A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Spike Antigens and Antibodies
2.2. Commercial Antigens
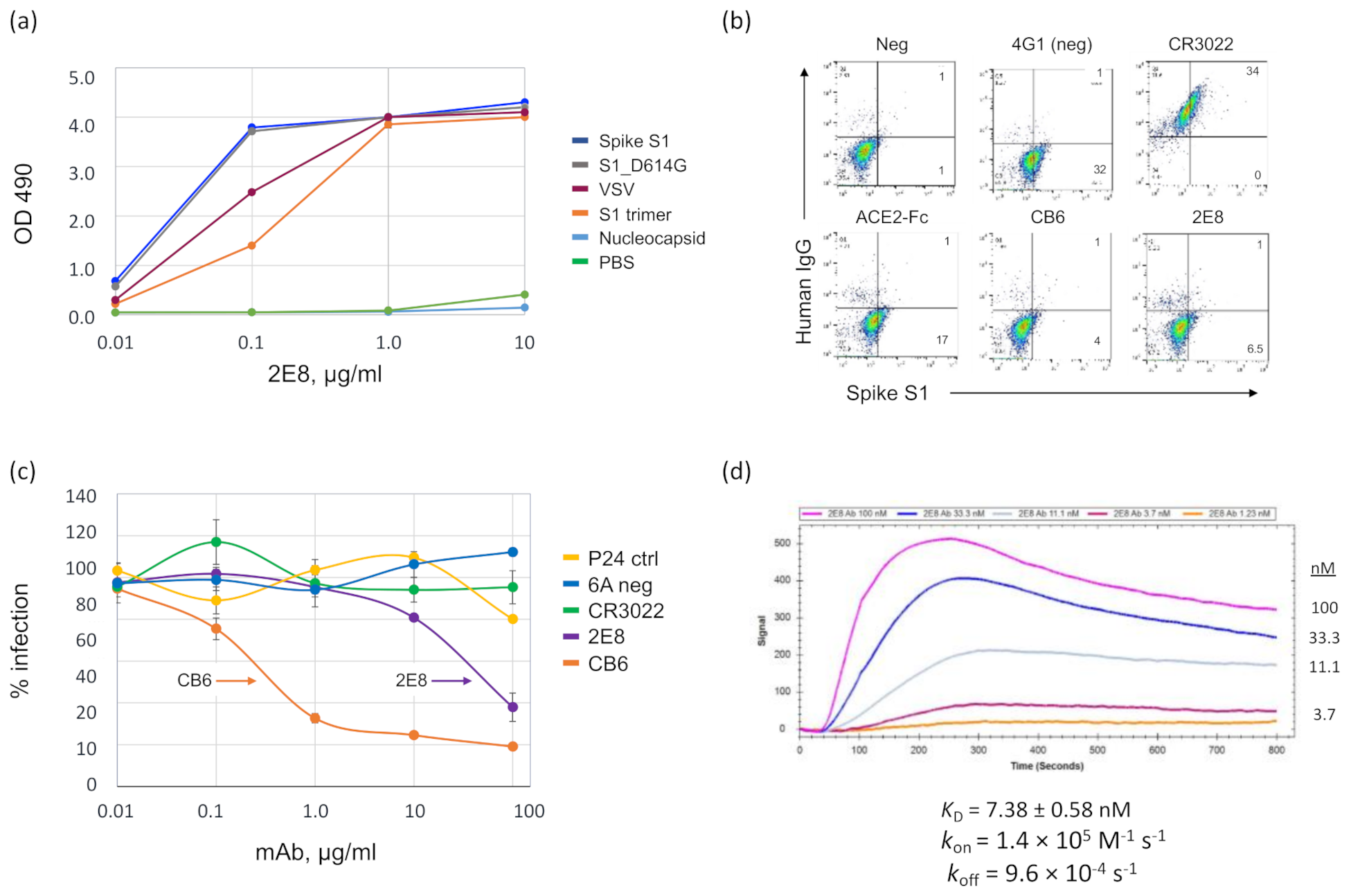
2.3. Discovery of the 2E8 Human Monoclonal Antibody
2.4. Production of Recombinant Antibodies
2.5. Surface Plasmon Resonance (SPR) Spectroscopy
2.6. ELISAs
2.7. Flow Cytometry-Based Receptor-Binding Inhibition Assay
2.8. Pseudotyped SARS-CoV-2 Antibody Neutralization Assay
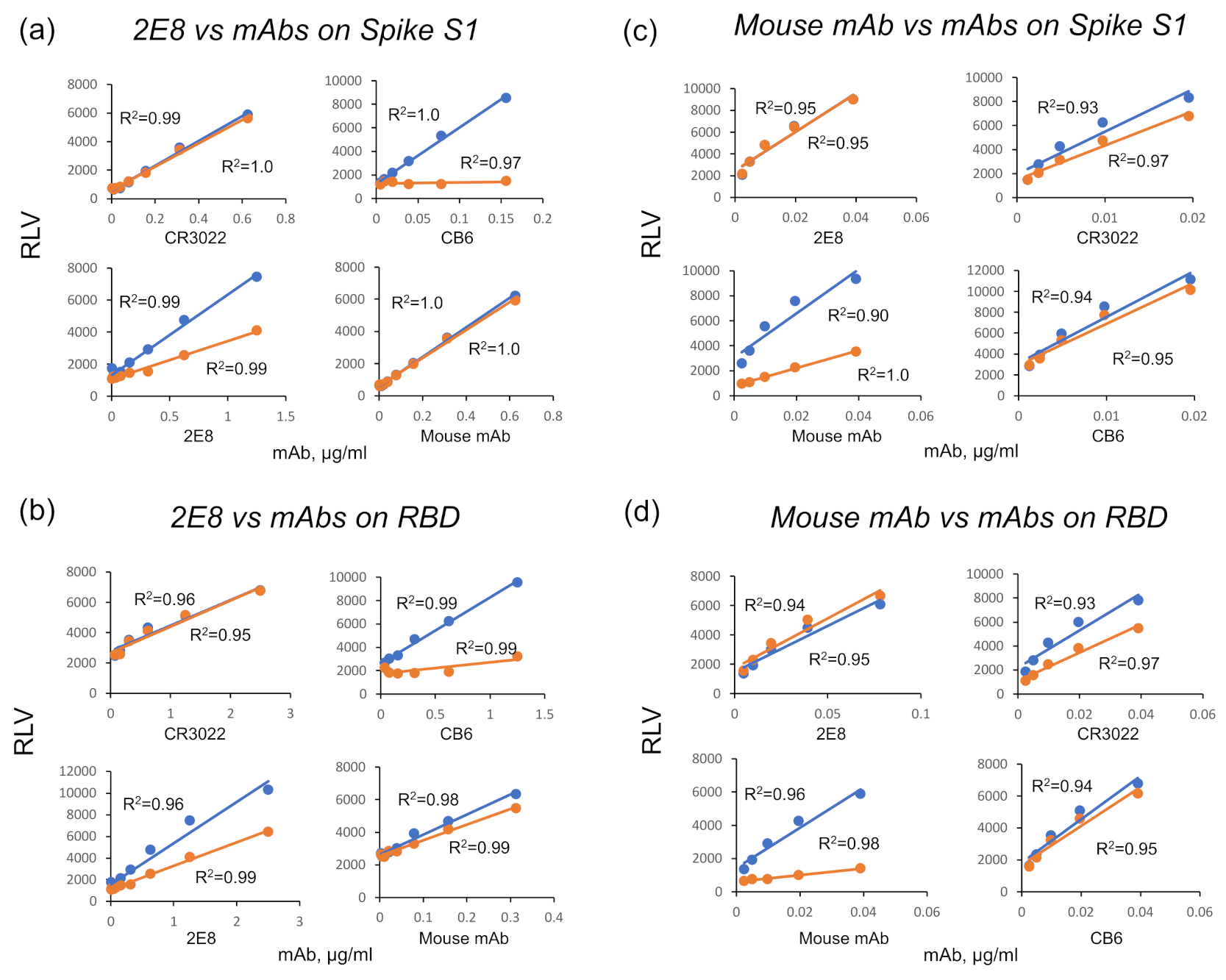
2.9. Epitope Binning
3. Results
3.1. A Human mAb That Neutralizes SARS-CoV-2 through Spike RBD Binding
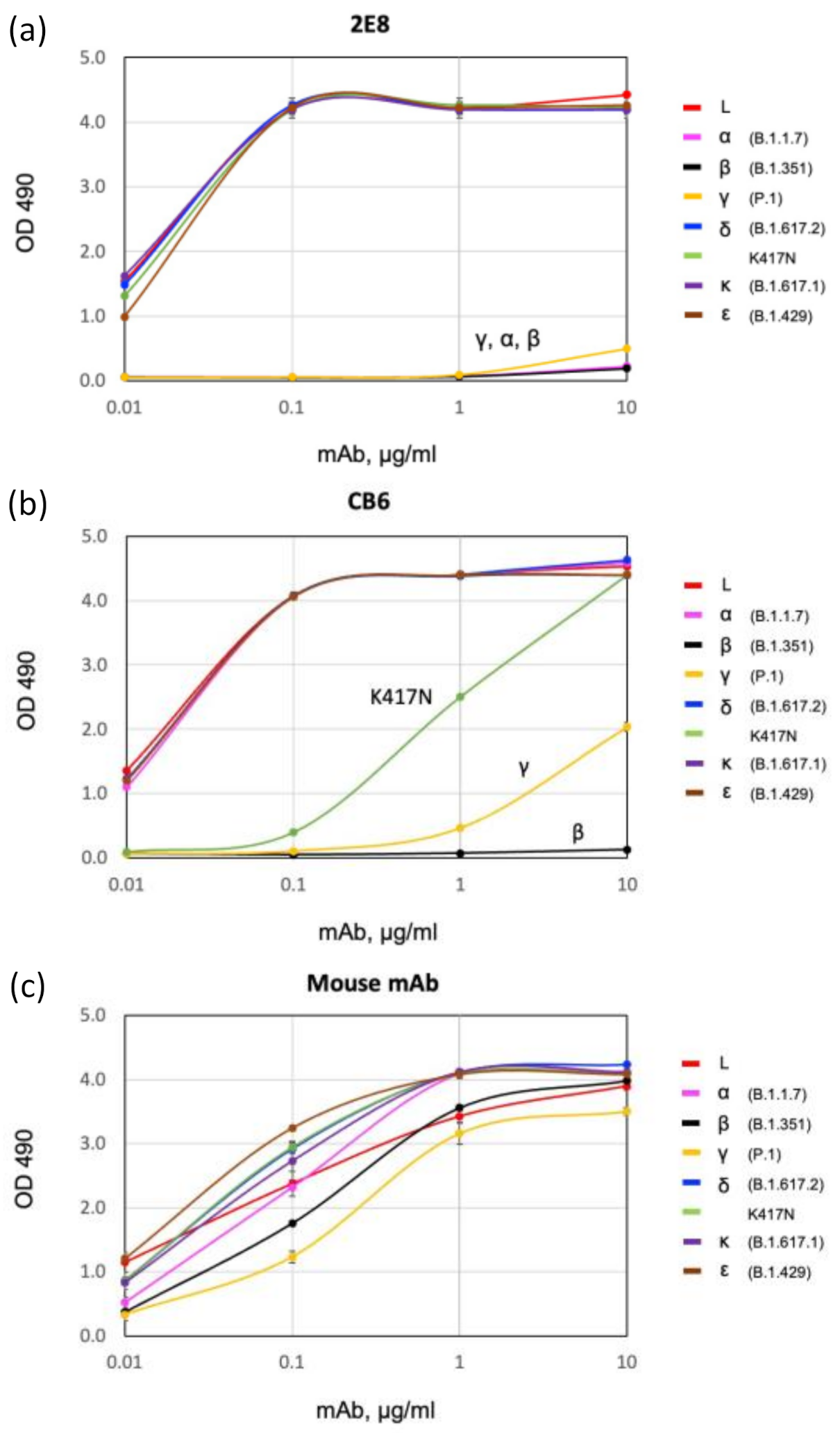
3.2. Binding of 2E8 to Important SARS-CoV-2 Variants
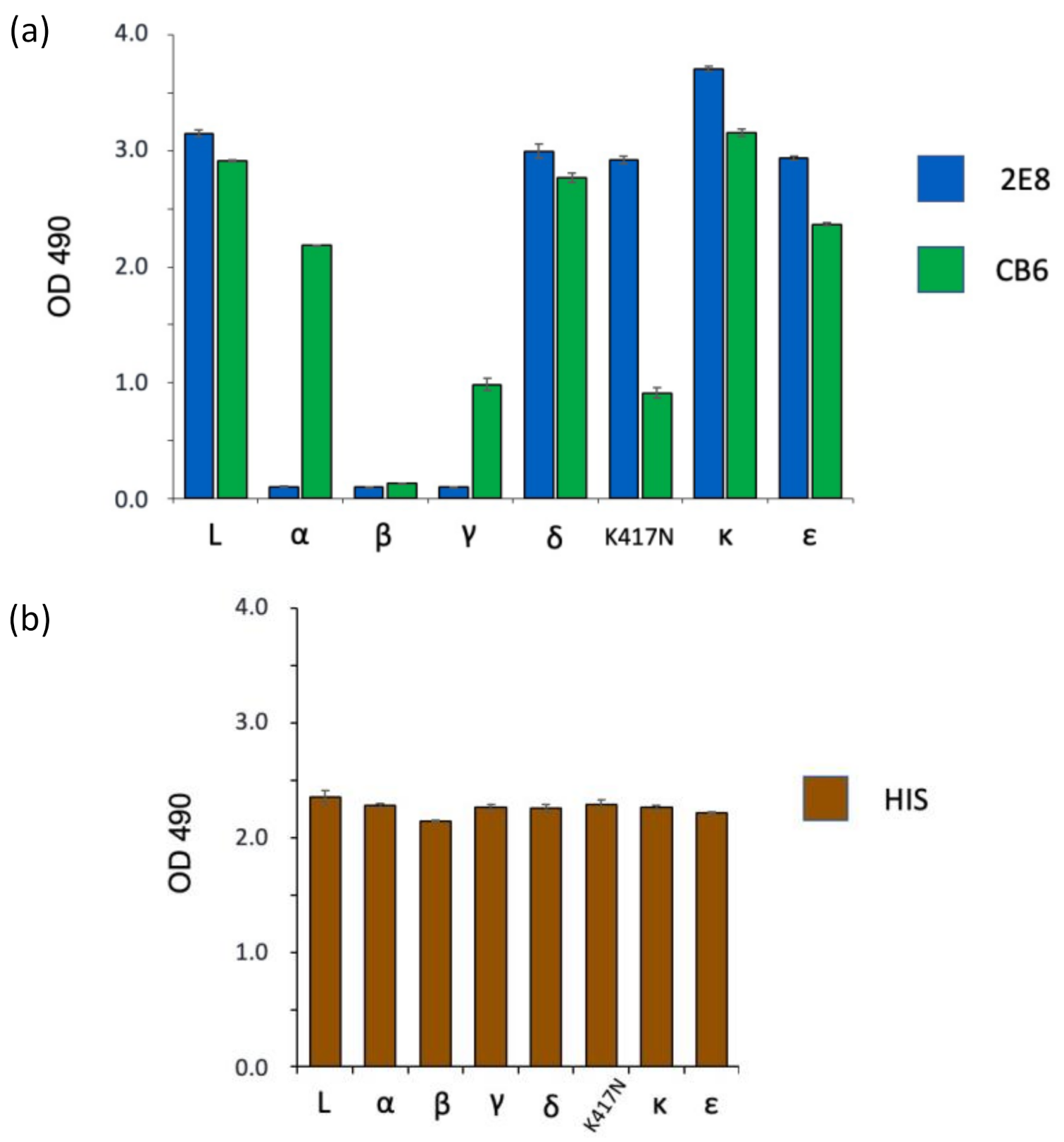
3.3. Spike Variant Binding in a Sandwich ELISA
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plante, J.A.; Mitchell, B.M.; Plante, K.S.; Debbink, K.; Weaver, S.C.; Menachery, V.D. The variant gambit: COVID-19’s next move. Cell Host Microbe 2021, 29, 508–515. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- CDC. SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (accessed on 22 October 2021).
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Greninger, A.L. Test it earlier, result it faster, makes us stronger: How rapid viral diagnostics enable therapeutic success. Curr. Opin. Virol. 2021, 49, 111–116. [Google Scholar] [CrossRef]
- Mercer, T.R.; Salit, M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021, 22, 415–426. [Google Scholar] [CrossRef]
- Eli Lilly and Company. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab; Revised 16 September 2021. Available online: https://www.fda.gov/media/145802/download (accessed on 11 November 2021).
- Martin, D.P.; Weaver, S.; Tegally, H.; San, J.E.; Shank, S.D.; Wilkinson, E.; Lucaci, A.G.; Giandhari, J.; Naidoo, S.; Pillay, Y.; et al. The emergence and ongoing convergent evolution of the SARS-CoV-2 N501Y lineages. Cell 2021, 184, 5189–5200.e7. [Google Scholar] [CrossRef]
- Supasa, P.; Zhou, D.; Dejnirattisai, W.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Nutalai, R.; Tuekprakhon, A.; et al. Reduced Neutralization of SARS-CoV-2 B.1.1.7 Variant by Convalescent and Vaccine Sera. Cell 2021, 184, 2201–2211.e7. [Google Scholar] [CrossRef]
- Zhu, X.; Mannar, D.; Srivastava, S.S.; Berezuk, A.M.; Demers, J.P.; Saville, J.W.; Leopold, K.; Li, W.; Dimitrov, D.S.; Tuttle, K.S.; et al. Cryo-electron microscopy structures of the N501Y SARS-CoV-2 spike protein in complex with ACE2 and 2 potent neutralizing antibodies. PLoS Biol. 2021, 19, e3001237. [Google Scholar] [CrossRef] [PubMed]
- Zahradnik, J.; Marciano, S.; Shemesh, M.; Zoler, E.; Harari, D.; Chiaravalli, J.; Meyer, B.; Rudich, Y.; Li, C.; Marton, I.; et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat. Microbiol. 2021, 6, 1188–1198. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.; Hozumi, Y.; Yin, C.; Wei, G.W. Emerging vaccine-breakthrough SARS-CoV-2 variants. arXiv 2021, arXiv:2109.04509v1. [Google Scholar]
- Scheepers, C.; Everatt, J.; Amoako, D.G.; Tegally, H.; Wibmer, C.K.; Mnguni, A.; Ismail, A.; Mahlangu, B.; Lambson, B.E.; Richardson, S.I. Emergence and phenotypic characterization of C.1.2, a globally detected lineage that rapidly accumulated mutations of concern. medRxiv 2021. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Franco-Munoz, C.; Alvarez-Diaz, D.A.; Ruiz-Moreno, H.A.; Usme-Ciro, J.A.; Prada, D.A.; Reales-Gonzalez, J.; Corchuelo, S.; Herrera-Sepulveda, M.T.; Naizaque, J.; et al. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect. Genet. Evol. 2021, 95, 105038. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Vogels, C.B.F.; Yildirim, I.; Rothman, J.E.; Lu, P.; Monteiro, V.; Gelhausen, J.R.; Campbell, M.; Silva, J.; Tabachikova, A.; et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Kimura, I.; Shirakawa, K.; Takaori-Kondo, A.; Nakada, T.-A.; Kaneda, A. Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ooi, K.H.; Liu, M.M.; Tay, J.W.D.; Teo, S.Y.; Kaewsapsak, P.; Jin, S.; Lee, C.K.; Hou, J.; Maurer-Stroh, S.; Lin, W.; et al. An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing. Nat. Commun. 2021, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.; Destras, G.; Gaymard, A.; Stefic, K.; Marlet, J.; Eymieux, S.; Regue, H.; Semanas, Q.; d’Aubarede, C.; Billaud, G.; et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Eurosurveillance 2021, 26, 2100008. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Breban, M.I.; Ott, I.M.; Alpert, T.; Petrone, M.E.; Watkins, A.E.; Kalinich, C.C.; Earnest, R.; Rothman, J.E.; Goes de Jesus, J.; et al. Multiplex qPCR discriminates variants of concern to enhance global surveillance of SARS-CoV-2. PLoS Biol. 2021, 19, e3001236. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef]
- Harmon, A.; Chang, C.; Salcedo, N.; Sena, B.; Herrera, B.B.; Bosch, I.; Holberger, L.E. Validation of an at-home direct antigen rapid test for COVID-19. JAMA Netw. Open 2021, 4, e2126931. [Google Scholar] [CrossRef] [PubMed]
- Heidt, B.; Siqueira, W.F.; Eersels, K.; Dilien, H.; van Grinsven, B.; Fujiwara, R.T.; Cleij, T.J. Point of care diagnostics in resource-limited settings: A review of the present and future of PoC in its most needed environment. Biosensors 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 rapid antigen tests: Promises and challenges. Lancet Infect. Dis. 2021, 21, e290–e295. [Google Scholar] [CrossRef]
- Larremore, D.B.; Wilder, B.; Lester, E.; Shehata, S.; Burke, J.M.; Hay, J.A.; Tambe, M.; Mina, M.J.; Parker, R. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 2021, 7, eabd5393. [Google Scholar] [CrossRef] [PubMed]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef]
- Pickering, S.; Batra, R.; Merrick, B.; Snell, L.B.; Nebbia, G.; Douthwaite, S.; Reid, F.; Patel, A.; Kia Ik, M.T.; Patel, B.; et al. Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e461–e471. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, Y.; Si, Y.; Zhu, X.; Zhang, L.; Feng, H.; Tian, D.; Liao, Y.; Liu, T.; Lu, H.; et al. Rapid SARS-CoV-2 antigen detection potentiates early diagnosis of COVID-19 disease. Biosci. Trends 2021, 15, 93–99. [Google Scholar] [CrossRef]
- FDA. In Vitro Diagnostics EUAs—Antigen Diagnostic Tests for SARS-CoV-2. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2 (accessed on 22 October 2021).
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Kurup, D.; Wirblich, C.; Feldmann, H.; Marzi, A.; Schnell, M.J. Rhabdovirus-based vaccine platforms against henipaviruses. J. Virol. 2015, 89, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Puligedda, R.D.; Sharma, R.; Al-Saleem, F.H.; Kouiavskaia, D.; Velu, A.B.; Kattala, C.D.; Prendergast, G.C.; Lynch, D.R.; Chumakov, K.; Dessain, S.K. Capture and display of antibodies secreted by hybridoma cells enables fluorescent on-cell screening. MAbs 2019, 11, 546–558. [Google Scholar] [CrossRef]
- Adekar, S.P.; Jones, R.M.; Elias, M.D.; Al-Saleem, F.H.; Root, M.J.; Simpson, L.L.; Dessain, S.K. A human monoclonal antibody that binds serotype A botulinum neurotoxin. Hybridoma 2008, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tiller, T.; Meffre, E.; Yurasov, S.; Tsuiji, M.; Nussenzweig, M.C.; Wardemann, H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 2008, 329, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudicelli, V.; Brochet, X.; Lefranc, M.P. IMGT/V-QUEST: IMGT standardized analysis of the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences. Cold Spring Harb. Protoc. 2011, 2011, 695–715. [Google Scholar] [CrossRef]
- Vazquez-Lombardi, R.; Nevoltris, D.; Luthra, A.; Schofield, P.; Zimmermann, C.; Christ, D. Transient expression of human antibodies in mammalian cells. Nat. Protoc. 2018, 13, 99–117. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [Green Version]
- Tursi, S.A.; Puligedda, R.D.; Szabo, P.; Nicastro, L.K.; Miller, A.L.; Qiu, C.; Gallucci, S.; Relkin, N.R.; Buttaro, B.A.; Dessain, S.K.; et al. Salmonella Typhimurium biofilm disruption by a human antibody that binds a pan-amyloid epitope on curli. Nat. Commun. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saleem, F.H.; Sharma, R.; Puligedda, R.D.; Elias, M.; Kattala, C.D.; Simon, P.M.; Simpson, L.L.; Dessain, S.K. RBC adherence of immune complexes containing botulinum toxin improves neutralization and macrophage uptake. Toxins 2017, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Puligedda, R.D.; Kouiavskaia, D.; Al-Saleem, F.H.; Kattala, C.D.; Nabi, U.; Yaqoob, H.; Bhagavathula, V.S.; Sharma, R.; Chumakov, K.; Dessain, S.K. Characterization of human monoclonal antibodies that neutralize multiple poliovirus serotypes. Vaccine 2017, 35, 5455–5462. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Ge, J.; Ren, W.; Zhang, R.; Lan, J.; Ju, B.; Su, B.; Yu, F.; Chen, P.; et al. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity 2021, 54, 1611–1621.e1615. [Google Scholar] [CrossRef]
- Wang, P.; Casner, R.G.; Nair, M.S.; Wang, M.; Yu, J.; Cerutti, G.; Liu, L.; Kwong, P.D.; Huang, Y.; Shapiro, L.; et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe 2021, 29, 747–751.e744. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Zhou, D.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 2021, 184, 2939–2954.e2939. [Google Scholar] [CrossRef]
- Feuerstein, G.Z.; Mansfield, M.A.; Lelkes, P.I.; Alesci, S.; Marcinkiewicz, C.; Butlin, N.; Sternberg, M. The use of near-infrared light-emitting fluorescent nanodiamond particles to detect Ebola virus glycoprotein: Technology development and proof of principle. Int. J. Nanomed. 2020, 15, 7583–7599. [Google Scholar] [CrossRef] [PubMed]
- Cantera, J.L.; Cate, D.M.; Golden, A.; Peck, R.B.; Lillis, L.L.; Domingo, G.J.; Murphy, E.; Barnhart, B.C.; Anderson, C.A.; Alonzo, L.F.; et al. Screening antibodies raised against the spike glycoprotein of SARS-CoV-2 to support the development of rapid antigen assays. ACS Omega 2021, 6, 20139–20148. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, M.; Jung, Y.; Lee, S.K.; Lee, C.S.; Kim, J.; Kim, J.; Kim, N.H.; Kim, B.T.; Kim, H.G. A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2). Biosens. Bioelectron. 2021, 171, 112715. [Google Scholar] [CrossRef]
| Variant | Lineage | CDC Classification | RBD Mutation(s) |
|---|---|---|---|
| L | Wuhan-Hu-1 | Wild type | N/A |
| alpha | B.1.1.7 | VBM | N501Y |
| beta | B.1.351 | VBM | K417N, E484K, N501Y |
| gamma | P.1 | VBM | K417T, E484K, N501Y |
| delta | B.1.617.2 | VOC | L452R, T478K |
| kappa | B.1.617.1 | VBM | L452R, E484Q |
| epsilon | B.1.429 | VBM | L452R |
| Variant | Lineage | Tested | RBD Mutation(s) | 2E8 | CB6 |
|---|---|---|---|---|---|
| L | Wuhan-Hu-1 | RBD | N/A | ++ | ++ |
| alpha | B.1.1.7 | S1 | N501Y | - | ++ |
| beta | B.1.351 | S1 | K417N, E484K, N501Y | - | - |
| gamma | P.1 | S1 | K417T, E484K, N501Y | - | + |
| delta | B.1.617.2 | RBD | L452R, T478K | ++ | ++ |
| kappa | B.1.617.1 | RBD | L452R, E484Q | ++ | ++ |
| epsilon | B.1.429 | S1 | L452R | ++ | ++ |
| N/A | N/A | RBD | K417N | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puligedda, R.D.; Al-Saleem, F.H.; Wirblich, C.; Kattala, C.D.; Jović, M.; Geiszler, L.; Devabhaktuni, H.; Feuerstein, G.Z.; Schnell, M.J.; Sack, M.; et al. A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue. Diagnostics 2021, 11, 2092. https://doi.org/10.3390/diagnostics11112092
Puligedda RD, Al-Saleem FH, Wirblich C, Kattala CD, Jović M, Geiszler L, Devabhaktuni H, Feuerstein GZ, Schnell MJ, Sack M, et al. A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue. Diagnostics. 2021; 11(11):2092. https://doi.org/10.3390/diagnostics11112092
Chicago/Turabian StylePuligedda, Rama Devudu, Fetweh H. Al-Saleem, Cristoph Wirblich, Chandana Devi Kattala, Marko Jović, Laura Geiszler, Himani Devabhaktuni, Giora Z. Feuerstein, Matthias J. Schnell, Markus Sack, and et al. 2021. "A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue" Diagnostics 11, no. 11: 2092. https://doi.org/10.3390/diagnostics11112092
APA StylePuligedda, R. D., Al-Saleem, F. H., Wirblich, C., Kattala, C. D., Jović, M., Geiszler, L., Devabhaktuni, H., Feuerstein, G. Z., Schnell, M. J., Sack, M., Livornese, L. L., Jr., & Dessain, S. K. (2021). A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue. Diagnostics, 11(11), 2092. https://doi.org/10.3390/diagnostics11112092






