Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10
Abstract
:1. Introduction
2. Patients and Methods
2.1. Data Collection
2.1.1. Patients
2.1.2. Background Data
2.1.3. Oral Function Measurement
2.1.4. Postoperative Oral Dysfunction Classification (MK Classification)
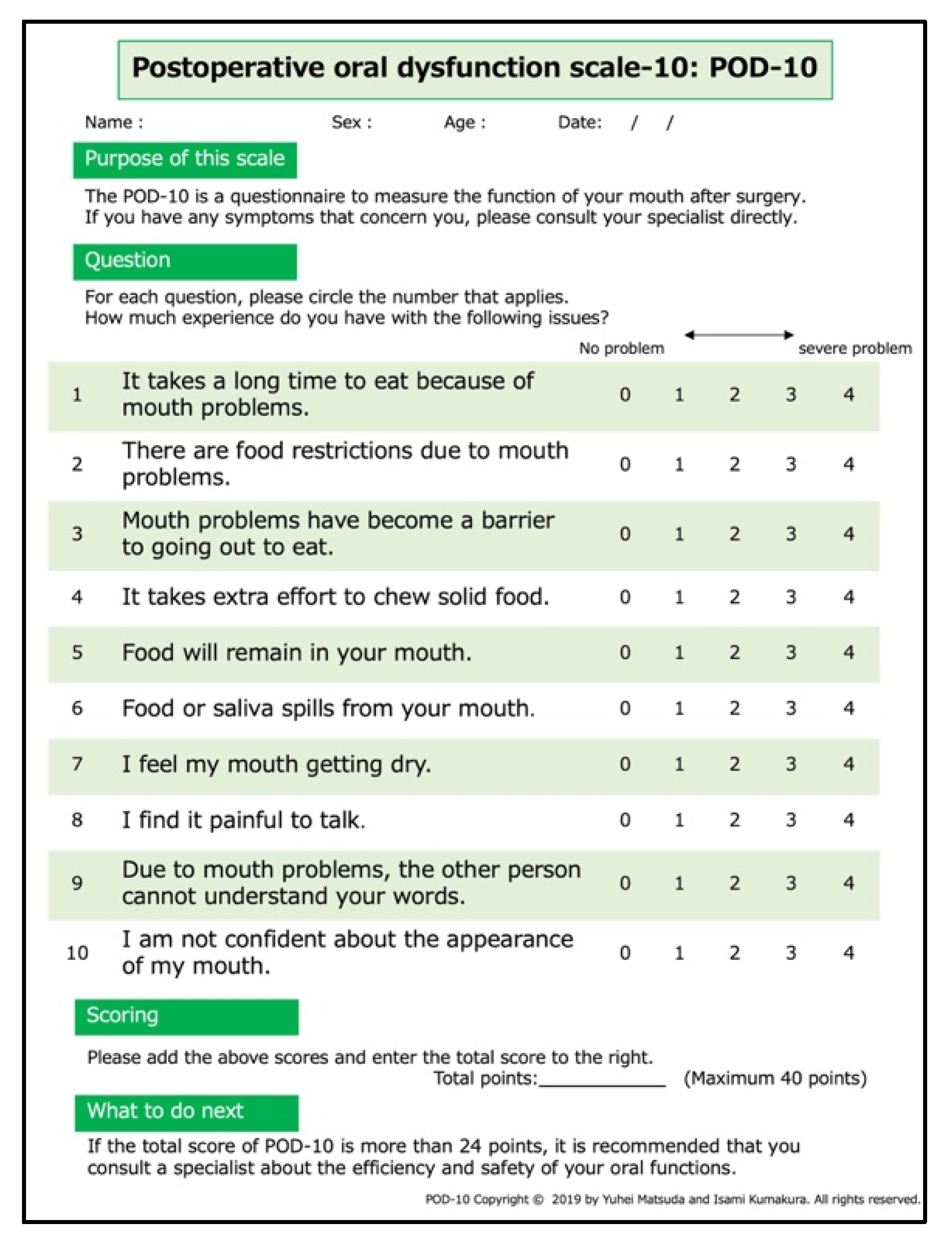
2.1.5. Postoperative Oral Dysfunction Scale-10
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Descriptive Statistics of Postoperative Oral Dysfunction Scale-10 and Ceiling and Floor Effect
3.3. Reliability
3.3.1. Internal Consistency
3.3.2. Reproducibility
3.4. Concurrent Validity
3.5. Discriminant Validity
3.6. Cut-Off Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef]
- Brennan, P.A.; Bradley, K.L.; Brands, M. Intensity-modulated radiotherapy in head and neck cancer—An update for oral and maxillofacial surgeons. Br. J. Oral Maxillofac. Surg. 2017, 55, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Noguchi, T.; Ariji, E.; Fushimi, C.; Fuwa, N.; Harada, H.; Hayashi, T.; Hayashi, R.; Honma, Y.; Miura, M.; et al. General rules for clinical and pathological studies on oral cancer (2nd edition): A synopsis. Int J. Clin. Oncol. 2021, 26, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Okui, T.; Karino, M.; Aoi, N.; Okuma, S.; Hayashida, K.; Sakamoto, T.; Kanno, T. Postoperative oral dysfunction following oral cancer resection and reconstruction: A preliminary cross-sectional study. Oral Oncol. 2021, 121, 105468. [Google Scholar] [CrossRef]
- Rogers, S.N.; Lowe, D. Health-related quality of life after oral cancer treatment: 10-year outcomes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 144–149. [Google Scholar] [CrossRef]
- Hay, J.L.; Atkinson, T.M.; Reeve, B.B.; Mitchell, S.A.; Mendoza, T.R.; Willis, G.; Minasian, L.M.; Clauser, S.B.; Denicoff, A.; O’Mara, A.; et al. Cognitive interviewing of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Qual. Life Res. 2014, 23, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef]
- Florie, M.; Pilz, W.; Kremer, B.; Verhees, F.; Waltman, G.; Winkens, B.; Winter, N.; Baijens, L. EAT-10 Scores and Fiberoptic Endoscopic Evaluation of Swallowing in Head and Neck Cancer Patients. Laryngoscope 2021, 131, E45–E51. [Google Scholar] [CrossRef]
- Chen, S.C. Oral Dysfunction in Patients With Head and Neck Cancer: A Systematic Review. J. Nurs Res. 2019, 27, e58. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Shiga, K.; Katagiri, K.; Saito, D.; Oikawa, S.I.; Tsuchida, K.; Ikeda, A.; Miyaguchi, J.; Kusaka, T.; Yamada, H. Evaluation and comparison of oral function after resection of cancer of the upper gingiva in patients who underwent reconstruction surgery versus those treated with a prosthesis. BMC Oral Health 2021, 21, 347. [Google Scholar] [CrossRef]
- Lazarus, C.; Logemann, J.A.; Pauloski, B.R.; Rademaker, A.W.; Helenowski, I.B.; Vonesh, E.F.; Maccracken, E.; Mittal, B.B.; Vokes, E.E.; Haraf, D.J. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck 2007, 29, 632–637. [Google Scholar] [CrossRef]
- Li, W.T.; Hsieh, J.H.; Horng, S.Y.; Cheng, N.C.; Chien, H.F.; Chen, J.S.; Lai, H.S. Treatment and long-term follow-up of oral cancer postoperative sialorrhea with dermal sling operation. Ann. Plast. Surg. 2015, 74 (Suppl. S2), S113–S117. [Google Scholar] [CrossRef]
- Bachmann, A.S.; Hoche, S.; Peters, B.; Wiltfang, J.; Hertrampf, K. Effects of high-frequency speech therapy on speech-related quality of life and objective speech intelligibility of oral cancer patients. J. Craniomaxillofac. Surg. 2021, in press. [Google Scholar] [CrossRef]
- Barclay, C.W.; Foster, E.C.; Taylor, C.L. Restorative aspects of oral cancer reconstruction. Br. Dent. J. 2018, 225, 848–854. [Google Scholar] [CrossRef]
- Yamauchi, T.; Edahiro, A.; Watanabe, Y.; Murakami, M.; Satou, E.; Saito, H.; Sanjo, Y.; Sakai, K.; Takaki, S.; Kamiyama, I.; et al. Risk factors for postoperative dysphagia in oral cancer. Bull. Tokyo Dent. Coll. 2012, 53, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.J.; Roh, J.L.; Choi, K.H.; Kim, M.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Pretreatment Dysphagia Inventory and videofluorographic swallowing study as prognostic indicators of early survival outcomes in head and neck cancer. Cancer 2015, 121, 1588–1598. [Google Scholar] [CrossRef]
- Rylands, J.; Lowe, D.; Rogers, S.N. Outcomes by area of residence deprivation in a cohort of oral cancer patients: Survival, health-related quality of life, and place of death. Oral Oncol. 2016, 52, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Fujimoto, K.; Mochizuki, R.; Ishida, K.; Lee, B. Development and validation of the comprehensive assessment scale for chemotherapy-induced peripheral neuropathy in survivors of cancer. BMC Cancer 2019, 19, 904. [Google Scholar] [CrossRef]
- Gansterer, W.N.; Niederbrucker, G.; Strakova, H.; Schulze Grotthoff, S. Scalable and fault tolerant orthogonalization based on randomized distributed data aggregation. J. Comput. Sci. 2013, 4, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Shimozuma, K.; Ohashi, Y.; Takeuchi, A.; Aranishi, T.; Morita, S.; Kuroi, K.; Ohsumi, S.; Makino, H.; Mukai, H.; Katsumata, N.; et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support. Care Cancer 2009, 17, 1483–1491. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakai, I.; Takahashi, Y.; Maeda, T.; Kunii, Y.; Kurokochi, K. Development of a new measurement scale for interprofessional collaborative competency: A pilot study in Japan. J. Interprof. Care 2014, 28, 45–51. [Google Scholar] [CrossRef]
- Bofill-Soler, N.; Guillen-Sola, A.; Marco, E.; Nieto-Cadalso, S.; Barrera, M.C.; Pera-Cegarra, O.; Membrive, I.; Duran, X.; Foro, P. Is EAT-10 Useful to Assess Swallowing during the Chemo-Radiotherapy Phase in Patients with Head and Neck Cancer? A Pilot Study. Ann. Otol. Rhinol. Laryngol. 2021, 130, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Hiiemae, K.M.; Palmer, J.B. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 1999, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Namaki, S.; Tanaka, T.; Hara, Y.; Ohki, H.; Shinohara, M.; Yonhehara, Y. Videofluorographic evaluation of dysphagia before and after modification of the flap and scar in patients with oral cancer. J. Plast. Surg. Hand Surg. 2011, 45, 136–142. [Google Scholar] [CrossRef]
- Collan, J.; Lundberg, M.; Vaalavirta, L.; Back, L.; Kajanti, M.; Makitie, A.; Tenhunen, M.; Saarilahti, K. Patterns of relapse following surgery and postoperative intensity modulated radiotherapy for oral and oropharyngeal cancer. Acta Oncol. 2011, 50, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Irune, E.; Dwivedi, R.C.; Nutting, C.M.; Harrington, K.J. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat. Rev. 2014, 40, 1106–1117. [Google Scholar] [CrossRef] [PubMed]



| Type | Name | Definition | Reference Values for Diagnostic Criteria |
|---|---|---|---|
| I | Transport type | A condition in which dysfunction occurs during the oral preparatory and transit phases of swallowing due to treatment-induced damage to the tongue, palate, buccal mucosa, or oral floor. | Masticatory function (cut-off value: 83 mg/dL) EAT-10 (cut-off value: 12) Tongue pressure (cut-off value: 14 kPa) |
| II | Occlusion type | Conditions in which occlusion is impaired due to the loss of maxilla and mandibular or teeth from treatment. | Occlusal force (cut-off value: 230 N) |
| III | Oral hygiene type | Conditions in which the self-cleaning and antibacterial moisturizing functions of the oral cavity are impaired by treatment. | Number of microorganisms (cut-off value: 106.5 or more) Oral dryness (cut-off value: 27.0) Chief complaint of subjective oral health perception |
| Item | Category | n (%) or Median (IQR) |
|---|---|---|
| Age (years) | 71.0 (63.0–76.5) | |
| Sex | Male | 42 (67.7) |
| Female | 20 (32.3) | |
| BMI | 19.9 (18.1–23.4) | |
| Brinkman index | 0.0 (0.0–440.0) | |
| Alcohol consumption | None | 29 (46.8) |
| Social drinker | 5 (8.1) | |
| Regular drinker | 28 (45.2) | |
| Performance status | 0 | 46 (74.2) |
| 1 | 9 (14.5) | |
| 2 | 1 (1.6) | |
| 3 | 6 (9.7) | |
| Primary tumor site | Tongue | 25 (40.3) |
| Maxillary gingiva | 12 (19.4) | |
| Mandibular gingiva | 12 (19.4) | |
| Palate | 3 (4.8) | |
| Oral floor | 5 (8.1) | |
| Buccal mucosa | 2 (3.2) | |
| Central mandible | 2 (3.2) | |
| Lower lip | 1 (1.6) | |
| Tumor stage | I | 12 (19.4) |
| II | 8 (12.9) | |
| III | 11 (17.7) | |
| IV | 31 (50.0) | |
| Treatment | Surgery | 25 (40.3) |
| Surgery + RT | 10 (16.1) | |
| Surgery + CT | 4 (6.5) | |
| Surgery + CRT | 23 (37.1) | |
| Neck dissection (yes) | 42 (67.7) | |
| Reconstruction (yes) | 40 (64.5) | |
| Number of teeth | 17.0 (0.0–25.0) | |
| Oral function measurement | Microorganisms (Grade) | 4.0 (2.0–5.0) |
| Oral dryness | 24.6 (21.2–26.7) | |
| Occlusal force (N) | 270.4 (27.8–458.6) | |
| Tongue pressure (kPa) | 15.7 (5.3–25.0) | |
| Masticatory function (mg/dL) | 58.0 (12.0–159.0) | |
| EAT-10 | 16.5 (5.5–25.3) | |
| FOIS | 5.0 (5.0–6.0) | |
| RSST | 3.0 (3.0–4.0) | |
| MNA-SF | Normal nutritional status | 13 (21.0) |
| At the risk of malnutrition | 25 (40.3) | |
| Malnourished | 24 (38.7) | |
| Postoperative oral dysfunction | None | 17 (27.4) |
| Type I | 3 (4.8) | |
| Type II | 13 (21.0) | |
| Type III | 11 (17.7) | |
| Type I & II | 1 (1.6) | |
| Type II & III | 7 (11.3) | |
| Type I & III | 6 (9.7) | |
| Type I & II & III | 4 (6.5) |
| Question | Median (IQR) or Mean (SD) |
|---|---|
| Q1. It takes a long time to eat because of mouth problems. | 2.0 (1.0–3.0) |
| Q2. There are food restrictions due to mouth problems. | 2.5 (1.0–4.0) |
| Q3. Mouth problems have become a barrier to going out to eat. | 2.0 (1.0–3.0) |
| Q4. It takes extra effort to chew solid food. | 2.0 (1.0–3.0) |
| Q5. Food will remain in your mouth. | 2.0 (0.0–3.0) |
| Q6. Food or saliva spills from your mouth. | 1.0 (0.0–3.0) |
| Q7. I feel my mouth getting dry. | 2.0 (0.0–3.0) |
| Q8. I find it painful to talk | 2.0 (0.0–2.0) |
| Q9. Due to mouth problems, the other person cannot understand your words. | 1.0 (0.0–2.0) |
| Q10. I am not confident about the appearance of my mouth. | 1.0 (0.0–2.3) |
| Total score | 18.0 (10.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, Y.; Kumakura, I.; Okui, T.; Karino, M.; Aoi, N.; Okuma, S.; Takeda, M.; Hayashida, K.; Sakamoto, T.; Kanno, T. Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics 2021, 11, 2061. https://doi.org/10.3390/diagnostics11112061
Matsuda Y, Kumakura I, Okui T, Karino M, Aoi N, Okuma S, Takeda M, Hayashida K, Sakamoto T, Kanno T. Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics. 2021; 11(11):2061. https://doi.org/10.3390/diagnostics11112061
Chicago/Turabian StyleMatsuda, Yuhei, Isami Kumakura, Tatsuo Okui, Masaaki Karino, Noriaki Aoi, Satoe Okuma, Mayu Takeda, Kenji Hayashida, Tatsunori Sakamoto, and Takahiro Kanno. 2021. "Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10" Diagnostics 11, no. 11: 2061. https://doi.org/10.3390/diagnostics11112061
APA StyleMatsuda, Y., Kumakura, I., Okui, T., Karino, M., Aoi, N., Okuma, S., Takeda, M., Hayashida, K., Sakamoto, T., & Kanno, T. (2021). Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics, 11(11), 2061. https://doi.org/10.3390/diagnostics11112061







