Biochemical and Hematological Relationship with the Evaluation of Autonomic Dysfunction by Heart Rate Recovery in Patients with Asthma and Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zalewski, P.; Slomko, J.; Zawadka-Kunikowska, M. Autonomic dysfunction and chronic disease. Br. Med. Bull. 2018, 128, 61–74. [Google Scholar] [CrossRef]
- Kankaanranta, H.; Kauppi, P.; Tuomisto, L.E.; Ilmarinen, P. Emerging Comorbidities in Adult Asthma: Risks, Clinical Asso-ciations, and Mechanisms. Mediat. Inflamm. 2016, 2016, 3690628. [Google Scholar] [CrossRef]
- Remus Popa, A.; Fratila, O.; Rus, M.; Anca Corb Aron, R.; Mihai Vesa, C.; Pantis, C.; C Diaconu, C.; Bratu, O.; Bungau, S.; Nemeth, S. Risk factors for adiposity in the urban population and influence on the prevalence of overweight and obesity. Exp. Ther. Med. 2020, 20, 129–133. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504–515. [Google Scholar] [CrossRef]
- Debnath, S.; Levy, T.J.; Bellehsen, M.; Schwartz, R.M.; Barnaby, D.P.; Zanos, S.; Volpe, B.T.; Zanos, T.P. A method to quantify autonomic nervous system function in healthy, able-bodied individuals. Bioelectron. Med. 2021, 7, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Babes, E.E.; Zaha, D.C.; Tit, D.M.; Nechifor, A.C.; Bungau, S.; Andronie-Cioara, F.L.; Behl, T.; Stoicescu, M.; Munteanu, M.A.; Rus, M.; et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics 2021, 11, 850. [Google Scholar] [CrossRef]
- Agarwala, P.; Salzman, S.H. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest 2020, 157, 603–611. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Ha, D.; Fuster, M.; Ries, A.L.; Wagner, P.D.; Mazzone, P.J. Heart Rate Recovery as a Preoperative Test of Perioperative Complication Risk. Ann. Thorac. Surg. 2015, 100, 1954–1962. [Google Scholar] [CrossRef]
- Georgiopoulou, V.V.; Dimopoulos, S.; Sakellariou, D.; Papazachou, O.; Gerovasili, V.; Tasoulis, A.; Agapitou, V.; Vogiatzis, I.; Roussos, C.; Nanas, S. Cardiopulmonary Re-habilitation Enhances Heart Rate Recovery in Patients with COPD. Respir. Care 2012, 57, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Florêncio, R.; Fregonezi, G.; Brilhante, S.; Borghi-Silva, A.; Dias, F.; Resqueti, V. Heart Rate Variability at Rest and after the 6-Minute Walk Test (6MWT) in Children with Cystic Fibrosis. Braz. J. Phys. Ther. 2013, 17, 419–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Currie, K.D.; Rosen, L.M.; Millar, P.J.; McKelvie, R.S.; MacDonald, M.J. Heart Rate Recovery and Heart Rate Variability Are Unchanged in Patients with Coronary Artery Disease Following 12 Weeks of High-Intensity Interval and Moderate-Intensity Endurance Exercise Training. Appl. Physiol. Nutr. Metab. 2013, 38, 644–650. [Google Scholar] [CrossRef]
- Sydó, N.; Sydó, T.; Gonzalez Carta, K.A.; Hussain, N.; Farooq, S.; Murphy, J.G.; Merkely, B.; Lopez-Jimenez, F.; Allison, T.G. Prognostic Performance of Heart Rate Recovery on an Exercise Test in a Primary Prevention Population. J. Am. Heart Assoc. 2018, 7, e008143. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guide-lines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Doi, T.; Tsutsumimoto, K.; Nakakubo, S.; Kim, M.J.; Kurita, S.; Ishii, H.; Shimada, H. Predictivity of bioimpedance phase angle for incident disability in older adults. J. Cachexia Sarcopenia Muscle 2020, 11, 46–54. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Laudisio, D.; de Alteriis, G.; Graziadio, C.; Colao, A.; Savastano, S. Phase Angle as an Easy Diagnostic Tool of Meta-Inflammation for the Nutritionist. Nutrients 2021, 13, 1446. [Google Scholar] [CrossRef]
- Fan, Y.; Gu, X.; Zhang, H. Prognostic value of six-minute walk distance in patients with heart failure: A meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 664–667. [Google Scholar] [CrossRef]
- Jae, S.Y.; Ahn, E.S.; Heffernan, K.S.; Woods, J.A.; Lee, M.-K.; Park, W.H.; Fernhall, B. Relation of Heart Rate Recovery after Exercise to C-Reactive Protein and White Blood Cell Count. Am. J. Cardiol. 2007, 99, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R.J.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- van de Vegte, Y.J.; van der Harst, P.; Verweij, N. Heart Rate Recovery 10 Seconds After Cessation of Exercise Predicts Death. J. Am. Heart Assoc. 2018, 7, e008341. [Google Scholar] [CrossRef]
- Lamberts, R.P.; Swart, J.; Capostagno, B.; Noakes, T.D.; Lambert, M.I. Heart Rate Recovery as a Guide to Monitor Fatigue and Predict Changes in Performance Parameters. Scand. J. Med. Sci. Sports 2010, 20, 449–457. [Google Scholar] [CrossRef]
- Thayer, J.F.; Sternberg, E.M. Neural Aspects of Immunomodulation: Focus on the Vagus Nerve. Brain Behav. Immun. 2010, 24, 1223–1228. [Google Scholar] [CrossRef]
- Jae, S.Y.; Bunsawat, K.; Kunutsor, S.K.; Yoon, E.S.; Kim, H.J.; Kang, M.; Choi, Y.H.; Franklin, B.A. Relation of Exercise Heart Rate Recovery to Predict Cardiometabolic Syndrome in Men. Am. J. Cardiol. 2019, 123, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-K.; Gore, J.M. Relation of Heart Rate Recovery after Exercise to Insulin Resistance and Chronic Inflammation in Otherwise Healthy Adolescents and Adults: Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Clin. Res. Cardiol. 2015, 104, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Đurić, B.; Suzić, S. Heart Rate Recovery: Short Review of Methodology. Med. Podml. 2016, 67, 48–50. [Google Scholar] [CrossRef]
- Buchheit, M.; Papelier, Y.; Laursen, P.B.; Ahmaidi, S. Noninvasive assessment of cardiac parasympathetic function: Postexercise heart rate recovery or heart rate variability? Am. J. Physiol. Heart Circ. Physiol. 2007, 93, H8–H10. [Google Scholar] [CrossRef]
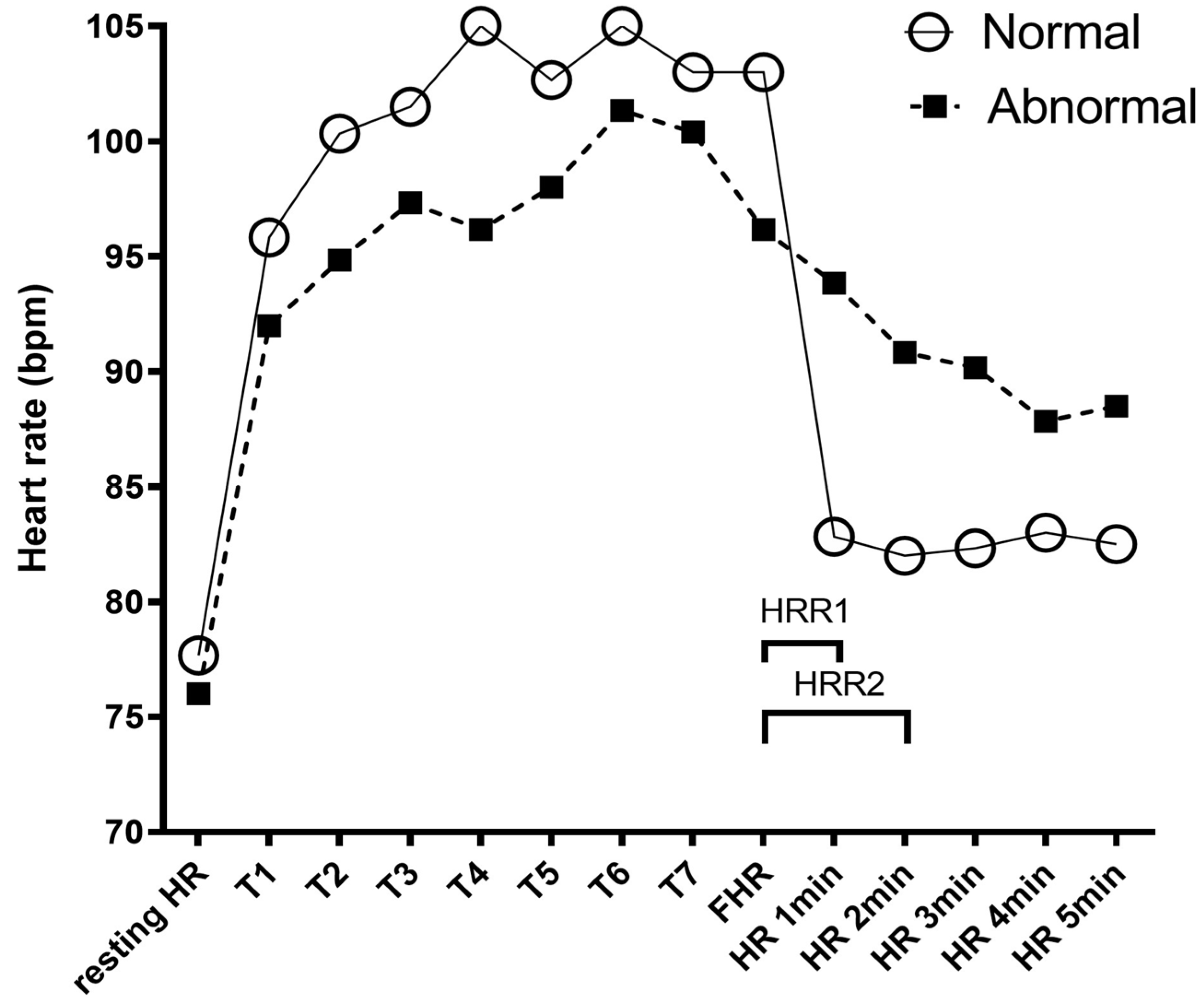
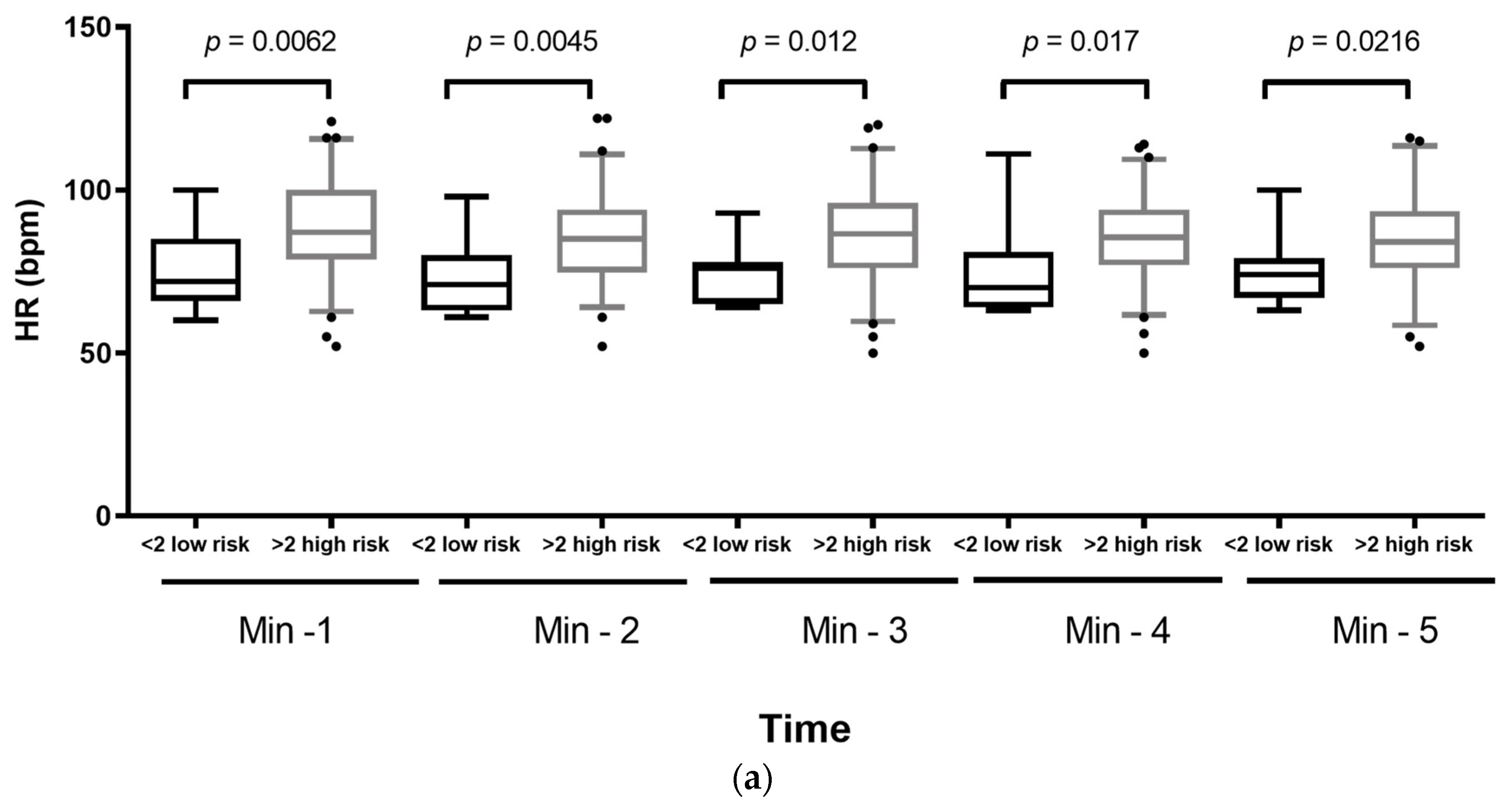
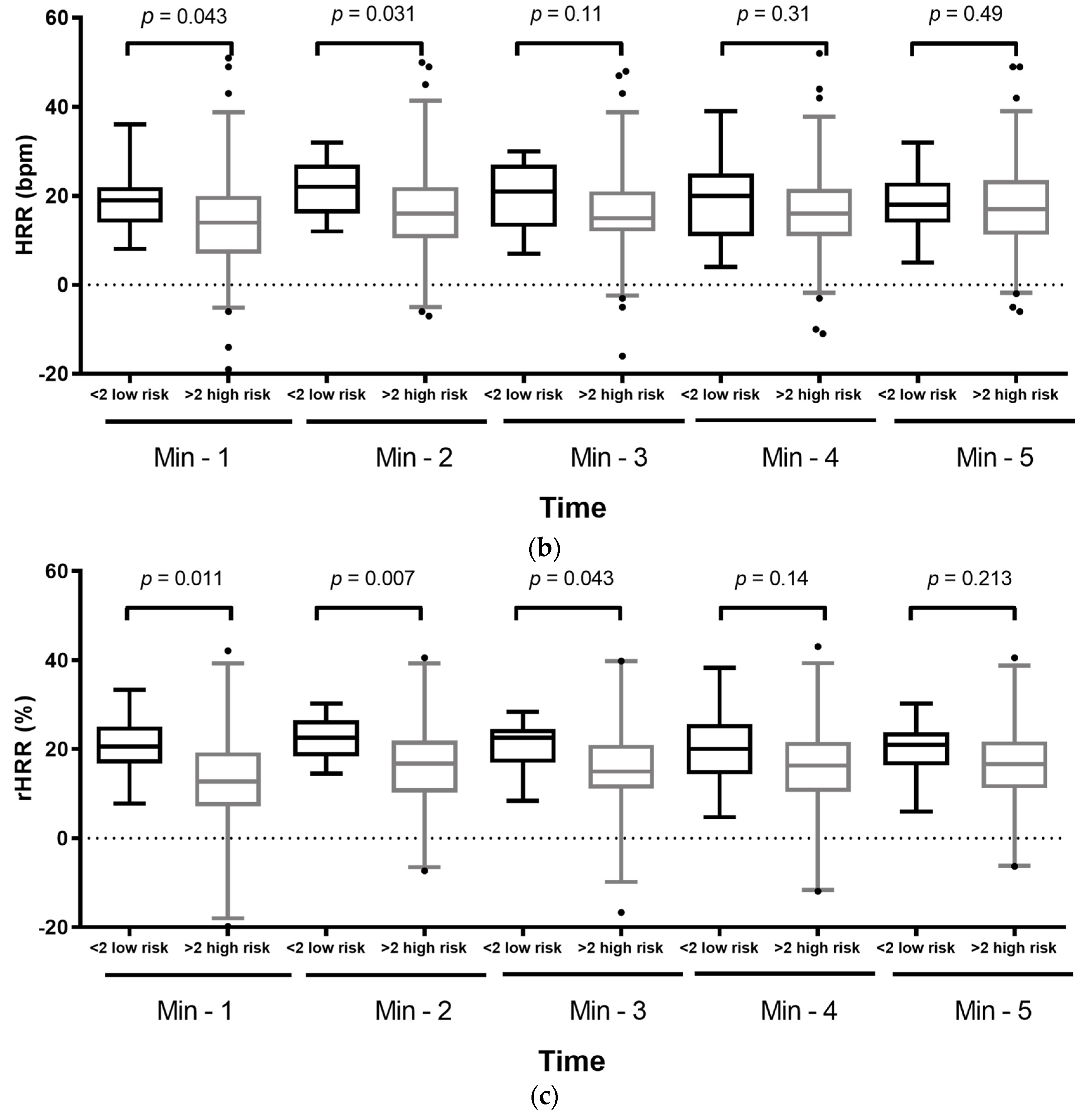
| Variable | Total (n = 78) | ||
| Median (IQR) | Min-Max | ||
| Age (Years) | 45 (34–53) | 25–67 | |
| Anthropometry | |||
| Height (m) | 1.59 (1.53–1.67) | 1.44–1.78 | |
| Weight (kg) | 71.55 (61.8–82.3) | 47–106.8 | |
| Waist Circumference (cm) | 92 (86–102) | 59–117.5 | |
| Neck Circumference (cm) | 35.75 (32.5–39) | 27–49 | |
| WHtR | 0.57 (0.53–0.65) | 0.39–0.79 | |
| BMI (kg/m2) | 27.8 (25–30.8) | 20.3–38.5 | |
| Variable | N (%) | Variable | N (%) |
| Sex | Asthma | ||
| Men | 28 (35.9) | No | 46 (58.7) |
| Women | 50 (64.1) | Yes | 32 (41.3) |
| BMI | Blood Pressure | ||
| Normal weight | 19 (24.4) | Normotensive | 29 (39.7) |
| Overweight | 30 (38.5) | Prehypertensive | 23 (31.5) |
| Obese | 29 (37.1) | Hypertensive | 21 (28.8) |
| Metabolic Syndrome | Castelli I index | ||
| No | 47 (60.3) | <4 low risk | 31 (40.3) |
| Yes | 31 (39.7) | >4 high risk | 46 (59.7) |
| Type 2 diabetes | Castelli II index | ||
| No | 59 (75.6) | <3 low risk | 50 (64.9) |
| Yes | 19 (24.4) | >3 high risk | 27 (35.1) |
| HOMA Index | Atherogenic coefficient | ||
| Without IR | 27 (39.1) | ||
| Suspected IR | 17 (24.6) | <2 low risk | 11 (14.3) |
| IR | 25 (36.2) | >2 high risk | 66 (85.7) |
| WHtR | AIP | ||
| <0.5 | 13 (16.9) | <0.24 low risk | 9 (11.7) |
| >0.5 | 64 (83.1) | >0.24 high risk | 68 (88.3) |
| Neck Circumference (cm) | Apo-Index | ||
| Normal weight | 31 (41.9) | low risk | 39 (65) |
| Overweight—Obese | 43 (58.9) | high risk | 21 (35) |
| Waist Circumference (cm) | HRR1 | ||
| Normal weight | 23 (29.9) | Normal | 57 (73.1) |
| Overweight—Obese | 54 (70.1) | Abnormal | 21 (26.9) |
| Phase angle (°) | HRR2 | ||
| >5.4 | 40 (51.3) | Normal | 58 (74.4) |
| <5.4 | 38 (48.7) | Abnormal | 20 (25.6) |
| Variable | Total (n = 78) | |
| Median (IQR) | Min-Max | |
| Respiratory-related measurements | ||
| SpO2 basal (%) | 95 (93–96) | 87–99 |
| SpO2 final (%) | 93 (91–94) | 75–98 |
| FeNO (ppb) | 15.8 (8.3–24.65) | 3.33–246 |
| Bioimpedance | ||
| SMM (Kg) | 18.7 (16.3–23) | 10.3–33.6 |
| Phase angle (°) | 5.5 (5.0–6.0) | 4.0–7.1 |
| Intracellular water (L) | 17.4 (15.5–21.9) | 10.4–29.4 |
| Hydration (%) | 77 (69.9–85.1) | 35.3–106.2 |
| Blood cell Counts | ||
| Leukocytes (103/µL−1) | 6.4 (5.7–7.3) | 3.9–16.9 |
| Neutrophils (103/µL−1) | 3.66 (3.2–4.3) | 1.8–5.8 |
| Lymphocytes (103/µL−1) | 1.97 (1.7–2.3) | 1.3–4.0 |
| Monocytes (103/µL−1) | 0.4 (0.4–0.5) | 0.23–0.9 |
| Eosinophils (103/µL−1) | 2.4 (1.3–3.5) | 0.4–13.5 |
| Basophils (103/µL−1) | 0.5 (0.4–1.2) | 0.2–1.2 |
| Erythrocytes (106/µL−1) | 5.18 (4.71–5.58) | 4.09–6.29 |
| Hemoglobin (gr/dL) | 15.2 (14.3–16.5) | 11.6–18.6 |
| Hematocrit (%) | 45.9 (42.7–49.9) | 36.5–56.6 |
| Blood Chemistry parameters | ||
| Glucose (mg/dL−1) | 97 (90–108.5) | 75–289 |
| HbA1c (%) | 5.7 (5.4–6.0) | 4.5–11.9 |
| Insulin (µU/mL−1) | 10.55 (7.6–16.2) | 2.9–34.5 |
| HOMA-IR | 2.46 (1.54–3.86) | 0.46–11.84 |
| LDL (mg/dL−1) | 120.9 (96–144) | 48.7–229 |
| No-HDL (mg/dL−1) | 150 (124.5–180.5) | 67–264 |
| Castelli-I index | 4.6 (3.5–5.3) | 0.048–7.1 |
| Castelli-II index | 2.79 (2.16–3.21) | 0.91–4.98 |
| AC | 3.6 (2.5–4.29) | 1.2–6.1 |
| CRP (mg/dL−1) | 0.15 (0.07–0.42) | 0.02–2.02 |
| Variable | HR (bpm) | HRR (bpm) | rHRR (%) | |||||||||
| 1 min | 2 min | 1 min | 2 min | 1 min | 2 min | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | |
| SpO2b (%) | −0.17 | 0.139 | −0.048 | 0.675 | 0.309 | 0.006 | 0.129 | 0.257 | 0.29 | 0.010 | 0.089 | 0.440 |
| SpO2f (%) | −0.23 | 0.046 | −0.195 | 0.087 | 0.059 | 0.602 | 0.017 | 0.884 | 0.09 | 0.425 | 0.045 | 0.696 |
| FeNO (ppb) | 0.001 | 0.990 | −0.05 | 0.666 | −0.22 | 0.060 | −0.209 | 0.070 | −0.17 | 0.145 | −0.14 | 0.237 |
| Variable | Total | Biomarker | Normal | Abnormal | p-Value | |||||||
| Median (IQR) | Median (IQR) | Median (IQR) | ||||||||||
| SpO2b (%) | 95 (93–96) | HRR1 | 95 (94–96) | 93 (92–95) | 0.005 | |||||||
| HRR2 | 95 (93–96) | 93.5 (92.5–95) | 0.198 | |||||||||
| SpO2f (%) | 93 (91–94) | HRR1 | 93 (92–95) | 92 (91–94) | 0.031 | |||||||
| HRR2 | 93.5 (92–94) | 92 (91–94) | 0.134 | |||||||||
| FeNO (ppb) | 15.8 (8.3–24.65) | HRR1 | 15.5 (8.3–22.7) | 18.2 (9.6–37.3) | 0.267 | |||||||
| HRR2 | 11.6 (8.3–23) | 18.6 (11–43.3) | 0.043 | |||||||||
| Variable | HR (bpm) | HRR (bpm) | rHRR (%) | |||||||||
| 1 min | 2 min | 1 min | 2 min | 1 min | 2 min | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | |
| SMM (Kg) | 0.146 | 0.203 | 0.119 | 0.297 | −0.207 | 0.069 | −0.143 | 0.212 | −0.234 | 0.039 | −0.192 | 0.092 |
| Phase angle (ϕ) | 0.229 | 0.043 | 0.237 | 0.037 | −0.226 | 0.047 | −0.216 | 0.057 | −0.261 | 0.021 | −0.275 | 0.015 |
| Intracellular Water (L) | 0.156 | 0.172 | 0.145 | 0.205 | −0.193 | 0.090 | −0.146 | 0.204 | −0.227 | 0.045 | −0.202 | 0.077 |
| Hydration (%) | −0.166 | 0.146 | −0.147 | 0.199 | 0.234 | 0.039 | 0.188 | 0.099 | 0.255 | 0.024 | 0.231 | 0.042 |
| Variable | Total | Biomarker | HRR | HRR | p-Value | |||||||
| Median (IQR) | Median (IQR) | Median (IQR) | ||||||||||
| Phase angle (°) | 5.5 (5–6) | HRR1 | 5.3 (5–5.9) | 5.7 (5–6.4) | 0.097 | |||||||
| HRR2 | 5.3 (4.9–5.9) | 5.7 (5.4–6.3) | 0.060 | |||||||||
| SMM (Kg) | 18.7 (16.3–23) | HRR1 | 18 (16.24–22) | 21.8 (17.1–28.9) | 0.039 | |||||||
| HRR2 | 18 (16–22.25) | 21.5 (17.3–26.4) | 0.158 | |||||||||
| Intracellular water (L) | 17.35 (15.5–21.9) | HRR1 | 17 (15.1–20.3) | 20.3 (16.2–26) | 0.038 | |||||||
| HRR2 | 17.1 (15.1–21) | 19.8 (16.4–23.9) | 0.134 | |||||||||
| Hydration (%) | 77 (69.9–85.1) | HRR1 | 79 (71.4–87.9) | 73 (64.2–82.3) | 0.035 | |||||||
| HRR2 | 79.8 (70–87.9) | 73 (67.5–81.5) | 0.082 | |||||||||
| Variable | HR (bpm) | HRR (bpm) | rHRR (%) | |||||||||
| 1 min | 2 min | 1 min | 2 min | 1 min | 2 min | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | |
| Leucocytes (103/µL−1) | 0.634 | 0.021 | 0.236 | 0.039 | −0.389 | 0.001 | −0.328 | 0.004 | −0.373 | 0.001 | −0.329 | 0.004 |
| Neutrophils (103/µL−1) | 0.209 | 0.068 | 0.279 | 0.014 | −0.234 | 0.041 | −0.303 | 0.007 | −0.255 | 0.025 | −0.328 | 0.004 |
| Lymphocytes (103/µL−1) | 0.192 | 0.095 | 0.115 | 0.321 | −0.299 | 0.008 | −0.198 | 0.085 | −0.262 | 0.021 | −0.182 | 0.114 |
| Monocytes (103/µL−1) | 0.195 | 0.089 | 0.048 | 0.678 | −0.371 | 0.001 | −0.158 | 0.169 | −0.32 | 0.005 | −0.115 | 0.319 |
| Erytrocytes (106/µL−1) | 0.173 | 0.134 | 0.127 | 0.272 | −0.312 | 0.006 | −0.209 | 0.068 | −0.325 | 0.004 | −0.233 | 0.042 |
| Hemoglobin (gr/dL) | 0.121 | 0.290 | 0.077 | 0.507 | −0.319 | 0.005 | −0.246 | 0.030 | −0.309 | 0.006 | −0.234 | 0.041 |
| Hematocrit (%) | 0.146 | 0.207 | 0.121 | 0.295 | −0.293 | 0.009 | −0.244 | 0.032 | −0.297 | 0.009 | −0.247 | 0.030 |
| Variable | Total | Biomarker | Normal | Abnormal | p-Value | |||||||
| Median (IQR) | Median (IQR) | Median (IQR) | ||||||||||
| Leucocytes (103/µL−1) | 6.4 (5.7–7.3) | HRR1 | 6.1 (5.5–6.95) | 7.3 (6.4–8.3) | 0.0008 | |||||||
| HRR2 | 6.3 (5.6–7.2) | 6.85 (6.05–7.65) | 0.0654 | |||||||||
| Neutrophils (103/µL−1) | 3.7 (3.2–4.3) | HRR1 | 3.6 (3–4.1) | 3.9 (3.5–4.8) | 0.0214 | |||||||
| HRR2 | 3.6 (3.1–4.2) | 3.85 (3.3–4.6) | 0.2379 | |||||||||
| Lymphocytes (103/µL−1) | 2 (1.7–2.3) | HRR1 | 1.9 (1.59–2.27) | 2.2 (1.8–2.6) | 0.0254 | |||||||
| HRR2 | 1.9 (1.6–2.3) | 2.2 (1.85–2.4) | 0.1331 | |||||||||
| Monocytes (103/µL−1) | 0.4 (0.4–0.5) | HRR1 | 0.4 (0.335–0.5) | 0.5 (0.4–0.7) | 0.0117 | |||||||
| HRR2 | 0.4 (0.36–0.5) | 0.4 (0.4–0.55) | 0.2697 | |||||||||
| Erytrocytes (106/µL−1) | 5.16 (4.7–5.58) | HRR1 | 5.01 (4.64–5.49) | 5.57 (5.21–5.68) | 0.0239 | |||||||
| HRR2 | 5.09 (4.67–5.57) | 5.33 (5.04–5.67) | 0.0955 | |||||||||
| Hemoglobin (gr/dL) | 15.2 (14.3–16.5) | HRR1 | 14.8 (13.7–16.25) | 16 (15.2–17) | 0.0199 | |||||||
| HRR2 | 14.9 (13.9–16.2) | 15.9 (14.9–17.05) | 0.0833 | |||||||||
| Hematocrit (%) | 45.9 (42.5–49.9) | HRR1 | 44.65 (41.5–49.75) | 47.9 (45.8–50.8) | 0.0228 | |||||||
| HRR2 | 44.8 (41.8–49) | 48 (45.3–51.1) | 0.0426 | |||||||||
| Variable | HR (bpm) | HRR (bpm) | rHRR (%) | |||||||||
| 1 min | 2 min | 1 min | 2 min | 1 min | 2 min | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | |
| Insulin | 0.316 | 0.008 | 0.264 | 0.029 | −0.233 | 0.054 | −0.105 | 0.380 | −0.248 | 0.040 | −0.154 | 0.210 |
| HOMA-IR | 0.350 | 0.003 | 0.299 | 0.013 | −0.247 | 0.041 | −0.105 | 0.390 | −0.268 | 0.026 | −0.166 | 0.172 |
| LDL | 0.266 | 0.020 | 0.183 | 0.111 | −0.144 | 0.211 | −0.046 | 0.689 | −0.200 | 0.082 | −0.089 | 0.440 |
| No-HDL | 0.225 | 0.049 | 0.157 | 0.173 | −0.128 | 0.267 | −0.066 | 0.567 | −0.175 | 0.128 | −0.097 | 0.404 |
| Castelli-1 | 0.259 | 0.023 | 0.260 | 0.023 | −0.164 | 0.155 | −0.188 | 0.101 | −0.209 | 0.069 | −0.241 | 0.035 |
| Castelli-2 | 0.230 | 0.044 | 0.210 | 0.072 | −0.132 | 0.251 | −0.118 | 0.307 | −0.170 | 0.139 | −0.160 | 0.167 |
| AC | 0.242 | 0.034 | 0.250 | 0.028 | −0.129 | 0.262 | −0.171 | 0.137 | −0.179 | 0.119 | −0.222 | 0.052 |
| CRP | 0.332 | 0.004 | 0.239 | 0.042 | −0.099 | 0.403 | −0.023 | 0.847 | −0.139 | 0.242 | −0.077 | 0.517 |
| Variable | Total | biomarker | Normal | Abnormal | p-Value | |||||||
| Median (IQR) | Median (IQR) | Median (IRQ) | ||||||||||
| HOMA-IR | 2.46 (1.54–3.85) | HRR1 | 2.35 (1.31–3.6) | 3.14 (1.95–4.98) | 0.052 | |||||||
| HRR2 | 2.37 (1.47–3.85) | 2.8 (1.68–4.32) | 0.383 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aztatzi-Aguilar, O.G.; Vargas-Domínguez, C.; Debray-Garcia, Y.; Ortega-Romero, M.S.; Almeda-Valdés, P.; Aguilar-Salinas, C.A.; Naranjo-Meneses, M.A.; Mena-Orozco, D.A.; Lam-Chung, C.E.; Cruz-Bautista, I.; et al. Biochemical and Hematological Relationship with the Evaluation of Autonomic Dysfunction by Heart Rate Recovery in Patients with Asthma and Type 2 Diabetes. Diagnostics 2021, 11, 2187. https://doi.org/10.3390/diagnostics11122187
Aztatzi-Aguilar OG, Vargas-Domínguez C, Debray-Garcia Y, Ortega-Romero MS, Almeda-Valdés P, Aguilar-Salinas CA, Naranjo-Meneses MA, Mena-Orozco DA, Lam-Chung CE, Cruz-Bautista I, et al. Biochemical and Hematological Relationship with the Evaluation of Autonomic Dysfunction by Heart Rate Recovery in Patients with Asthma and Type 2 Diabetes. Diagnostics. 2021; 11(12):2187. https://doi.org/10.3390/diagnostics11122187
Chicago/Turabian StyleAztatzi-Aguilar, O. Gamaliel, Claudia Vargas-Domínguez, Yazmin Debray-Garcia, Manolo S. Ortega-Romero, Paloma Almeda-Valdés, Carlos A. Aguilar-Salinas, M. Augusta Naranjo-Meneses, D. Abril Mena-Orozco, César E. Lam-Chung, Ivette Cruz-Bautista, and et al. 2021. "Biochemical and Hematological Relationship with the Evaluation of Autonomic Dysfunction by Heart Rate Recovery in Patients with Asthma and Type 2 Diabetes" Diagnostics 11, no. 12: 2187. https://doi.org/10.3390/diagnostics11122187
APA StyleAztatzi-Aguilar, O. G., Vargas-Domínguez, C., Debray-Garcia, Y., Ortega-Romero, M. S., Almeda-Valdés, P., Aguilar-Salinas, C. A., Naranjo-Meneses, M. A., Mena-Orozco, D. A., Lam-Chung, C. E., Cruz-Bautista, I., & Sierra-Vargas, M. P. (2021). Biochemical and Hematological Relationship with the Evaluation of Autonomic Dysfunction by Heart Rate Recovery in Patients with Asthma and Type 2 Diabetes. Diagnostics, 11(12), 2187. https://doi.org/10.3390/diagnostics11122187






