Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Data Collection
2.3. Study Variables
2.4. Assessments
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
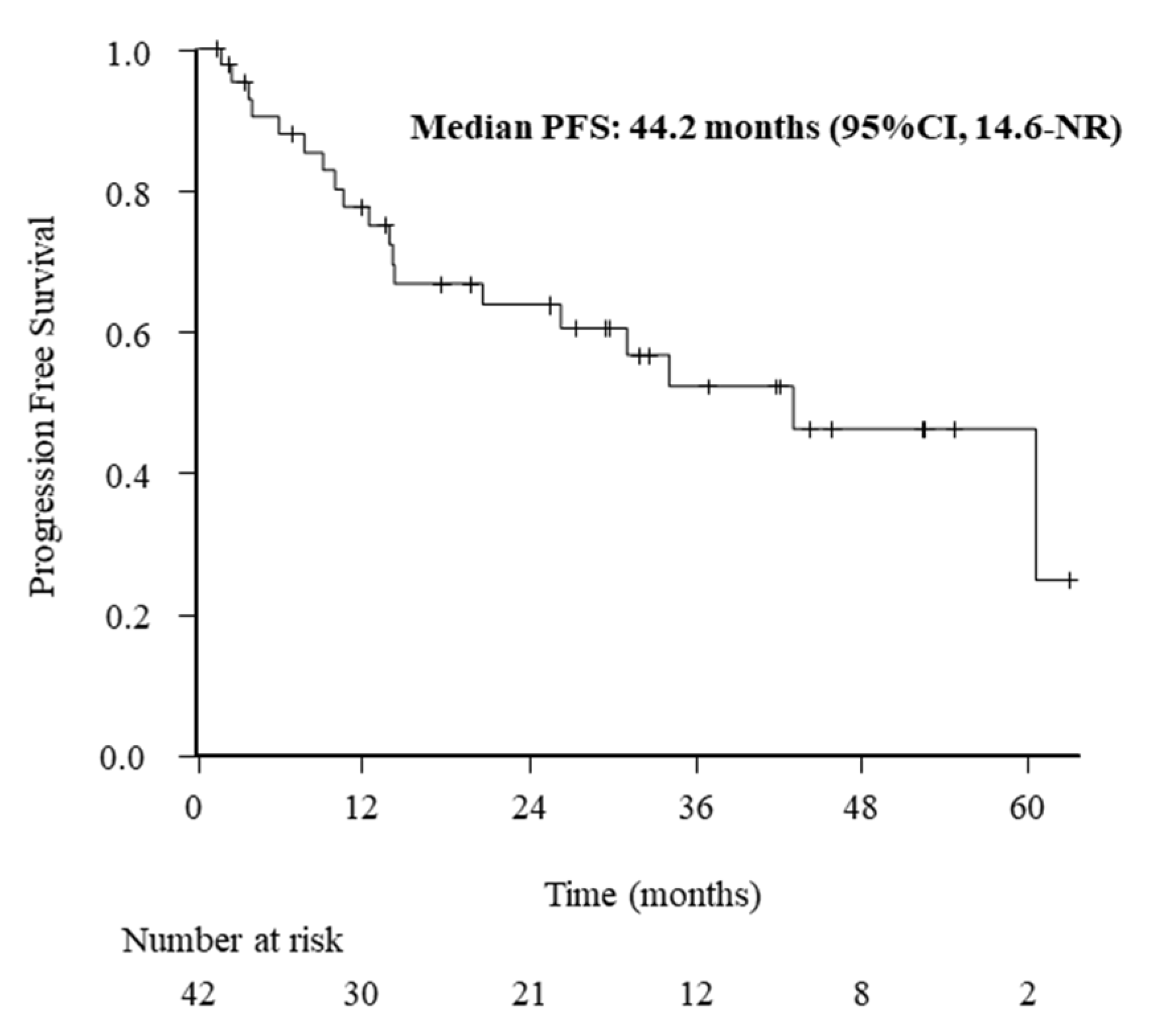
3.2. ORR and PFS Outcomes
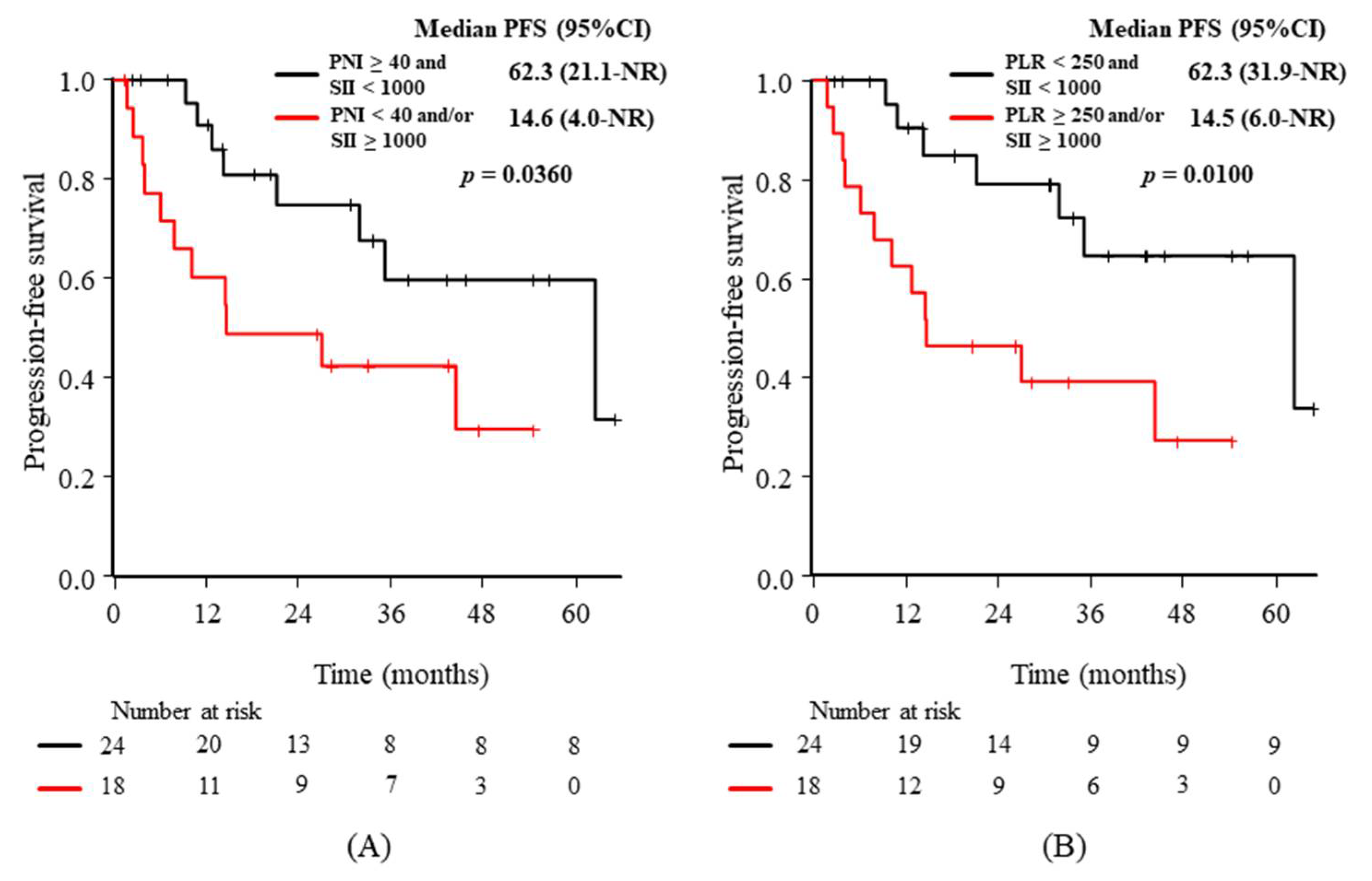
3.3. Relationships between the Baseline Characteristics or Candidate Predictive Biomarkers and PFS
3.4. Adverse Events (AEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Code Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Sakai, K.; Terashima, M.; Kaneda, H.; Hayashi, H.; Tanaka, K.; Okamoto, K.; Takahama, T.; Yoshida, T.; Iwasa, T.; et al. Clinical application of amplicon-based next-generation sequencing to therapeutic decision making in lung cancer. Ann. Oncol. 2015, 26, 2477–2482. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.-I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK -positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Coudert, B.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; De Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Iwama, E.; Goto, Y.; Murakami, H.; Harada, T.; Tsumura, S.; Sakashita, H.; Mori, Y.; Nakagaki, N.; Fujita, Y.; Seike, M.; et al. Alectinib for Patients with ALK Rearrangement–Positive Non–Small Cell Lung Cancer and a Poor Performance Status (Lung Oncology Group in Kyushu 1401). J. Thorac. Oncol. 2017, 12, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Nie, L.; Nong, L.; Cheng, Y. Primary resistance to alectinib in a patient with STRN-ALK -positive non-small cell lung cancer: A case report. Thorac. Cancer 2021, 12, 1927–1930. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Halazun, K.; Mirza, F.; Port, J.L.; Lee, P.C.; Neugut, A.I.; Altorki, N.K.; Abrams, J.A. Elevated Preoperative Neutrophil:Lymphocyte Ratio as a Predictor of Postoperative Disease Recurrence in Esophageal Cancer. Ann. Surg. Oncol. 2011, 18, 3362–3369. [Google Scholar] [CrossRef] [Green Version]
- Motomura, T.; Shirabe, K.; Mano, Y.; Muto, J.; Toshima, T.; Umemoto, Y.; Fukuhara, T.; Uchiyama, H.; Ikegami, T.; Yoshizumi, T.; et al. Neutrophil–lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via the inflammatory microenvironment. J. Hepatol. 2013, 58, 58–64. [Google Scholar] [CrossRef]
- Bhatti, I.; Peacock, O.; Lloyd, G.; Larvin, M.; Hall, R.I. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am. J. Surg. 2010, 200, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Y.; Wang, Z.; Yao, Y.; Chen, F.; Zhang, H.; Wang, Y.; Song, Y. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J. Thorac. Dis. 2013, 5, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-F.; Huang, Y.; Chen, Q.-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.-Y.; Liu, H.-L.; Ning, N.; Li, S.-Y.; Du, X.-H.; Li, R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol. Lett. 2016, 11, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Kanda, M.; Fujii, T.; Kodera, Y.; Nagai, S.; Takeda, S.; Nakao, A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2010, 98, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Chan, S.; Wong, G.L.-H.; Wong, V.W.S.; Chong, C.; Lai, P.B.S.; Chan, H.L.Y.; To, K.-F. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma after Surgical Resection. Ann. Surg. Oncol. 2015, 22, 4138–4148. [Google Scholar] [CrossRef]
- Jafri, S.H.; Shi, R.; Mills, G. Advanced lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013, 13, 158. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Kataoka, N.; Kunimatsu, Y.; Tachibana, Y.; Sugimoto, T.; Tani, N.; Sato, I.; Hirose, K.; Kato, D.; Takeda, T. Predictors of survival among Japanese patients receiving first-line chemoimmunotherapy for advanced non-small cell lung cancer. Thorac. Cancer 2021, 12, 97–105. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Zhai, E.-T.; Yuan, Y.; Wu, K.-M.; Xu, J.-B.; Peng, J.-J.; Chen, C.-Q.; He, Y.-L.; Cai, S.-R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef]
- Phan, T.T.; Ho, T.T.; Nguyen, H.T.; Nguyen, H.T.; Tran, T.B.; Nguyen, S.T. The prognostic impact of neutrophil to lymphocyte ratio in advanced non-small-cell lung cancer patients treated with EGFR TKI. Int. J. Gen. Med. 2018, 11, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Zhang, N.; Wang, Y.; Jiang, S.; Lu, M.; Huang, Y.; Ma, J.; Hu, C.; Hou, T. High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine 2019, 98, e16875. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Hong, L.; Sun, L.; Zhuang, H.; Sun, B.; Wang, H.; Zhang, X.; Ren, X. Platelet–lymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Future Oncol. 2017, 13, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, H.; Yang, G.; Yang, L.; Li, J.; Wang, Y. The value of blood biomarkers of progression and prognosis in ALK-positive patients with non-small cell lung cancer treated with crizotinib. Asia Pac. J. Clin. Oncol. 2020, 16, 63–69. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Eesfahani, K.; Agulnik, J.S.; Ecohen, V. A Systemic review of resistance mechanisms and ongoing clinical trials in ALK-Rearranged non-small cell lung cancer. Front. Oncol. 2014, 4, 174. [Google Scholar] [CrossRef] [Green Version]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, T.; Li, X.; Wang, Y.; Zhao, C.; Zhao, S.; Xi, L.; Zhang, S.; Liu, X.; Jia, Y.; et al. Clinical features ofBimdeletion polymorphism and its relation with crizotinib primary resistance in Chinese patients withALK/ROS1fusion-positive non-small cell lung cancer. Cancer 2017, 123, 2927–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, C.; Seo, S.; Kim, S.; Jang, S.; Park, K.; Song, J.; Lee, B.; Richards, M.; Bayliss, R.; Lee, D.; et al. Differential protein stability and clinical responses ofEML4-ALK fusion variants to various ALK inhibitors in advancedALK-rearranged non-small cell lung cancer. Ann. Oncol. 2017, 28, 791–797. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Karachaliou, N.; Berenguer, J.; Gimenez-Capitan, A.; Schellen, P.; Teixido, C.; Tannous, J.; Kuiper, J.L.; Drees, E.; Grabowska, M.; et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 2016, 7, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, F.; Deng, Q.; Xiao, D.; He, P.; Lin, X.; He, D. Predictive and prognostic value of phosphorylated c-KIT and PDGFRA in advanced non-small cell lung cancer harboring ALK fusion. Oncol. Lett. 2019, 17, 3071–3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef]
- Zer, A.; Moskovitz, M.; Hwang, D.M.; Hershko-Klement, A.; Fridel, L.; Korpanty, G.J.; Dudnik, E.; Peled, N.; Shochat, T.; Leighl, N.B.; et al. ALK -Rearranged Non–Small-Cell Lung Cancer Is Associated with a High Rate of Venous Thromboembolism. Clin. Lung Cancer 2017, 18, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Schindewolf, M.; Stanford, S.; Larsen, O.H.; Thiele, T. The Role of Platelets in Venous Thromboembolism. Semin. Thromb. Hemost. 2016, 42, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n (%) |
|---|---|
| Age (median, range) | 67 years (29–85) |
| Sex (female/male) | 20 (46.7%)/22 (52.4%) |
| Histology; AD/NSCLC-NOS | 40 (95.2%)/2 (4.8%) |
| ECOG-PS; 0–1/2/3 | 37 (88.1%)/3 (7.1%)/2 (4.8%) |
| Smoking; never/current or former | 12 (28.6%)/30 (71.4%) |
| Weight loss of ≥5%; (−)/(+) | 30 (71.4%)/12 (28.6%) |
| Biomarkers at baseline | |
| NLR (<5 vs. ≥5) | 29 (69.0%) vs. 13 (31.0%) |
| NLR (<3 vs. ≥3) | 19 (45.2%) vs. 23 (54.8%) |
| PLR (<250 vs. ≥250) | 27 (64.3%) vs. 15 (35.7%) |
| SII (<1000 vs. ≥1000) | 27 (64.3%) vs. 15 (35.7%) |
| ALI (≥18 vs. <18) | 27 (64.3%) vs. 15 (35.7%) |
| PNI (≥40 vs. <40) | 31 (71.4%) vs. 11 (28.6%) |
| Biomarkers 3 weeks after treatment | |
| NLR (<5 vs. ≥5) | 36 (85.7%) vs. 6 (14.3%) |
| NLR (<3 vs. ≥3) | 27 (64.3%) vs. 15 (35.7%) |
| PLR (<250 vs. ≥250) | 36 (85.7%) vs. 6 (14.3%) |
| SII (<1000 vs. ≥1000) | 27 (64.3%) vs. 15 (35.7%) |
| ALI (≥18 vs. <18) | 33 (78.6%) vs. 9 (21.4%) |
| PNI (≥40 vs. <40) | 33 (78.6%) vs. 9 (21.4%) |
| PFS | |||
|---|---|---|---|
| mPFS (Months, 95% CI) | HR (95% CI) | p-Value | |
| Age (<70 vs. ≥70 years) | 44.2 (14.6–NR) vs. 35.1 (7.8–NR) | 1.29 (0.52–3.23) | 0.585 |
| Sex (female vs. male) | 44.2 (21.1–NR) vs. NR (9.2–NR) | 1.33 (0.53–3.32) | 0.541 |
| Smoking (− vs. +) | NR (14.4–NR) vs. 35.1 (10.1–62.3) | 2.28 (0.75–6.98) | 0.136 |
| ECOG-PS (<2 vs. ≥2) | 44.2 (21.1–NR) vs. 10.7 (1.7–NR) | 3.75 (1.04–13.49) | 0.0300 |
| BW loss of ≥5% (− vs. +) | 35.1 (14.6–NR) vs. 44.2 (6.0–NR) | 1.12 (0.42–2.98) | 0.827 |
| Before treatment | |||
| NLR (<5 vs. ≥5) | 62.3 (14.2–NR) vs. 26.9 (4.0–NR) | 1.61 (0.64–4.01) | 0.307 |
| NLR (<3 vs. ≥3) | 62.3 (10.7–NR) vs. 44.2 (14.2–NR) | 1.14 (0.46–2.83) | 0.783 |
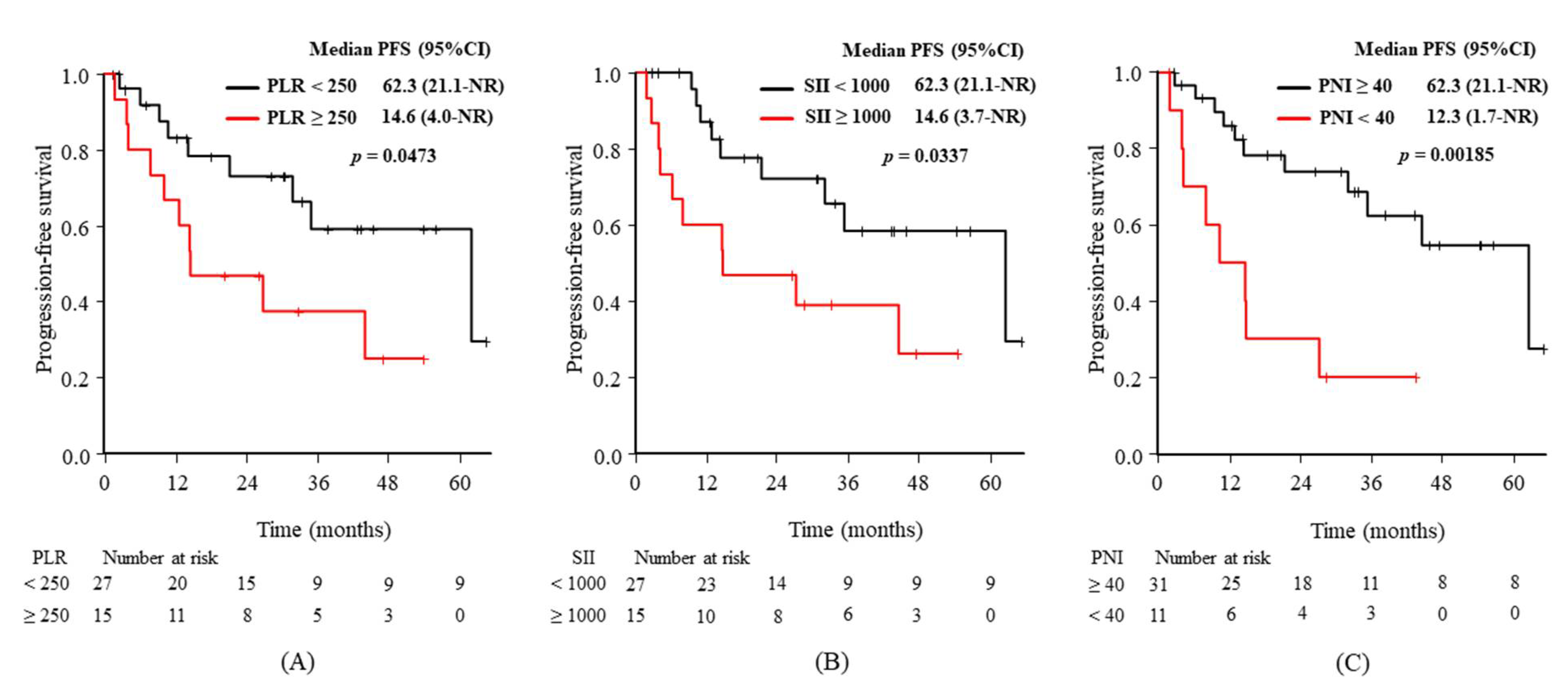
| PLR (<250 vs. ≥250) | 62.3 (21.1–NR) vs. 14.6 (4.0–NR) | 2.49 (0.98–6.34) | 0.0473 |
| SII (<1000 vs. ≥1000) | 62.3 (21.1–NR) vs. 14.6 (3.7–NR) | 2.65 (1.04–6.72) | 0.0337 |
| ALI (≥18 vs. <18) | 35.1 (14.2–NR) vs. 44.2 (4.0–NR) | 0.98 (0.95–1.01) | 0.917 |
| PNI (≥40 vs. <40) | 62.3 (31.9–NR) vs. 12.3 (1.7–NR) | 4.15 (1.57–10.9) | 0.00185 |
| [After 3 weeks] | |||
| NLR (<5 vs. ≥5) | 44.2 (14.4–NR) vs. 17.9 (1.7–NR) | 1.53 (0.49–4.77) | 0.459 |
| NLR (<3 vs. ≥3) | 44.2 (26.9–NR) vs. 14.6 (4.0–NR) | 1.89 (0.75–4.78) | 0.174 |
| PLR (<250 vs. ≥250) | 44.2 (14.6–NR) vs. 7.8 (1.7–NR) | 1.98 (0.56–7.05) | 0.284 |
| SII (<1000 vs. ≥1000) | 44.2 (26.9–NR) vs. 14.6 (1.7–NR) | 2.85 (0.97–8.43) | 0.0473 |
| ALI (≥18 vs. <18) | 35.1 (14.2–NR) vs. 44.2 (4.0–NR) | 2.00 (0.53–7.46) | 0.304 |
| PNI (≥40 vs. <40) | 62.3 (26.9–NR) vs. 14.6 (1.7–NR) | 3.04 (1.09–8.46) | 0.0125 |
| Any Grade (%) | Grade 2 (%) | |
|---|---|---|
| Any event | 10 (23.8) | 2 (4.8) |
| Renal dysfunction | 4 (9.5) | 1 (2.4) |
| Edema | 2 (4.8) | 0 (0) |
| Peripheral neuropathy | 2 (4.8) | 0 (0) |
| Anemia | 1 (2.4) | 0 (0) |
| Bradycardia | 1 (2.4) | 0 (0) |
| Skin toxicity | 1 (2.4) | 0 (0) |
| Liver dysfunction | 1 (2.4) | 1 (2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, T.; Yamada, T.; Tanimura, K.; Nakano, T.; Ishida, M.; Tachibana, Y.; Shiotsu, S.; Horiuchi, S.; Hibino, M.; Okada, A.; et al. Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib. Diagnostics 2021, 11, 2170. https://doi.org/10.3390/diagnostics11122170
Takeda T, Yamada T, Tanimura K, Nakano T, Ishida M, Tachibana Y, Shiotsu S, Horiuchi S, Hibino M, Okada A, et al. Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib. Diagnostics. 2021; 11(12):2170. https://doi.org/10.3390/diagnostics11122170
Chicago/Turabian StyleTakeda, Takayuki, Tadaaki Yamada, Keiko Tanimura, Takayuki Nakano, Masaki Ishida, Yusuke Tachibana, Shinsuke Shiotsu, Shigeto Horiuchi, Makoto Hibino, Asuka Okada, and et al. 2021. "Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib" Diagnostics 11, no. 12: 2170. https://doi.org/10.3390/diagnostics11122170
APA StyleTakeda, T., Yamada, T., Tanimura, K., Nakano, T., Ishida, M., Tachibana, Y., Shiotsu, S., Horiuchi, S., Hibino, M., Okada, A., Chihara, Y., & Takayama, K. (2021). Prognostic Markers of Survival among Japanese Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Receiving First-Line Alectinib. Diagnostics, 11(12), 2170. https://doi.org/10.3390/diagnostics11122170






