Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10
Abstract
1. Introduction
2. Patients and Methods
2.1. Data Collection
2.1.1. Patients
2.1.2. Background Data
2.1.3. Oral Function Measurement
2.1.4. Postoperative Oral Dysfunction Classification (MK Classification)
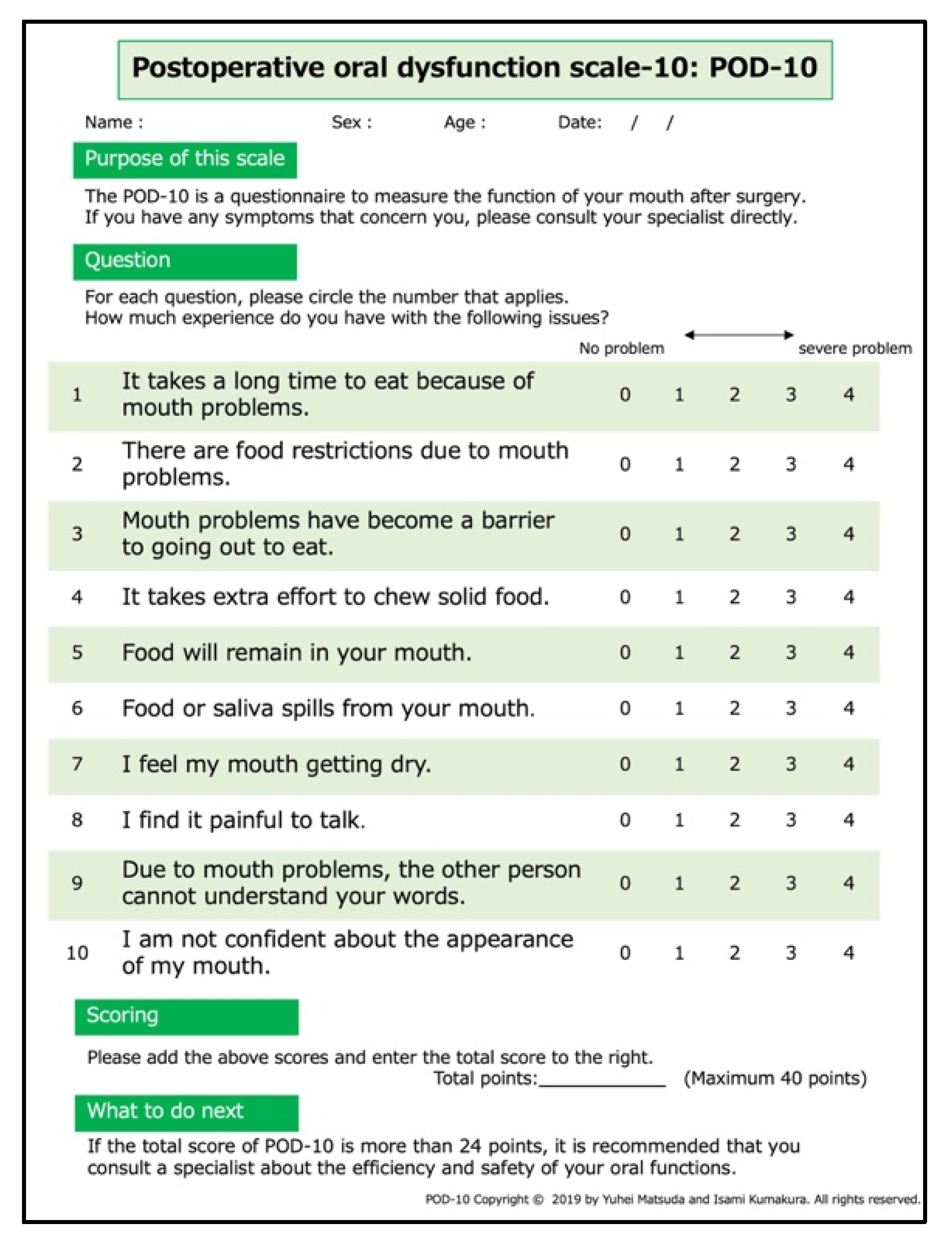
2.1.5. Postoperative Oral Dysfunction Scale-10
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Descriptive Statistics of Postoperative Oral Dysfunction Scale-10 and Ceiling and Floor Effect
3.3. Reliability
3.3.1. Internal Consistency
3.3.2. Reproducibility
3.4. Concurrent Validity
3.5. Discriminant Validity
3.6. Cut-Off Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef]
- Brennan, P.A.; Bradley, K.L.; Brands, M. Intensity-modulated radiotherapy in head and neck cancer—An update for oral and maxillofacial surgeons. Br. J. Oral Maxillofac. Surg. 2017, 55, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Noguchi, T.; Ariji, E.; Fushimi, C.; Fuwa, N.; Harada, H.; Hayashi, T.; Hayashi, R.; Honma, Y.; Miura, M.; et al. General rules for clinical and pathological studies on oral cancer (2nd edition): A synopsis. Int J. Clin. Oncol. 2021, 26, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Okui, T.; Karino, M.; Aoi, N.; Okuma, S.; Hayashida, K.; Sakamoto, T.; Kanno, T. Postoperative oral dysfunction following oral cancer resection and reconstruction: A preliminary cross-sectional study. Oral Oncol. 2021, 121, 105468. [Google Scholar] [CrossRef]
- Rogers, S.N.; Lowe, D. Health-related quality of life after oral cancer treatment: 10-year outcomes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 144–149. [Google Scholar] [CrossRef]
- Hay, J.L.; Atkinson, T.M.; Reeve, B.B.; Mitchell, S.A.; Mendoza, T.R.; Willis, G.; Minasian, L.M.; Clauser, S.B.; Denicoff, A.; O’Mara, A.; et al. Cognitive interviewing of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Qual. Life Res. 2014, 23, 257–269. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef]
- Florie, M.; Pilz, W.; Kremer, B.; Verhees, F.; Waltman, G.; Winkens, B.; Winter, N.; Baijens, L. EAT-10 Scores and Fiberoptic Endoscopic Evaluation of Swallowing in Head and Neck Cancer Patients. Laryngoscope 2021, 131, E45–E51. [Google Scholar] [CrossRef]
- Chen, S.C. Oral Dysfunction in Patients With Head and Neck Cancer: A Systematic Review. J. Nurs Res. 2019, 27, e58. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Shiga, K.; Katagiri, K.; Saito, D.; Oikawa, S.I.; Tsuchida, K.; Ikeda, A.; Miyaguchi, J.; Kusaka, T.; Yamada, H. Evaluation and comparison of oral function after resection of cancer of the upper gingiva in patients who underwent reconstruction surgery versus those treated with a prosthesis. BMC Oral Health 2021, 21, 347. [Google Scholar] [CrossRef]
- Lazarus, C.; Logemann, J.A.; Pauloski, B.R.; Rademaker, A.W.; Helenowski, I.B.; Vonesh, E.F.; Maccracken, E.; Mittal, B.B.; Vokes, E.E.; Haraf, D.J. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck 2007, 29, 632–637. [Google Scholar] [CrossRef]
- Li, W.T.; Hsieh, J.H.; Horng, S.Y.; Cheng, N.C.; Chien, H.F.; Chen, J.S.; Lai, H.S. Treatment and long-term follow-up of oral cancer postoperative sialorrhea with dermal sling operation. Ann. Plast. Surg. 2015, 74 (Suppl. S2), S113–S117. [Google Scholar] [CrossRef]
- Bachmann, A.S.; Hoche, S.; Peters, B.; Wiltfang, J.; Hertrampf, K. Effects of high-frequency speech therapy on speech-related quality of life and objective speech intelligibility of oral cancer patients. J. Craniomaxillofac. Surg. 2021, in press. [Google Scholar] [CrossRef]
- Barclay, C.W.; Foster, E.C.; Taylor, C.L. Restorative aspects of oral cancer reconstruction. Br. Dent. J. 2018, 225, 848–854. [Google Scholar] [CrossRef]
- Yamauchi, T.; Edahiro, A.; Watanabe, Y.; Murakami, M.; Satou, E.; Saito, H.; Sanjo, Y.; Sakai, K.; Takaki, S.; Kamiyama, I.; et al. Risk factors for postoperative dysphagia in oral cancer. Bull. Tokyo Dent. Coll. 2012, 53, 67–74. [Google Scholar] [CrossRef]
- Yang, C.J.; Roh, J.L.; Choi, K.H.; Kim, M.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Pretreatment Dysphagia Inventory and videofluorographic swallowing study as prognostic indicators of early survival outcomes in head and neck cancer. Cancer 2015, 121, 1588–1598. [Google Scholar] [CrossRef]
- Rylands, J.; Lowe, D.; Rogers, S.N. Outcomes by area of residence deprivation in a cohort of oral cancer patients: Survival, health-related quality of life, and place of death. Oral Oncol. 2016, 52, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Fujimoto, K.; Mochizuki, R.; Ishida, K.; Lee, B. Development and validation of the comprehensive assessment scale for chemotherapy-induced peripheral neuropathy in survivors of cancer. BMC Cancer 2019, 19, 904. [Google Scholar] [CrossRef]
- Gansterer, W.N.; Niederbrucker, G.; Strakova, H.; Schulze Grotthoff, S. Scalable and fault tolerant orthogonalization based on randomized distributed data aggregation. J. Comput. Sci. 2013, 4, 480–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Shimozuma, K.; Ohashi, Y.; Takeuchi, A.; Aranishi, T.; Morita, S.; Kuroi, K.; Ohsumi, S.; Makino, H.; Mukai, H.; Katsumata, N.; et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support. Care Cancer 2009, 17, 1483–1491. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakai, I.; Takahashi, Y.; Maeda, T.; Kunii, Y.; Kurokochi, K. Development of a new measurement scale for interprofessional collaborative competency: A pilot study in Japan. J. Interprof. Care 2014, 28, 45–51. [Google Scholar] [CrossRef]
- Bofill-Soler, N.; Guillen-Sola, A.; Marco, E.; Nieto-Cadalso, S.; Barrera, M.C.; Pera-Cegarra, O.; Membrive, I.; Duran, X.; Foro, P. Is EAT-10 Useful to Assess Swallowing during the Chemo-Radiotherapy Phase in Patients with Head and Neck Cancer? A Pilot Study. Ann. Otol. Rhinol. Laryngol. 2021, 130, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Hiiemae, K.M.; Palmer, J.B. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 1999, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Namaki, S.; Tanaka, T.; Hara, Y.; Ohki, H.; Shinohara, M.; Yonhehara, Y. Videofluorographic evaluation of dysphagia before and after modification of the flap and scar in patients with oral cancer. J. Plast. Surg. Hand Surg. 2011, 45, 136–142. [Google Scholar] [CrossRef]
- Collan, J.; Lundberg, M.; Vaalavirta, L.; Back, L.; Kajanti, M.; Makitie, A.; Tenhunen, M.; Saarilahti, K. Patterns of relapse following surgery and postoperative intensity modulated radiotherapy for oral and oropharyngeal cancer. Acta Oncol. 2011, 50, 1119–1125. [Google Scholar] [CrossRef]
- Irune, E.; Dwivedi, R.C.; Nutting, C.M.; Harrington, K.J. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat. Rev. 2014, 40, 1106–1117. [Google Scholar] [CrossRef] [PubMed]



| Type | Name | Definition | Reference Values for Diagnostic Criteria |
|---|---|---|---|
| I | Transport type | A condition in which dysfunction occurs during the oral preparatory and transit phases of swallowing due to treatment-induced damage to the tongue, palate, buccal mucosa, or oral floor. | Masticatory function (cut-off value: 83 mg/dL) EAT-10 (cut-off value: 12) Tongue pressure (cut-off value: 14 kPa) |
| II | Occlusion type | Conditions in which occlusion is impaired due to the loss of maxilla and mandibular or teeth from treatment. | Occlusal force (cut-off value: 230 N) |
| III | Oral hygiene type | Conditions in which the self-cleaning and antibacterial moisturizing functions of the oral cavity are impaired by treatment. | Number of microorganisms (cut-off value: 106.5 or more) Oral dryness (cut-off value: 27.0) Chief complaint of subjective oral health perception |
| Item | Category | n (%) or Median (IQR) |
|---|---|---|
| Age (years) | 71.0 (63.0–76.5) | |
| Sex | Male | 42 (67.7) |
| Female | 20 (32.3) | |
| BMI | 19.9 (18.1–23.4) | |
| Brinkman index | 0.0 (0.0–440.0) | |
| Alcohol consumption | None | 29 (46.8) |
| Social drinker | 5 (8.1) | |
| Regular drinker | 28 (45.2) | |
| Performance status | 0 | 46 (74.2) |
| 1 | 9 (14.5) | |
| 2 | 1 (1.6) | |
| 3 | 6 (9.7) | |
| Primary tumor site | Tongue | 25 (40.3) |
| Maxillary gingiva | 12 (19.4) | |
| Mandibular gingiva | 12 (19.4) | |
| Palate | 3 (4.8) | |
| Oral floor | 5 (8.1) | |
| Buccal mucosa | 2 (3.2) | |
| Central mandible | 2 (3.2) | |
| Lower lip | 1 (1.6) | |
| Tumor stage | I | 12 (19.4) |
| II | 8 (12.9) | |
| III | 11 (17.7) | |
| IV | 31 (50.0) | |
| Treatment | Surgery | 25 (40.3) |
| Surgery + RT | 10 (16.1) | |
| Surgery + CT | 4 (6.5) | |
| Surgery + CRT | 23 (37.1) | |
| Neck dissection (yes) | 42 (67.7) | |
| Reconstruction (yes) | 40 (64.5) | |
| Number of teeth | 17.0 (0.0–25.0) | |
| Oral function measurement | Microorganisms (Grade) | 4.0 (2.0–5.0) |
| Oral dryness | 24.6 (21.2–26.7) | |
| Occlusal force (N) | 270.4 (27.8–458.6) | |
| Tongue pressure (kPa) | 15.7 (5.3–25.0) | |
| Masticatory function (mg/dL) | 58.0 (12.0–159.0) | |
| EAT-10 | 16.5 (5.5–25.3) | |
| FOIS | 5.0 (5.0–6.0) | |
| RSST | 3.0 (3.0–4.0) | |
| MNA-SF | Normal nutritional status | 13 (21.0) |
| At the risk of malnutrition | 25 (40.3) | |
| Malnourished | 24 (38.7) | |
| Postoperative oral dysfunction | None | 17 (27.4) |
| Type I | 3 (4.8) | |
| Type II | 13 (21.0) | |
| Type III | 11 (17.7) | |
| Type I & II | 1 (1.6) | |
| Type II & III | 7 (11.3) | |
| Type I & III | 6 (9.7) | |
| Type I & II & III | 4 (6.5) |
| Question | Median (IQR) or Mean (SD) |
|---|---|
| Q1. It takes a long time to eat because of mouth problems. | 2.0 (1.0–3.0) |
| Q2. There are food restrictions due to mouth problems. | 2.5 (1.0–4.0) |
| Q3. Mouth problems have become a barrier to going out to eat. | 2.0 (1.0–3.0) |
| Q4. It takes extra effort to chew solid food. | 2.0 (1.0–3.0) |
| Q5. Food will remain in your mouth. | 2.0 (0.0–3.0) |
| Q6. Food or saliva spills from your mouth. | 1.0 (0.0–3.0) |
| Q7. I feel my mouth getting dry. | 2.0 (0.0–3.0) |
| Q8. I find it painful to talk | 2.0 (0.0–2.0) |
| Q9. Due to mouth problems, the other person cannot understand your words. | 1.0 (0.0–2.0) |
| Q10. I am not confident about the appearance of my mouth. | 1.0 (0.0–2.3) |
| Total score | 18.0 (10.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, Y.; Kumakura, I.; Okui, T.; Karino, M.; Aoi, N.; Okuma, S.; Takeda, M.; Hayashida, K.; Sakamoto, T.; Kanno, T. Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics 2021, 11, 2061. https://doi.org/10.3390/diagnostics11112061
Matsuda Y, Kumakura I, Okui T, Karino M, Aoi N, Okuma S, Takeda M, Hayashida K, Sakamoto T, Kanno T. Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics. 2021; 11(11):2061. https://doi.org/10.3390/diagnostics11112061
Chicago/Turabian StyleMatsuda, Yuhei, Isami Kumakura, Tatsuo Okui, Masaaki Karino, Noriaki Aoi, Satoe Okuma, Mayu Takeda, Kenji Hayashida, Tatsunori Sakamoto, and Takahiro Kanno. 2021. "Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10" Diagnostics 11, no. 11: 2061. https://doi.org/10.3390/diagnostics11112061
APA StyleMatsuda, Y., Kumakura, I., Okui, T., Karino, M., Aoi, N., Okuma, S., Takeda, M., Hayashida, K., Sakamoto, T., & Kanno, T. (2021). Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics, 11(11), 2061. https://doi.org/10.3390/diagnostics11112061







