizures. Brain Behav. 2020, 10, e01565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingaman, W.E. Surgery for focal cortical dysplasia. Neurology 2004, 62, S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Najm, I.M.; Sarnat, H.B. Review: The international consensus classification of Focal Cortical Dysplasia—A critical update 2018. Neuropathol. Appl. Neurobiol. 2018, 44, 18–31. [Google Scholar] [CrossRef]
- Giulioni, M.; Martinoni, M. About focal cortical dysplasia (FCD) type IIIa. Epilepsy Res. 2014, 108, 1955–1957. [Google Scholar] [CrossRef]
- Tahta, A.; Turgut, M. Focal cortical dysplasia: Etiology, epileptogenesis, classification, clinical presentation, imaging, and management. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2020, 36, 2939–2947. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y. Multimodal noninvasive evaluation in MRI-negative operculoinsular epilepsy. J. Neurosurg. 2019, 132, 1334–1344. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Nanda, S.K. Focal Cortical Dysplasia and Refractory Epilepsy: Role of Multimodality Imaging and Outcome of Surgery. Am. J. Neuroradiol. 2019, 40, 892–898. [Google Scholar] [CrossRef]
- Feng, C.; Zhao, H. Detecting focal cortical dysplasia lesions from FLAIR-negative images based on cortical thickness. Biomed. Eng. Online 2020, 19, 13. [Google Scholar] [CrossRef] [Green Version]
- Jafari-Khouzani, K.; Elisevich, K. Contribution of Quantitative Amygdalar MR FLAIR Signal Analysis for Lateralization of Mesial Temporal Lobe Epilepsy. J. Neuroimaging 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Dworetzky, B.A.; Reinsberger, C. The role of the interictal EEG in selecting candidates for resective epilepsy surgery. Epilepsy Behav. 2011, 20, 167–171. [Google Scholar] [CrossRef]
- Jackson, G.D.; Badawy, R.A. Selecting patients for epilepsy surgery: Identifying a structural lesion. Epilepsy Behav. 2011, 20, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Korsakova, M.B.; Kozlova, A.B. Features of ictal and interictal electrical activity in assessment of the epileptogenic zone in children with focal cortical dysplasias. Zhurnal Vopr. Neirokhirurgii Im. NN Burd. 2019, 83, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Kim, S.Y. Surgical outcome and predictive factors of epilepsy surgery in pediatric isolated focal cortical dysplasia. Epilepsy Res. 2018, 139, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Jung, N.Y. Factors Related to the Clinical Outcomes of Surgery for Extra-Temporal Lobe Epilepsy: Long-Term Follow-Up Results. World Neurosurg. 2018, 115, e645–e649. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q. Comparison of seizure outcomes and safety between anterior temporal lobotomy and lobectomy in patients with temporal lobe epilepsy. Neurol. Res. 2020, 42, 164–169. [Google Scholar] [CrossRef]
- Cossu, M.; d’Orio, P. Focal Cortical Dysplasia IIIa in Hippocampal Sclerosis-Associated Epilepsy: Anatomo-Electro-Clinical Profile and Surgical Results from a Multicentric Retrospective Study. Neurosurgery 2020, 88, 384–393. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Miyata, H. Surgical Pathology of Adulthood Epilepsy. Brain Nerve 2017, 69, 1091–1104. [Google Scholar] [PubMed]
- Thom, M.; Blümcke, I. Long-term epilepsy-associated tumors. Brain Pathol. 2012, 22, 350–379. [Google Scholar] [CrossRef]
- Detre, J.A.; Rao, H. Applications of arterial spin labeled MRI in the brain. J. Magn. Reson. Imaging 2012, 35, 1026–1037. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Herten, A. Hemorrhage from cerebral cavernous malformations: The role of associated developmental venous anomalies. Neurology 2020, 95, e89–e96. [Google Scholar] [CrossRef] [PubMed]
- Palkopoulou, M.; Bakola, E. Cerebral cavernous malformation in a patient with pontine hemorrhage: A case study. Clin. Pract. 2020, 10, 1211. [Google Scholar] [CrossRef]
- Blümcke, I.; Coras, R. Review: Challenges in the histopathological classification of ganglioglioma and DNT: Microscopic agreement studies and a preliminary genotype-phenotype analysis. Neuropathol. Appl. Neurobiol. 2019, 45, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Castro Castro, J.; Lista Martínez, O. Papillary glioneuronal tumor. A case report. Neurocirugia 2019, 30, 144–148. [Google Scholar] [CrossRef]
- Ahmad, R.; Maiworm, M. Cortical Changes in Epilepsy Patients with Focal Cortical Dysplasia: New Insights with T2 Mapping. J. Magn. Reson. Imaging 2020, 52, 1783–1789. [Google Scholar] [CrossRef]
- Rossini, L.; Garbelli, R. Seizure activity per se does not induce tissue damage markers in human neocortical focal epilepsy. Ann. Neurol. 2017, 82, 331–341. [Google Scholar] [CrossRef]
- West, S.; Nevitt, S.J. Surgery for epilepsy. Cochrane Database Syst. Rev. 2019, 6, Cd010541. [Google Scholar] [CrossRef]
- Seifer, G.; Blenkmann, A. Noninvasive approach to focal cortical dysplasias: Clinical, EEG, and neuroimaging features. Epilepsy Res. Treat. 2012, 2012, 736784. [Google Scholar] [CrossRef]
- Chassoux, F.; Devaux, B. Stereoelectroencephalography in focal cortical dysplasia: A 3D approach to delineating the dysplastic cortex. Brain A J. Neurol. 2000, 123 Pt 8, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S.; Wendling, F. Local and remote epileptogenicity in focal cortical dysplasias and neurodevelopmental tumours. Brain 2009, 132 Pt 11, 3072–3086. [Google Scholar] [CrossRef] [Green Version]
- Besson, P.; Andermann, F. Surface-based texture and morphological analysis detects subtle cortical dysplasia. Med. Image Comput. Comput. Assist. Interv. 2008, 11 Pt 1, 645–652. [Google Scholar] [PubMed] [Green Version]
- Pail, M.; Marecek, R. The role of voxel-based morphometry in the detection of cortical dysplasia within the temporal pole in patients with intractable mesial temporal lobe epilepsy. Epilepsia 2012, 53, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.T.; Murayi, R. Prospective use of MRI post-processing in the surgical treatment of MRI-negative orbitofrontal epilepsy. J. Neurol. Sci. 2020, 414, 116828. [Google Scholar] [CrossRef]
- Ishibashi, H.; Simos, P.G.; Papanicolaou, A.C. Localization of ictal and interictal bursting epileptogenic activity in focal cortical dysplasia: Agreement of magnetoencephalography and electrocorticography. Neurol. Res. 2002, 24, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Clemenceau, S. Intraoperative Ultrasound Shear-Wave Elastography in Focal Cortical Dysplasia Surgery. J. Clin. Med. 2021, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Gennari, A.G. Advanced intraoperative ultrasound (ioUS) techniques in focal cortical dysplasia (FCD) surgery: A preliminary experience on a case series. Clin. Neurol. Neurosurg. 2020, 198, 106188. [Google Scholar] [CrossRef]
- Sacino, M.F.; Ho, C.Y. The role of intraoperative MRI in resective epilepsy surgery for peri-eloquent cortex cortical dysplasias and heterotopias in pediatric patients. Neurosurg. Focus 2016, 40, E16. [Google Scholar] [CrossRef]
| Total | Class 1/1a | Class 2–6 | p-Value | |
|---|---|---|---|---|
| Age at epilepsy onset * | 12.5 (6.0–19.0) | 13 (7–20) | 11 (4–18) | 0.145 |
| Duration of epilepsy * | 10 (4–16) | 9 (3–15) | 12 (6–18) | 0.004 |
| Gender n (%) | 0.340 | |||
| Females | 95 (35.7%) | 52 (59.8%) | 35 (40.2%) | |
| Males | 171 (64.3%) | 119 (66.5%) | 62 (71.3%) | |
| Children (≤12 y) | 133 (50.0%) | 84 (46.9%) | 49 (56.3%) | 0.191 |
| FS | 42 (15.8%) | 24 (13.5%) | 18 (20.9%) | 0.150 |
| Seizure types | 0.314 | |||
| Focal onset | 51 (19.2) | 32 (17.9%) | 19(21.8%) | |
| Generalized onset | 139 (52.3%) | 96 (53.6%) | 43 (49.4%) | |
| Focal to bilateral tonic-clonic | 63 (23.7%) | 40 (22.3%) | 23 (26.4%) | |
| Unknown onset | 13 (4.9%) | 11 (6.1%) | 2 (2.3%) | |
| Histology | 0.288 | |||
| FCD Ⅲa | 183 (68.8%) | 118 (65.9%) | 65 (74.7%) | |
| FCD Ⅲb | 61 (22.9%) | 46 (25.7%) | 15 (17.2%) | |
| FCD Ⅲc | 22 (8.3%) | 15 (8.4%) | 7 (8.0%) | |
| FCD | 0.904 | |||
| Ia | 21 (8.2%) | 15 (8.7%) | 6 (7.3%) | 0.932 |
| Ib | 217 (85.1%) | 147 (85%) | 70 (85.4%) | |
| Ic | 17 (6.7%) | 11 (6.4%) | 6 (7.3%) | |
| Lesion location | ||||
| Temporal lobe | 229 (86.1%) | 15 (86.6%) | 74 (85.1%) | |
| Extre-temporal lobe | 12 (4.5%) | 8 (4.5%) | 4 (4.6%) | |
| Multiple lesion | 25 (9.4%) | 16 (8.9%) | 9 (10.3%) | |
| Side of surgery | 0.896 | |||
| Left | 118 (44.4%) | 80 (44.7%) | 38 (43.7%) | |
| Right | 148 (55.6%) | 99 (55.3%) | 49 (56.3%) | |
| Procedures | 0.858 | |||
| Temporal lesionectomy | 172 (64.7%) | 11 (64.2%) | 57 (65.5%) | |
| Anterior temporal lobectomy | 51 (19.2%) | 34 (19.0%) | 17 (19.5%) | |
| Extratemporal lobectomy | 10 (3.8%) | 8 (4.5%) | 2 (2.3%) | |
| Multilobar lesionectomy | 33 (12.4%) | 22 (12.3%) | 11 (12.6%) | |
| Incomplete resection | 40 (15.0%) | 12 (6.7%) | 28 (32.2%) | 0.000 |
| MRI features of FCD | 0.680 | |||
| Cortex atrophy/thickening | 90 (13.5%) | 59 (13.4%) | 31 (13.8%) | |
| Indistinctness of the gray–white matter junction | 176 (66.2%) | 118(65.9%) | 58 (66.7%) | 1.000 |
| Intensity | 166 (62.4%) | 116 (64.8%) | 50 (57.5%) | 0.281 |
| MRI Negative | 36 (13.5%) | 19 (10.6%) | 17 (19.5%) | 0.056 |
| Total | FCD Subtype | FCD-Associated Tumor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCD Ia | FCD Ib | FCD Ic | GG | DNT | PNT | AG | GG+DNT | CCM | AVM | ||
| FCD IIIa | 183 | 15 (8.6%) | 147 (84.0%) | 13 (7.4%) | / | / | / | / | / | / | / |
| FCD IIIb | 61 | 5 (8.6%) | 49 (84.5%) | 4 (6.9%) | 41 (67.2%) | 14 (22.9%) | 1 (1.6%) | 1 (1.6%) | 1 (1.6%) | / | / |
| FCD IIIc | 22 | 1 (4.5%) | 21 (95.5%) | 0 | / | / | / | / | / | 15 (68.2%) | 7 (31.8%) |
| Factors | OR (95% CI) | p Value |
|---|---|---|
| Age at epilepsy onset | 0.99 (0.96–1.03) | 0.617 |
| Duration of epilepsy | 1.03 (0.99–1.07) | 0.132 |
| Febrile convulsions | 0.73 (0.32–1.64) | 0.441 |
| MRI Negative | 0.34 (0.45–0.81) | 0.015 |
| Complete removed | 0.12 (0.05–0.29) | <0.001 |
| Seizure Types | ||
| Focal onset | 1 | 0.394 |
| Generalized onset | 1.19 (0.20–6.90) | 0.850 |
| Focal to bilateral tonic-clonic | 2.12 (0.42–10.9) | 0.364 |
| Unknown onset | 2.47 (0.32–1.63) | 0.295 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Deng, D.; Zhou, C.; Li, H.; Guan, X.; Fang, L.; Cai, Q.; Wang, W.; Zhou, Q. Focal Cortical Dysplasia Type Ⅲ Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome. Diagnostics 2021, 11, 2225. https://doi.org/10.3390/diagnostics11122225
Wang X, Deng D, Zhou C, Li H, Guan X, Fang L, Cai Q, Wang W, Zhou Q. Focal Cortical Dysplasia Type Ⅲ Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome. Diagnostics. 2021; 11(12):2225. https://doi.org/10.3390/diagnostics11122225
Chicago/Turabian StyleWang, Xiaozhuan, Dabiao Deng, Chengqian Zhou, Honglin Li, Xueqin Guan, Liguang Fang, Qinxin Cai, Wensheng Wang, and Quan Zhou. 2021. "Focal Cortical Dysplasia Type Ⅲ Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome" Diagnostics 11, no. 12: 2225. https://doi.org/10.3390/diagnostics11122225
APA StyleWang, X., Deng, D., Zhou, C., Li, H., Guan, X., Fang, L., Cai, Q., Wang, W., & Zhou, Q. (2021). Focal Cortical Dysplasia Type Ⅲ Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome. Diagnostics, 11(12), 2225. https://doi.org/10.3390/diagnostics11122225





