The Effect of Limiting the Scan Range of Computed Tomography Pulmonary Angiography (to Reduce Radiation Exposure) on the Detection of Pulmonary Embolism: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Literature Search
2.3. Data Extraction
2.4. Quality Assessment
2.5. Outcome Measures
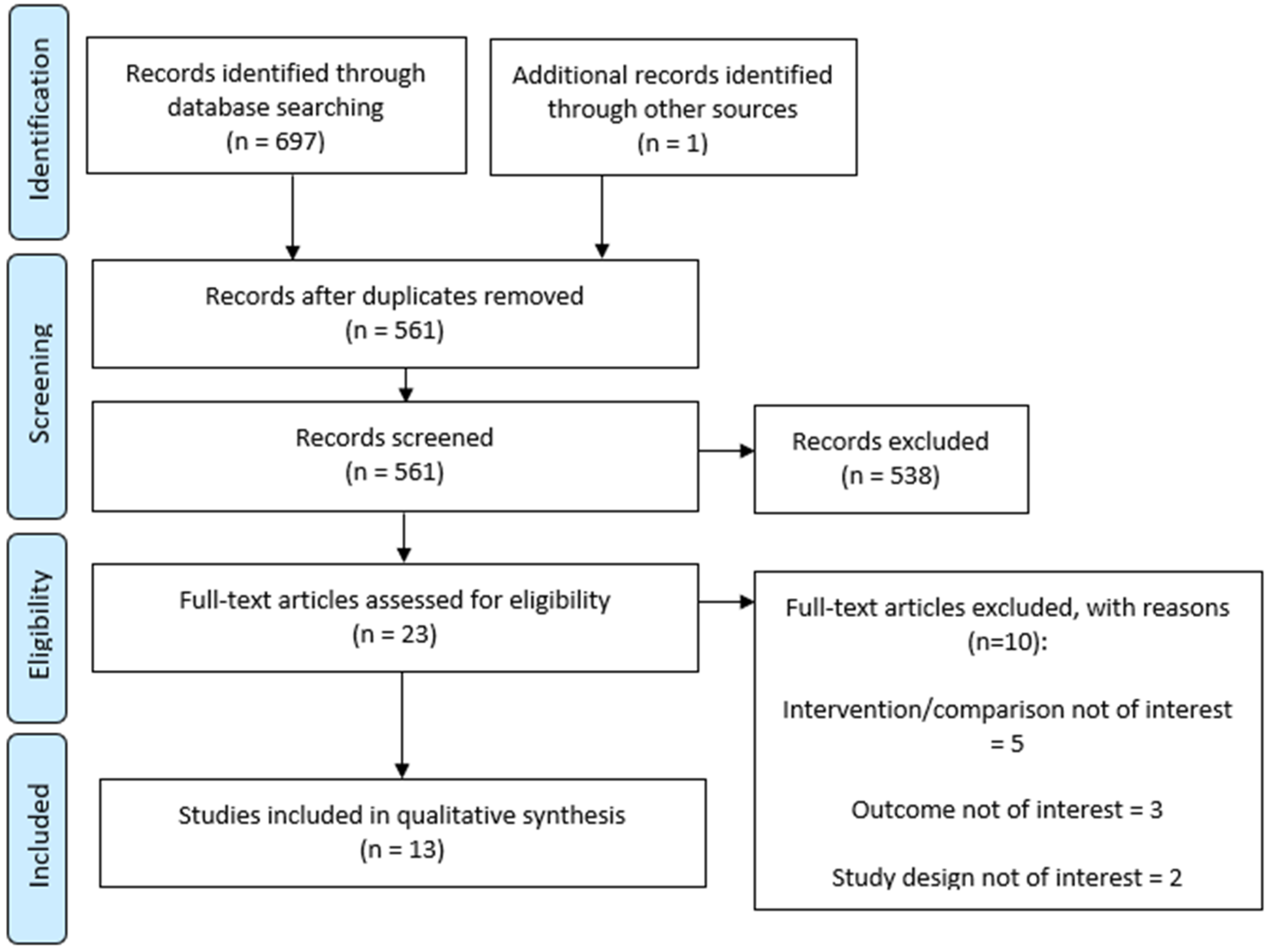
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Giordano, N.J.; Jansson, P.S.; Young, M.N.; Hagan, K.A.; Kabrhel, C. Epidemiology, pathophysiology, stratification, and natural history of pulmonary embolism. Tech. Vasc. Interv. Radiol. 2017, 20, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Estrada, Y.M.R.M.; Oldham, S.A. CTPA as the gold standard for the diagnosis of pulmonary embolism. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 557–563. [Google Scholar] [CrossRef]
- Wiener, R.S.; Schwartz, L.M.; Woloshin, S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch. Intern. Med. 2011, 171, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, M.; Subedi, S.K.; Ahmed, A.; Khan, J.; Dawood, T.; Ríos-Bedoya, C.F.; Bachuwa, G. Computed tomography pulmonary angiography is overused to diagnose pulmonary embolism in the emergency department of academic community hospital. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, C.; Foley, S.; Rowan, M.; Ewins, K.; NiAinle, F.; MacMahon, P. The OPTICA study (optimised computed tomography pulmonary angiography in pregnancy quality and safety study): Rationale and design of a prospective trial assessing the quality and safety of an optimised CTPA protocol in pregnancy. Thromb. Res. 2019, 177, 172–179. [Google Scholar] [CrossRef]
- Kline, J.A.; Courtney, D.M.; Beam, D.M.; King, M.C.; Steuerwald, M. Incidence and predictors of repeated computed tomographic pulmonary angiography in emergency department patients. Ann. Emerg. Med. 2009, 54, 41–48. [Google Scholar] [CrossRef]
- Champion, N.; Hogan, S.; Flemming, J. Assessing the prevalence of incidental findings identified by CTPA in women of reproductive age. Emerg. Med. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Refaat, R.; El-Shinnawy, M.A. Does the anatomic distribution of acute pulmonary emboli at MDCT pulmonary angiography in oncology-population differ from that in non-oncology counterpart? Egypt. J. Radiol. Nucl. Med. 2013, 44, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Dobler, C.C. Overdiagnosis of pulmonary embolism: Definition, causes and implications. Breathe 2019, 15, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar]
- Atalay, M.K.; Walle, N.L.; Grand, D.J.; Mayo-Smith, W.W.; Cronan, J.J.; Egglin, T.K. Scan length optimization for pulmonary embolism at CT angiography: Analysis based on the three-dimensional spatial distribution of 370 emboli in 100 patients. Clin. Radiol. 2011, 66, 405–411. [Google Scholar] [CrossRef]
- Shahir, K.; McCrea, J.M.; Lozano, L.A.S.; Goodman, L.R. Reduced z-axis technique for CT Pulmonary angiography in pregnancy-validation for practical use and dose reduction. Emerg. Radiol. 2015, 22, 651–656. [Google Scholar] [CrossRef]
- Kallen, J.A.; Coughlin, B.F.; O’Loughlin, M.T.; Stein, B. Reduced Z-axis coverage multidetector CT angiography for suspected acute pulmonary embolism could decrease dose and maintain diagnostic accuracy. Emerg. Radiol. 2010, 17, 31–35. [Google Scholar] [CrossRef]
- Atalay, M.K.; Walle, N.L.; Egglin, T.K. Prevalence and nature of excluded findings at reduced scan length CT angiography for pulmonary embolism. J. Cardiovasc. Comput. Tomogr. 2011, 5, 325–332. [Google Scholar] [CrossRef]
- Shahir, K.; Goodman, L.R.; Lam, C.A.; Midia, E.C. Dose reduction of 69% for computed tomography pulmonary angiography: Reduced z-axis computed tomography pulmonary angiography retains accuracy in those younger than 40 years. J. Comput. Assist. Tomogr. 2013, 37, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Tanabe, N.; Funabashi, N.; Takaoka, H.; Ikari, J.; Toyama, S.; Shimizu, H.; Hoshino, S.; Sugiura, T.; Saito, M.; et al. Detailed distribution of acute pulmonary thromboemboli: Direct evidence for reduction of acquisition length and radiation dose for triple rule-out CT angiography. Int. J. Cardiol. 2011, 147, 234–238. [Google Scholar] [CrossRef]
- Ho, A.; Mathew, S.; Harden, S. CTPAs: Reducing radiation dose without compromising diagnostic accuracy. Clin. Radiol. 2019, 74, e17. [Google Scholar] [CrossRef]
- Michalakis, N.; Keyzer, C.; De Maertelaer, V.; Tack, D.; Gevenois, P.A. Reduced z-axis coverage in multidetector-row CT pulmonary angiography decreases radiation dose and diagnostic accuracy of alternative diseases. Br. Inst. Radiol. 2014, 87, 20130546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Bhatti, J.; Rabinowitz, D. Tailoring CT pulmonary angiogram to different clinical scenarios. J. Med. Imaging Radiat. Oncol. 2019, 63, 53–54. [Google Scholar]
- Hendriks, B.M.F.; Schnerr, R.S.; Milanese, G.; Jeukens, C.R.L.P.N.; Niesen, S.; Eijsvoogel, N.G.; Wildberger, J.E.; Das, M. Computed tomography pulmonary angiography during pregnancy: Radiation dose of commonly used protocols and the effect of scan length optimization. Korean J. Radiol. 2019, 20, 313–322. [Google Scholar] [CrossRef]
- Atweh, L.; Orth, R.C.; Seghers, V.; Zhang, W.; Guillerman, R.P. Limited Z-axis coverage strategy for reducing radiation dose of CT pulmonary angiography for the diagnosis of pulmonary embolism in children. Pediatr. Radiol. 2012, 42, S268. [Google Scholar]
- Cowell, D.; Sheridan, S. Limited range CTPA—A safe way to reduce dose? Clin. Radiol. 2012, 67, S4. [Google Scholar] [CrossRef]
- Patel, H.; Coughlin, B.F.; LaFrance, T.; O’Donnell-Moran, G. Comparison of Full Chest CTA with Limited CTA and Triple Rule-out CTA for PE Detection and Effective Dose Implications. In Proceedings of the Radiological Society of North America Annual Meeting, Chicago, IL, USA, 25–30 November 2007. [Google Scholar]
- Carrier, M.; Righini, M.; Wells, P.S.; Perrier, A.; Anderson, D.R.; Rodger, M.A.; Pleasance, S.; Le Gal, G. Subsegmental pulmonary embolism diagnosed by computed tomography: Incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J. Thromb. Haemost. 2010, 8, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.V.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Cogley, J.R.; Ghobrial, P.M.; Chandrasekaran, B.; Allen, S.B. Pulmonary embolism evaluation in the pregnant patient: A review of current imaging approaches. Semin. Ultrasound CT MRI 2012, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Niemann, T.; Nicolas, G.; Roser, H.W.; Müller-Brand, J.; Bongartz, G. Imaging for suspected pulmonary embolism in pregnancy-what about the fetal dose? A comprehensive review of the literature. Insights Imaging 2010, 1, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, J.; Reed, W. A guide to foetal dose minimisation in computed tomography pulmonary angiography of the pregnant patient. Radiographer 2012, 59, 56–60. [Google Scholar] [CrossRef]
- Hofmann, B. Diagnosing overdiagnosis: Conceptual challenges and suggested solutions. Eur. J. Epidemiol. 2014, 29, 599–604. [Google Scholar] [CrossRef]
- Demircioğlu, A.; Stein, M.C.; Kim, M.-S.; Geske, H.; Quinsten, A.S.; Blex, S.; Umutlu, L.; Nassenstein, K. Detecting the pulmonary trunk in CT scout views using deep learning. Sci. Rep. 2021, 11, 10215. [Google Scholar] [CrossRef] [PubMed]
| Study (Journal) | Study Type | Publication | Country | Study Groups | Study Period | Patient Selection |
|---|---|---|---|---|---|---|
| Kallen et al., 2010 [13] | Retrospective cohort | Peer-reviewed article | USA | N/A | July 2005–February 2008 | All patients who underwent CTPA |
| Uehara et al., 2011 [16] | Retrospective cohort | Peer-reviewed article | Japan | N/A | January 2005–December 2006 | Consecutive patients with high risk of PE based on symptoms and clinical data (low PaO2, low PaCO2, increased D-dimer, ECG, ultrasonography) |
| Shahir, K et al., 2013 [15] | Case-control | Peer-reviewed article | USA | Study: PE-positive CTPA Control: Randomly selected negative CTPA | 2006–2008 | Patients between 18–40 years old who underwent CTPA (first scan only if multiple). PE-positive scans selected first, then randomly selected controls (normal exam, non-PE related findings) chosen from same selection population. |
| Michalakis et al., 2014 [18] | Prospective cohort | Peer-reviewed article | Belgium | N/A | September 2010–July 2012 | Consecutive patients who underwent CTPA based on clinical suspicion of PE |
| Shahir et al., 2015 [12] | Retrospective cohort | Peer-reviewed article | USA | N/A | 2004–2012 | All pregnant women who underwent CTPA |
| Atalay et al., 2011 [11] (Clin Rad) | Retrospective cohort | Peer-reviewed article | USA | N/A | January 2005–March 2006 | Consecutive patients positive for acute PE |
| Atalay et al., 2011 [14] (J Cardiol Comput Tomogr) | Retrospective cohort | Peer-reviewed article | USA | N/A | February 2010–March 2010 | Patients who presented with chest pain, hypoxemia, tachycardia, shortness of breath or variations of these as indication for their CTPA |
| Hendriks et al., 2019 [20] | Retrospective cohort | Peer-reviewed article | Netherlands | N/A | Not specified | Consecutive non-pregnant female patients who underwent CTPA |
| Patel et al., 2007 [23] | Retrospective cohort | Conference abstract | Not specified | N/A | Not specified | Not specified |
| Cowell & Sheridan, 2012 [22] | Retrospective cohort | Conference abstract | Not specified | N/A | January 2012–April 2012 | Patients who underwent CTPA |
| Atweh et al., 2012 [21] | Retrospective cohort | Conference abstract | Not specified | N/A | 2005–2011 | All pediatric patients (0–25 years) with a PE-positive CTPA |
| Ho et al., 2019 [17] | Retrospective and prospective cohort | Conference abstract | England | A: Patients who underwent CTPA (retrospective; n = 153) B: Patients assessed for dose for standard and reduced scan coverage (prospective; n = 23) | A: Nov 2018 B: December 2018–January 2019 | Patients who underwent CTPA |
| Chen et al., 2019 [19] | Retrospective cohort | Conference abstract | Australia | N/A | Not specified | Consecutive patients with a PE-positive CTPA |
| Study (Journal) | CT Scan Type | Reduced Scan Window Used | Was the Optimal Scan Range Evaluated to Capture All PE Diagnoses/Filling Defects? | Reduction in Mean Scan Length (cm, % Reduction) | Reduction in Radiation Dose (%) |
|---|---|---|---|---|---|
| Kallen et al., 2010 [13] | 64-row MDCT | Above the aortic arch to below inferior-most aspect of the heart | N | 9.6 (37%) | N/A |
| Uehara et al., 2011 [16] | 16-slice MDCT | Top of aortic arch to below the under surface of the heart | N | 21.90% | 22 |
| Shahir et al., 2013 [15] | 16-row and 64-row MDCT | Top of aortic arch to below the level of the heart | N | 11 (42%); calculated based on 15 different consecutive patients who underwent CTPA | 60; based on different 15 consecutive patients who underwent CTPA) |
| Michalakis et al., 2014 [18] | 16-section and 64-row MDCT | 10 cm scan length starting from the bottom of aortic arch | N | 19.6 (52%) | 69 |
| Shahir et al., 2015 [12] | 16-row and 64-row MDCT | Top of aortic arch to below the level of the heart | N | 15 (42%) | 71; calculated based on 36 consecutive non-pregnant adult patients who underwent CTPA |
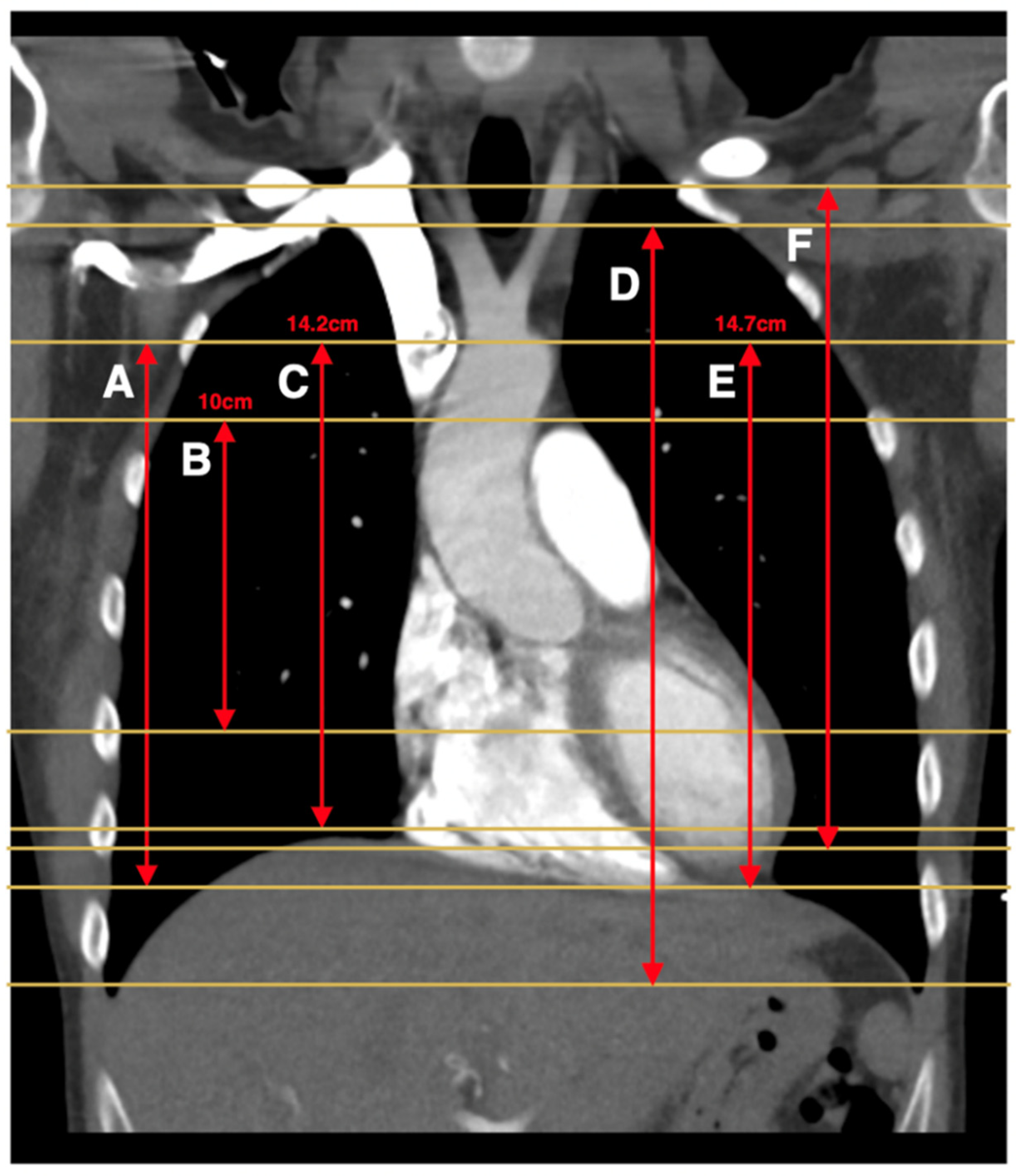
| Atalay et al., 2011 [11] (Clin Rad) | 16-row MDCT | A: 14.2 cm scan length centered 4.1 cm below the carina (capture at least one PE) B: Top of aorta to bottom of the heart | A: Y B: N | A: 11.7 (44%) B: 9.9 (38%) | N/A |
| Atalay et al., 2011 [14] (J Cardiol Comput Tomogr) | 16-row and 64-row MDCT | 14.2 cm scan length centered 4.1 cm below the carina (based on previous study which optimized scan length to capture all PE) | N | 13.8 (49%) | N/A |
| Hendriks et al., 2019 [20] | A: 64-slice MDCT B: 2 × 128-slice DSCT C: 2 × 128-slice DSCT D: 2 × 192-slice DSCT | A-B: Lung apex to the top of the most caudal diaphragm | A-B: N | A: 33% B: 30% C: 30% D: 31% | A: 26% B: 25% C: 26% D: 23% |
| Patel et al., 2007 [23] | Not specified | Top of the aortic arch to below the heart | N | N/A | 48 |
| Cowell & Sheridan, 2012 [22] | Not specified | Superior aspect of the aortic arch to the inferior aspect of the heart | N | Not specified | Not specified |
| Atweh et al., 2012 [21] | Not specified | A: Patients without congenital heart disease: 14 cm scan length centered 3.5 cm below the carina (captures 100% of all filling defects) B: Patients with CHD: 8 cm scan length centered 5 cm below the carina (captures at least 1 filling defect) | A: Y B: Y | A: 20% B: 40% | Not specified |
| Ho et al., 2019 [17] | Not specified | Humeral heads to lung bases (excludes lung apices) | N | A & B: 14.9 cm (49.6%) | A: N/A B: 21 |
| Chen et al., 2019 [19] | Not specified | 14.7 cm length starting superiorly at the top of the aortic arch (no rationale provided) | N | N/A | N/A |
| Study (Journal) | Total Number of CTPAs Analysed | Number of CTPAs Excluded | Reasons for Exclusion | Number of CTPAs Included | PE-Positive CTPAs (% of Included Scans) | Number of PE Diagnoses Missed with Reduced Scan Window (% of All PEs) | Number of Filling Defects Missed | Total Number of Other Findings | Number of Other Findings Missed with Reduced Scan Window (% of All Other Findings) |
|---|---|---|---|---|---|---|---|---|---|
| Kallen et al. 2010 [13] | 1734 | 0 | Not applicable | 1734 | 295 (17) | 0 | Not specified | Not specified | Not specified |
| Uehara et al., 2011 [16] | 75 | 0 | Not applicable | 75 | 75 (100) | 0 | 2 (2.6%) | Not specified | Not specified |
| Shahir et al., 2013 [15] | 878 | 678 (77%) | Suboptimal opacification of pulmonary arteries, compromised evaluation due to breathing artefact (n = 112); negative for PE; not randomly selected for control group | 200 | 86 (43.7) | 0 | Not specified | 392 (1.96 findings per scan) | 7 (1.8) |
| Michalakis et al., 2014 [18] | 253 | 6 (0.023%) | Poor arterial enhancement | 247 | 57 (23.4) | 1 (1.8; sub-segmental) | Not specified | 343 (1.39 findings per scan) | 48 (14) |
| Shahir et al., 2015 [12] | 95 | 11 (8.64%) | Suboptimal contrast opacification; respiratory motion artefacts | 84 | 2 (2.3; segmental [n = 2]) | 0 | Not specified | 76 (0.9 findings per scan) | 4 (5.26) |
| Atalay et al., 2011 [11] (Clin Rad) | 95 | 11 (8.64%) | Suboptimal contrast opacification; respiratory motion artefacts | 84 | 2 (2.3; segmental [n = 2]) | 0 | Not specified | 76 (0.9 findings per scan) | 4 (5.26) |
| Atalay et al., 2011 [14] (J Cardiol Comput Tomogr) | 95 | 11 (8.64%) | Suboptimal contrast opacification; respiratory motion artefacts | 84 | 2 (2.3; segmental [n = 2]) | 0 | Not specified | 76 (0.9 findings per scan) | 4 (5.26) |
| Hendriks et al., 2019 [20] | 95 | 11 (8.64%) | Suboptimal contrast opacification; respiratory motion artefacts | 84 | 2 (2.3; segmental [n = 2]) | 0 | Not specified | 76 (0.9 findings per scan) | 4 (5.26) |
| Patel et al., 2007 [23] | 95 | 11 (8.64%) | Suboptimal contrast opacification; respiratory motion artefacts | 84 | 2 (2.3; segmental [n = 2]) | 0 | Not specified | 76 (0.9 findings per scan) | 4 (5.26) |
| Cowell & Sheridan, 2012 [22] | 200 | 161 (80.5%) | Negative for PE | 39 | 39 (100) | 0 | Not specified | Not specified | 3 patients with other findings (number of missed findings not specified) |
| Atweh et al., 2012 [21] | Not specified | Not specified | Negative for PE | 45 | 45 (100) | A: 0 B: 0 | A: 0 B: Not specified | Not specified | Not specified |
| Ho et al., 2019 [17] | A: 153 B: 23 | Not specified | Not specified | A: 153 B: 23 | A: 29 (19) B: Not specified | 0 | Not specified | Not specified | Not specified |
| Chen et al., 2019 [19] | 200 | Not specified | Not specified | 200 | 200 (100) | 1 (0.5; sub-segmental) | 2 (1%) | Not specified | Not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaw, A.; Nguyen, R.; Lam, L.; Kaplan, A.; Dobler, C.C. The Effect of Limiting the Scan Range of Computed Tomography Pulmonary Angiography (to Reduce Radiation Exposure) on the Detection of Pulmonary Embolism: A Systematic Review. Diagnostics 2021, 11, 2179. https://doi.org/10.3390/diagnostics11122179
Zaw A, Nguyen R, Lam L, Kaplan A, Dobler CC. The Effect of Limiting the Scan Range of Computed Tomography Pulmonary Angiography (to Reduce Radiation Exposure) on the Detection of Pulmonary Embolism: A Systematic Review. Diagnostics. 2021; 11(12):2179. https://doi.org/10.3390/diagnostics11122179
Chicago/Turabian StyleZaw, Amayar, Rebecca Nguyen, Leon Lam, Anthony Kaplan, and Claudia C. Dobler. 2021. "The Effect of Limiting the Scan Range of Computed Tomography Pulmonary Angiography (to Reduce Radiation Exposure) on the Detection of Pulmonary Embolism: A Systematic Review" Diagnostics 11, no. 12: 2179. https://doi.org/10.3390/diagnostics11122179
APA StyleZaw, A., Nguyen, R., Lam, L., Kaplan, A., & Dobler, C. C. (2021). The Effect of Limiting the Scan Range of Computed Tomography Pulmonary Angiography (to Reduce Radiation Exposure) on the Detection of Pulmonary Embolism: A Systematic Review. Diagnostics, 11(12), 2179. https://doi.org/10.3390/diagnostics11122179






