Digital Pathology Transformation in a Supraregional Germ Cell Tumour Network
Abstract
:1. Introduction
1.1. Background
1.2. Testicular Germ Cell Networks
1.3. Aims
2. Materials and Methods
2.1. Aims
2.2. Pre-Deployment Assessment of Perceptions of Transition to and Utility of DP
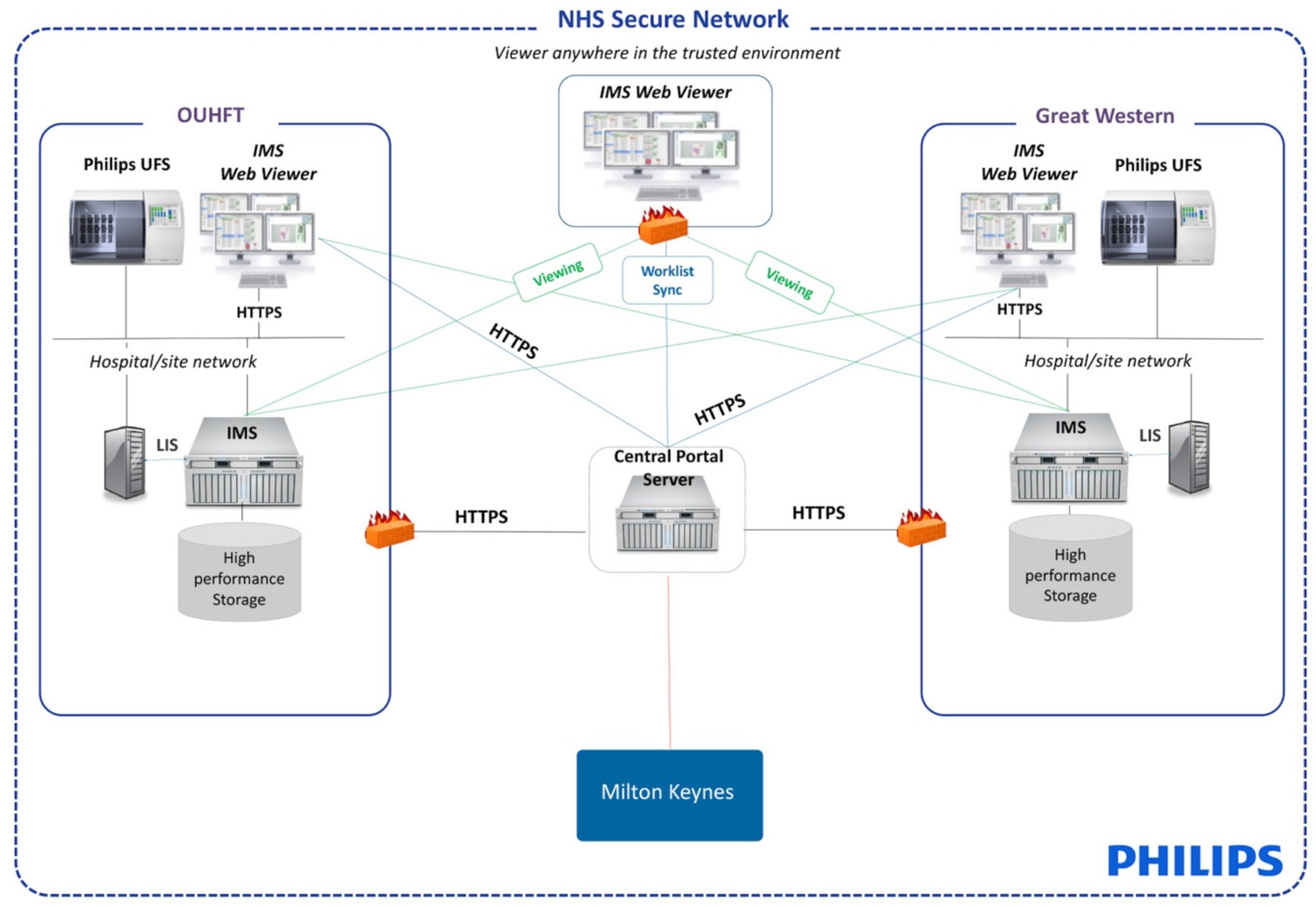
2.3. Integration of DP Platform into Laboratories
2.4. Validation and DP Reporting of Cases
2.5. Germ Cell Tumour Network—User Experience Surveys
2.5.1. Survey 1: The Impact of Access to DP in the Setting of the TVGCTN, on the Laboratory Staff, Administrative Staff, and Histopathologists within OUHFT, MKUHFT, and GWHFT
2.5.2. Survey 2: For the Members of the TVGCT MDT
3. Results
3.1. Pre-Deployment Assessment of Perceptions of Transition to and Utility of DP
3.2. Integration of DP Platform into Laboratories
3.3. The Impact of COVID-19
3.4. Validation and DP Reporting of Cases
3.5. Germ Cell Tumour Network—User Experience Surveys
Survey 1: The Impact of Access to DP in the Setting of the TVGCTN, on the Laboratory staff, Administrative Staff, and Histopathologists within OUHFT, MKUHFT, and GWHFT
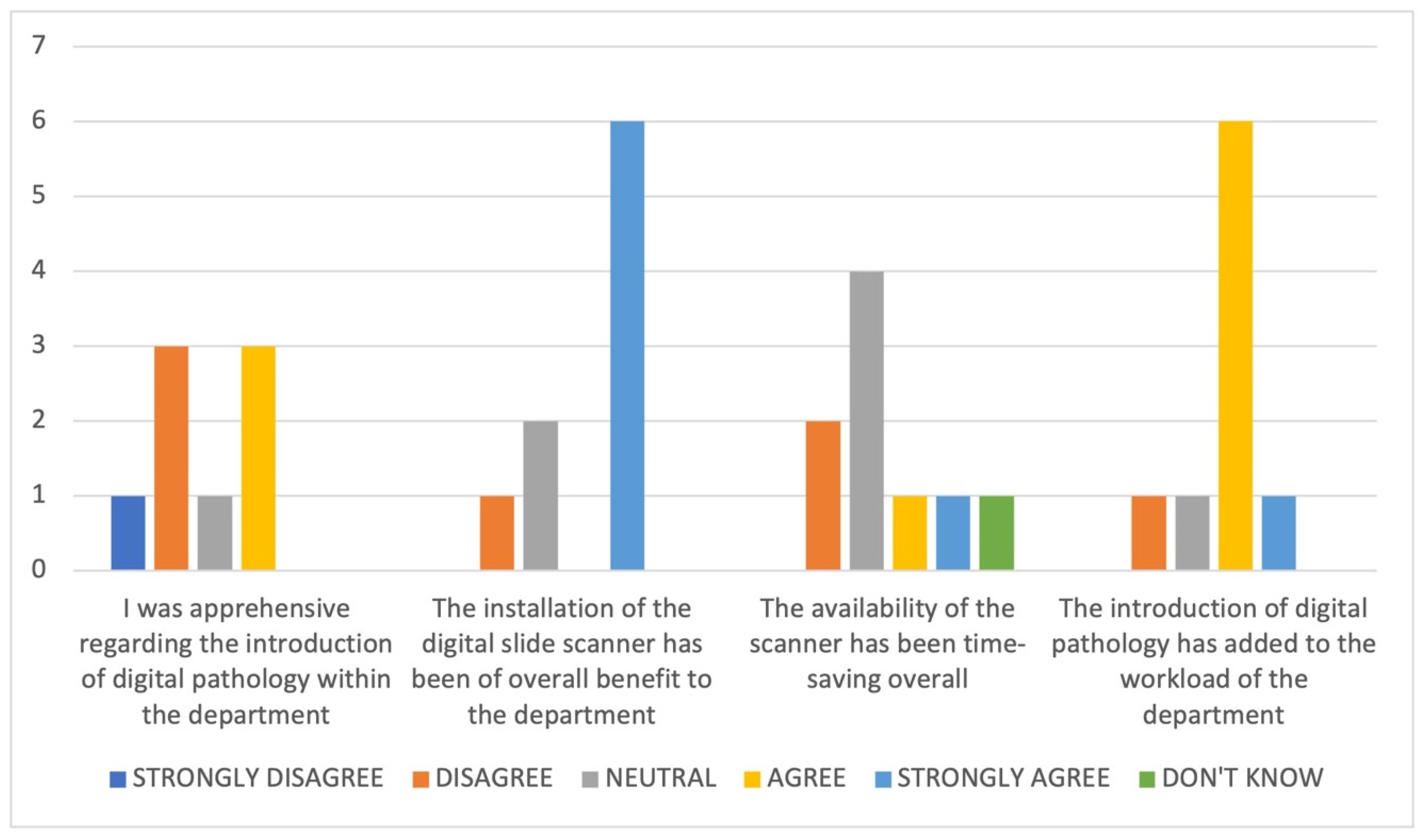
3.6. Impact of the Introduction of DP on the Department
- The new process has taken time to adjust to, with things taking much longer;
- Time is needed to clean and prepare slides for scanning;
- There is no real advantage whilst the GS are still being posted;
- An issue with the link between one of the hubs’ peripheral sites and the central site at OUHFT created problems with DP case review;
- The workflow and expansion of the service is a work in progress but will be of benefit in the long term;
- Workflow issues such as out of focus images and missing slides and issues such as storage, training, integration, and cost need to be resolved.
Survey 2: The members of the TVGCT MDT
‘… I still feel it is important to be able to review images at the MDT and particularly useful for me as a clinician to have interaction with pathologists and understand the pathology. Impressive to see the digital images.’
4. Discussion
4.1. The Move to DP
4.2. The Benefits of Supraregional Working in Testicular Germ Cell Tumour Management
4.3. Digitisation of Oxford and the Supraregional Network
4.4. COVID-19
4.5. Feedback on the DP Experience of the Wider TVGCT Network
4.6. Future Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stathonikos, N.; Veta, M.; Huisman, A.; van Diest, P.J. Going fully digital: Perspective of a Dutch academic pathology lab. J. Pathol. Inform. 2013, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Thorstenson, S.; Molin, J.; Lundström, C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: Digital pathology experiences 2006–2013. J. Pathol. Inform. 2014, 5, 14. [Google Scholar]
- Babawale, M.; Gunavardhan, A.; Walker, J.; Corfield, T.; Huey, P.; Savage, A.; Bansal, A.; Atkinson, M.; Abdelsalam, H.; Raweily, E.; et al. Verification and Validation of Digital Pathology (Whole Slide Imaging) for Primary Histopathological Diagnosis: All Wales Experience. J. Pathol. Inform. 2021, 12, 4. [Google Scholar]
- Retamero, J.A.; Aneiros-Fernandez, J.; del Moral, R.G. Complete Digital Pathology for Routine Histopathology Diagnosis in a Multicenter Hospital Network. Arch. Pathol. Lab. Med. 2020, 144, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef]
- Lim, S.J.; Gurusamy, K.; O’Connor, D.; Shaaban, A.M.; Brierley, D.; Lewis, I.; Harrison, D.; Kendall, T.J.; Robinson, M. Recommendations for cellular and molecular pathology input into clinical trials: A systematic review and meta-aggregation. J. Pathol. Clin. Res. 2021, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Heallth and Care Excellence. Improving Outcomes in Urological Cancers. Available online: https://www.nice.org.uk/guidance/csg2 (accessed on 16 April 2021).
- Turnquist, C.; Roberts-Gant, S.; Hemsworth, H.; White, K.; Browning, L.; Rees, G.; Roskell, D.; Verrill, C. On the Edge of a Digital Pathology Transformation: Views from a Cellular Pathology Laboratory Focus Group. J. Pathol. Inform. 2019, 10, 37. [Google Scholar] [CrossRef]
- Evans, A.J.; Salama, M.E.; Henricks, W.H.; Pantanowitz, L. Implementation of Whole Slide Imaging for Clinical Purposes: Issues to Consider From the Perspective of Early Adopters. Arch. Pathol. Lab. Med. 2017, 141, 944–959. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.; Furness, P.; Igali, L.; Snead, D.; Treanor, D. Royal College of Pathologists Best Practice Recommendations for Implementing Digital Pathology January 2018. Available online: https://www.rcpath.org/uploads/assets/f465d1b3-797b-4297-b7fedc00b4d77e51/Best-practice-recommendations-for-implementing-digital-pathology.pdf (accessed on 16 April 2021).
- Williams, B.J.; Bottoms, D.; Treanor, D. Future-proofing pathology: The case for clinical adoption of digital pathology. J. Clin. Pathol. 2017, 70, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Browning, L.; Colling, R.; Rittscher, J.; Winter, L.; McEntyre, N.; Verrill, C. Implementation of digital pathology into diagnostic practice: Perceptions and opinions of histopathology trainees and implications for training. J. Clin. Pathol. 2020, 73, 223–227. [Google Scholar] [CrossRef]
- Wilkins, B. The Retention and Storage of Pathological Records and Specimens: Guidence from the Royal College of Pathologists and the Institute of Biomedical Science. 2015. Available online: https://www.rcpath.org/uploads/assets/049ea966-df5c-4a9f-9353ba24a69bb808/The-retention-and-storage-of-pathological-records-and-specimens-5th-edition.pdf (accessed on 16 June 2021).
- Williams, B.J.; Brettle, D.; Aslam, M.; Barrett, P.; Bryson, G.; Cross, S.; Snead, D.; Verrill, C.; Clarke, E.; Wright, A.; et al. Guidance for Remote Reporting of Digital Pathology Slides During Periods of Exceptional Service Pressure: An Emergency Response from the UK Royal College of Pathologists. J. Pathol. Inform. 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.; Fryer, E.; Roskell, D.; White, K.; Colling, R.; Rittscher, J.; Verrill, C. Role of digital pathology in diagnostic histopathology in the response to COVID-19: Results from a survey of experience in a UK tertiary referral hospital. J. Clin. Pathol. 2021, 74, 129–132. [Google Scholar] [CrossRef]
- Browning, L.; Colling, R.; Rakha, E.; Rajpoot, N.; Rittscher, J.; James, J.A.; Salto-Tellez, M.; Snead, D.R.J.; Verrill, C. Digital pathology and artificial intelligence will be key to supporting clinical and academic cellular pathology through COVID-19 and future crises: The PathLAKE consortium perspective. J. Clin. Pathol. 2020, 74, 443–447. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Pathologists. Review of the Categorisation of Discrepancies in Histopathology. 2008. Available online: https://www.rcpath.org/uploads/assets/554f0249-a9dc-4c23-a9cfd6b2baa2c9b1/Review-of-the-categorisation-of-discrepancies-in-histopathology-2008.pdf (accessed on 24 June 2021).
- Bell, J. Life Sciences Industrial Strategy—A Report to the Government from the Life Sciences Sector. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/650447/LifeSciencesIndustrialStrategy_acc2.pdf (accessed on 24 June 2021).
- Williams, B.J.; Bottoms, D.; Clark, D.; Treanor, D. Future-proofing pathology part 2: Building a business case for digital pathology. J. Clin. Pathol. 2019, 72, 198–205. [Google Scholar] [CrossRef]
- Azam, A.S.; Miligy, I.M.; Kimani, P.K.; Maqbool, H.; Hewitt, K.; Rajpoot, N.M.; Snead, D.R. Diagnostic concordance and discordance in digital pathology: A systematic review and meta-analysis. J. Clin. Pathol. 2020, 74, 448–455. [Google Scholar] [CrossRef]
- Vodovnik, A. Distance reporting in digital pathology: A study on 950 cases. J. Pathol. Inform. 2015, 6, 18. [Google Scholar] [CrossRef]
- Lujan, G.M.; Savage, J.; Shana’ah, A.; Yearsley, M.; Thomas, D.; Allenby, P.; Otero, J.; Limbach, A.L.; Cui, X.; Scarl, R.; et al. Digital Pathology Initiatives and Experience of a Large Academic Institution During the Coronavirus Disease 2019 (COVID-19) Pandemic. Arch. Pathol. Lab. Med. 2021, 145, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Delaney, R.J.; Sayers, C.D.; Walker, M.A.; Mead, G.M.; Theaker, J.M. The continued value of central histopathological review of testicular tumours. Histopathology 2005, 47, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Purshouse, K.; Watson, R.A.; Church, D.N.; Richardson, C.; Crane, G.; Traill, Z.; Sullivan, M.; Roberts, I.; Browning, L.; Turner, G.; et al. Value of Supraregional Multidisciplinary Review for the Contemporary Management of Testicular Tumors. Clin. Genitourin. Cancer 2017, 15, 152–156. [Google Scholar] [CrossRef] [PubMed]
- van Diest, P.J.; Huisman, A.; van Ekris, J.; Meijer, J.; Willems, S.; Hofhuis, H.; Verbeek, X.; van der Wel, M.; Vos, S.; Leguit, R.; et al. Pathology Image Exchange: The Dutch Digital Pathology Platform for Exchange of Whole-Slide Images for Efficient Teleconsultation, Telerevision, and Virtual Expert Panels. JCO Clin. Cancer Inform. 2019, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, M.G.; Reuter, V.E.; Hameed, M.R.; Tan, L.K.; Chiang, S.; Sigel, C.; Hollmann, T.; Giri, D.; Samboy, J.; Moradel, C.; et al. Whole slide imaging equivalency and efficiency study: Experience at a large academic center. Mod. Pathol. 2019, 32, 916–928. [Google Scholar] [CrossRef]
- Baidoshvili, A.; Bucur, A.; van Leeuwen, J.; van der Laak, J.; Kluin, P.; van Diest, P.J. Evaluating the benefits of digital pathology implementation: Time savings in laboratory logistics. Histopathology 2018, 73, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Quiroga-Garza, G.M.; Bien, L.; Heled, R.; Laifenfeld, D.; Linhart, C.; Sandbank, J.; Albrecht Shach, A.; Shalev, V.; Vecsler, M.; et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: A blinded clinical validation and deployment study. Lancet Digital Health 2020, 2, e407–e416. [Google Scholar] [CrossRef]
- Campanella, G.; Hanna, M.G.; Geneslaw, L.; Miraflor, A.; Werneck Krauss Silva, V.; Busam, K.J.; Brogi, E.; Reuter, V.E.; Klimstra, D.S.; Fuchs, T.J. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 2019, 25, 1301–1309. [Google Scholar] [CrossRef]
- Ghosh, A.; Sirinukunwattana, K.; Khalid Alham, N.; Browning, L.; Colling, R.; Protheroe, A.; Protheroe, E.; Jones, S.; Aberdeen, A.; Rittscher, J.; et al. The Potential of Artificial Intelligence to Detect Lymphovascular Invasion in Testicular Cancer. Cancers 2021, 13, 1325. [Google Scholar] [CrossRef]
- Linder, N.; Taylor, J.C.; Colling, R.; Pell, R.; Alveyn, E.; Joseph, J.; Protheroe, A.; Lundin, M.; Lundin, J.; Verrill, C. Deep learning for detecting tumour-infiltrating lymphocytes in testicular germ cell tumours. J. Clin. Pathol. 2019, 72, 157–164. [Google Scholar] [CrossRef]
- Colling, R.; Pitman, H.; Oien, K.; Rajpoot, N.; Macklin, P.; Snead, D.; Sackville, T.; Verrill, C. Artificial intelligence in digital pathology: A roadmap to routine use in clinical practice. J. Pathol. 2019, 249, 143–150. [Google Scholar] [CrossRef]


| Details (Tissue Type/Specimen Type/Preparation/Stain). |
|---|
| Mixed germ cell tumour (embryonal carcinoma, yolk sac tumour, teratoma) Rete testis stroma invasion Hilar soft tissue invasion LVI |
| Leiomyosarcoma |
| Spermatocytic tumour Referral |
| Leydig Cell Tumour Various IHC (calretinin, CD99, inhibin, MelanA, panCK, OCT3/4, PLAP, CD30, AFP, Ki-67) |
| Seminoma Rete testis stroma invasion Hilar soft tissue invasion Spermatic cord invasion IHC (OCT3/4, C-Kit, D2-40, LCA, CD30, panCK) |
| Seminoma Tumour smearing artefact |
| Regressed germ cell tumour OCT3/4 IHC |
| Mixed germ cell tumour (seminoma, embryonal carcinoma) GCNIS |
| Mixed germ cell tumour (seminoma, embryonal carcinoma) LVI Rete testis stroma invasion GCNIS Various IHC Comment: Challenging case was originally said to have hilar soft tissue invasion but was later revised to LVI in hilar soft tissue only. |
| Benign: Atrophy |
| Mixed germ cell tumour (teratoma, embryonal carcinoma, yolk sac tumour) LVI |
| Seminoma GCNIS Separate soft tissue deposit in the cord (M1) Comment: Described as no unequivocal LVI, therefore challenging. |
| Diffuse large B-cell lymphoma |
| Seminoma LVI in cord Comment: Difficult case—differential diagnosis of LVI in cord versus a soft tissue deposit (M1). |
| Seminoma Partial orchidectomy |
| Mixed germ cell tumour (embryonal carcinoma, yolk sac tumour, teratoma) LVI |
| Seminoma Hilar soft tissue invasion Comment: LVI assessment very difficult, needed IHC (D2-40). |
| Mixed germ cell tumour (yolk sac tumour, teratoma, seminoma) Comment: Various IHC needed to clarify tumour components. |
| Seminoma Comment: Challenging case. Focal rete testis stroma invasion added after MDT review. |
| Benign: Abscess |
| RPLND: Viable yolk sac tumour and post chemotherapy teratoma |
| Benign: Infarct/torsion Referral |
| Partial orchidectomy Choriocarcinoma Embryonal carcinoma Teratoma Seminoma Yolk sac tumour Referral Mega slides |
| Metastasis Biopsy Seminoma Various IHC Referral |
| Regressed germ cell tumour—scar Referral Challenging case Very focal GCNIS |
| Mediastinal biopsy Seminoma IHC |
| A | ||||
| Pathologist | Preferred Method of Reporting—Digital (%) | Preferred Method of Reporting—Glass (%) | Preferred Method of Reporting—Either | Not Recorded (%) |
| 1 | 67 | 12 | 19 | 2 |
| 2 | 0 | 24 | 76 | 0 |
| 3 | 100 | 0 | 0 | 0 |
| B | ||||
| Pathologist | Confidence Score 4 (%) | Confidence Score 5 (%) | Confidence Score 6 (%) | Confidence Score 7 (%) |
| 1 | 1 | 1 | 8 | 88 |
| 2 | 0 | 35 | 53 | 12 |
| 3 | 0 | 0 | 18 | 82 |
| C | ||||
| Pathologist | Confidence Score 4 (%) | Confidence Score 5 (%) | Confidence Score 6 (%) | Confidence Score 7 (%) |
| 1 | 0 | 1 | 10 | 87 |
| 2 | 0 | 0 | 35 | 65 |
| 3 | 0 | 0 | 9 | 91 |
| Questions to Those Involved in Slide Scanning | ||||||
| Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree | Don’t Know | |
| Scanning of GS has been easy to introduce within the department | 0/5 | 1/5 | 1/5 | 3/5 | 0/5 | 0/5 |
| Scanning of GS has not impacted significantly on my workload | 0/5 | 2/5 | 2/5 | 1/5 | 0/5 | 0/5 |
| Scanning of slides is efficient | 0/5 | 1/5 | 1/5 | 2/5 | 1/5 | 0/5 |
| Scanning of slides fits into a lean workflow | 0/5 | 1/5 | 3/5 | 0/5 | 1/5 | 0/5 |
| I understand how my role to enable scanning of these cases fits into the overall strategy for digitising this TVGCTN | 0/5 | 0/5 | 1/5 | 3/5 | 1/5 | 0/5 |
| The training I have received has been timely and sufficient to give me confidence in this role | 0/5 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 |
| I would like additional training for this role | 1/5 | 0/5 | 1/5 | 1/5 | 0/5 | 0/5 |
| Questions to Those Involved in the Administration of Slides for MDT Referral | ||||||
| Scanning of GS has not impacted significantly on my workload | 0/4 | 1/4 | 2/4 | 1/4 | 0/4 | 0/4 |
| Now that slides are scanned, packing and sending cases in the workflow is less pressurised as pathologists can very quickly access the slides digitally | 0/4 | 2/4 | 1/4 | 0/4 | 1/4 | 0/4 |
| I understand how my role to enable scanning of these cases fits into the overall strategy for digitising this TVGCTN | 0/4 | 0/4 | 1/4 | 2/4 | 1/4 | 0/4 |
| I feel more comfortable in the knowledge that cases are digitally scanned as well as physically posted to another department | 0/4 | 0/4 | 1/4 | 2/4 | 1/4 | 0/4 |
| The availability of DP has made my role (in the TVGCTN setting) easier | 1/3 | 0/3 | 2/3 | 0/3 | 0/3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colling, R.; Protheroe, A.; Sullivan, M.; Macpherson, R.; Tuthill, M.; Redgwell, J.; Traill, Z.; Molyneux, A.; Johnson, E.; Abdullah, N.; et al. Digital Pathology Transformation in a Supraregional Germ Cell Tumour Network. Diagnostics 2021, 11, 2191. https://doi.org/10.3390/diagnostics11122191
Colling R, Protheroe A, Sullivan M, Macpherson R, Tuthill M, Redgwell J, Traill Z, Molyneux A, Johnson E, Abdullah N, et al. Digital Pathology Transformation in a Supraregional Germ Cell Tumour Network. Diagnostics. 2021; 11(12):2191. https://doi.org/10.3390/diagnostics11122191
Chicago/Turabian StyleColling, Richard, Andrew Protheroe, Mark Sullivan, Ruth Macpherson, Mark Tuthill, Jacqueline Redgwell, Zoe Traill, Angus Molyneux, Elizabeth Johnson, Niveen Abdullah, and et al. 2021. "Digital Pathology Transformation in a Supraregional Germ Cell Tumour Network" Diagnostics 11, no. 12: 2191. https://doi.org/10.3390/diagnostics11122191
APA StyleColling, R., Protheroe, A., Sullivan, M., Macpherson, R., Tuthill, M., Redgwell, J., Traill, Z., Molyneux, A., Johnson, E., Abdullah, N., Taibi, A., Mercer, N., Haynes, H. R., Sackville, A., Craft, J., Reis, J., Rees, G., Soares, M., Roberts, I. S. D., ... Verrill, C. (2021). Digital Pathology Transformation in a Supraregional Germ Cell Tumour Network. Diagnostics, 11(12), 2191. https://doi.org/10.3390/diagnostics11122191






