IsoMAG—An Automated System for the Immunomagnetic Isolation of Squamous Cell Carcinoma-Derived Circulating Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Blood Samples
2.3. Immobilization of EpCAM Antibodies on Immunomagnetic Beads
2.4. Manual Cell Isolation by Immunomagnetic Beads
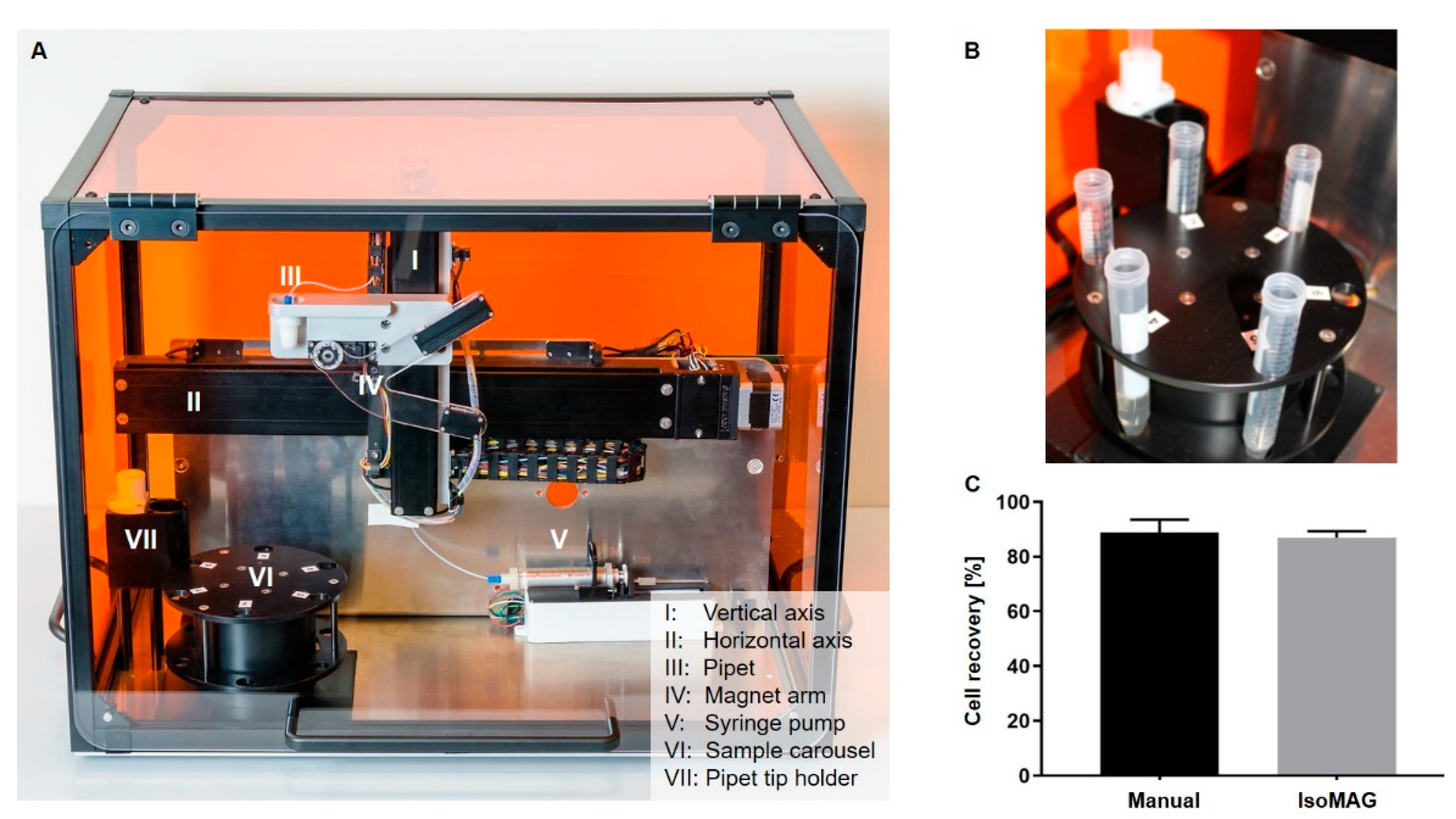
2.5. Automated CTC Enrichment Using the IsoMAG Device
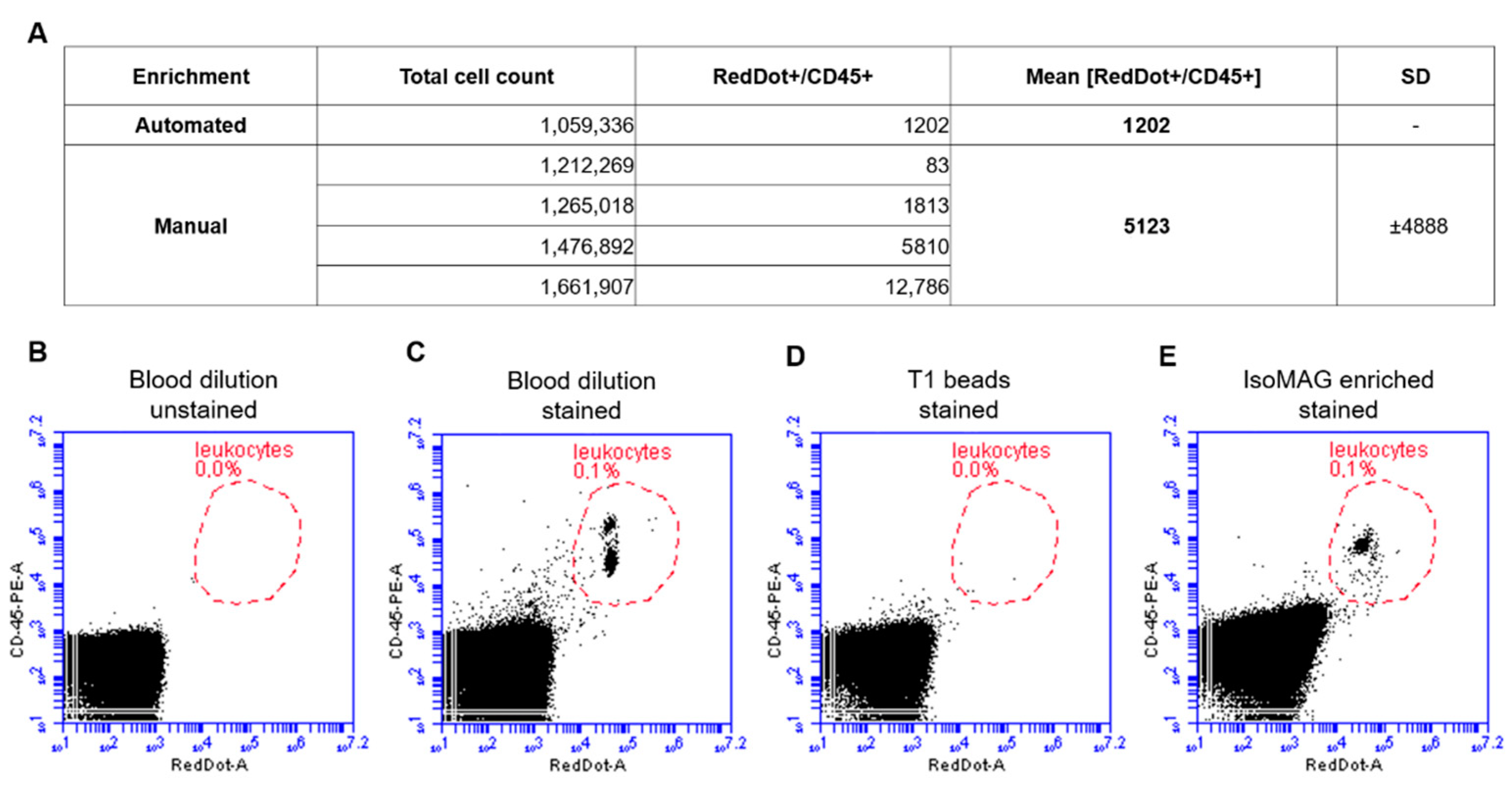
2.6. Characterization of Leukocyte Contamination in IsoMAG Isolates
2.7. Immunostaining of IsoMAG Isolated CTCs
2.8. Statistical Analysis
3. Results
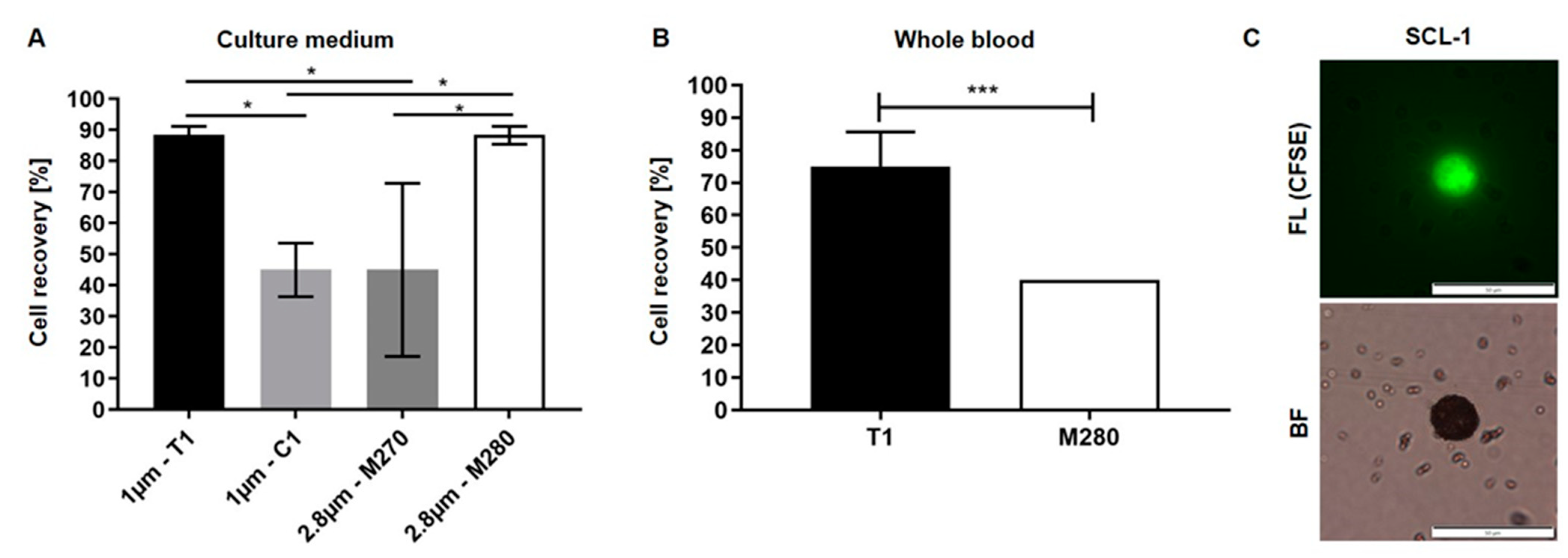
3.1. Optimization of Biofunctionalized Beads for Cell Enrichment
3.2. Reduction of Blood Cell Contamination
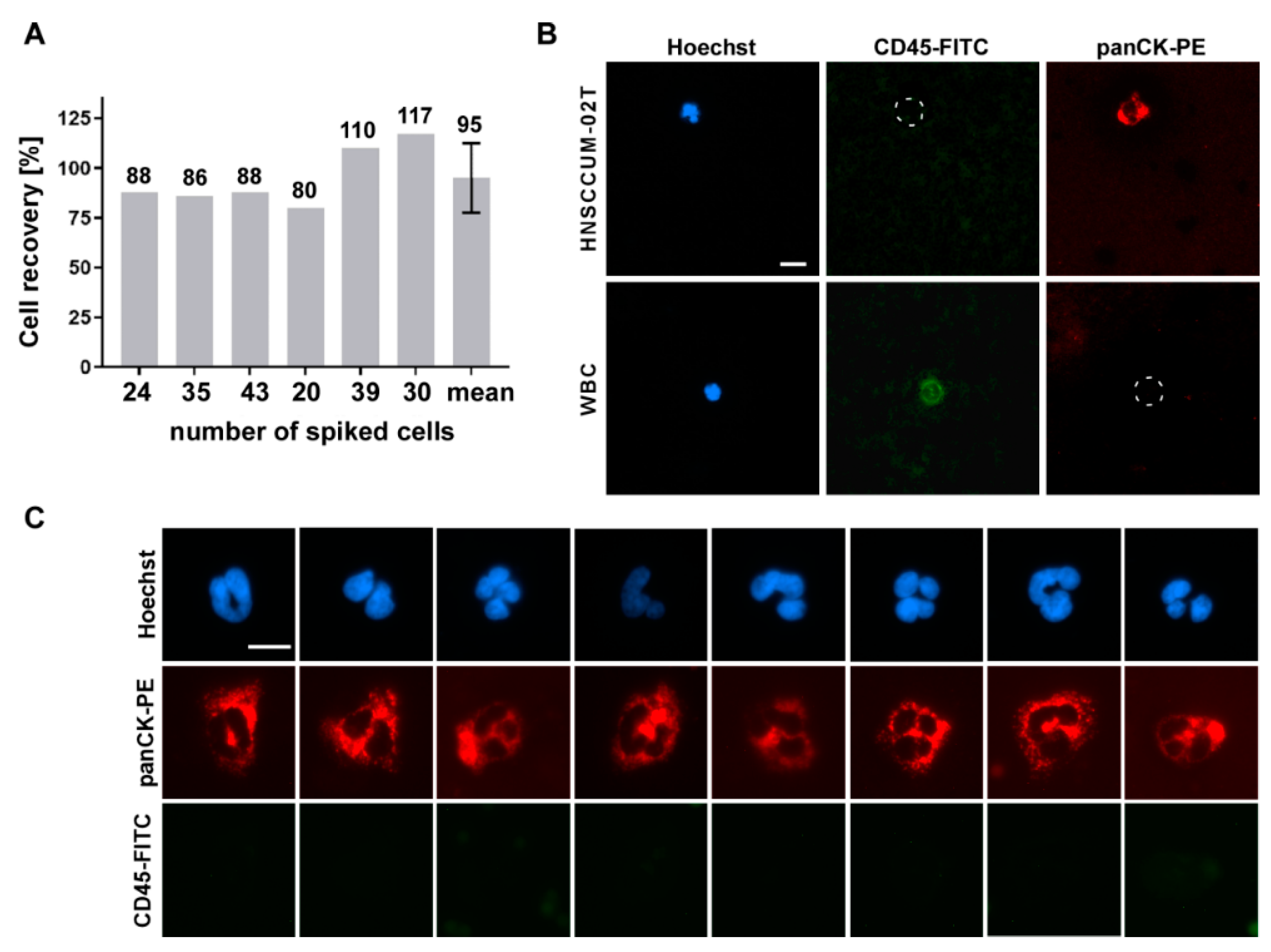
3.3. Automated Enrichment of Head and Neck Cancer Cells
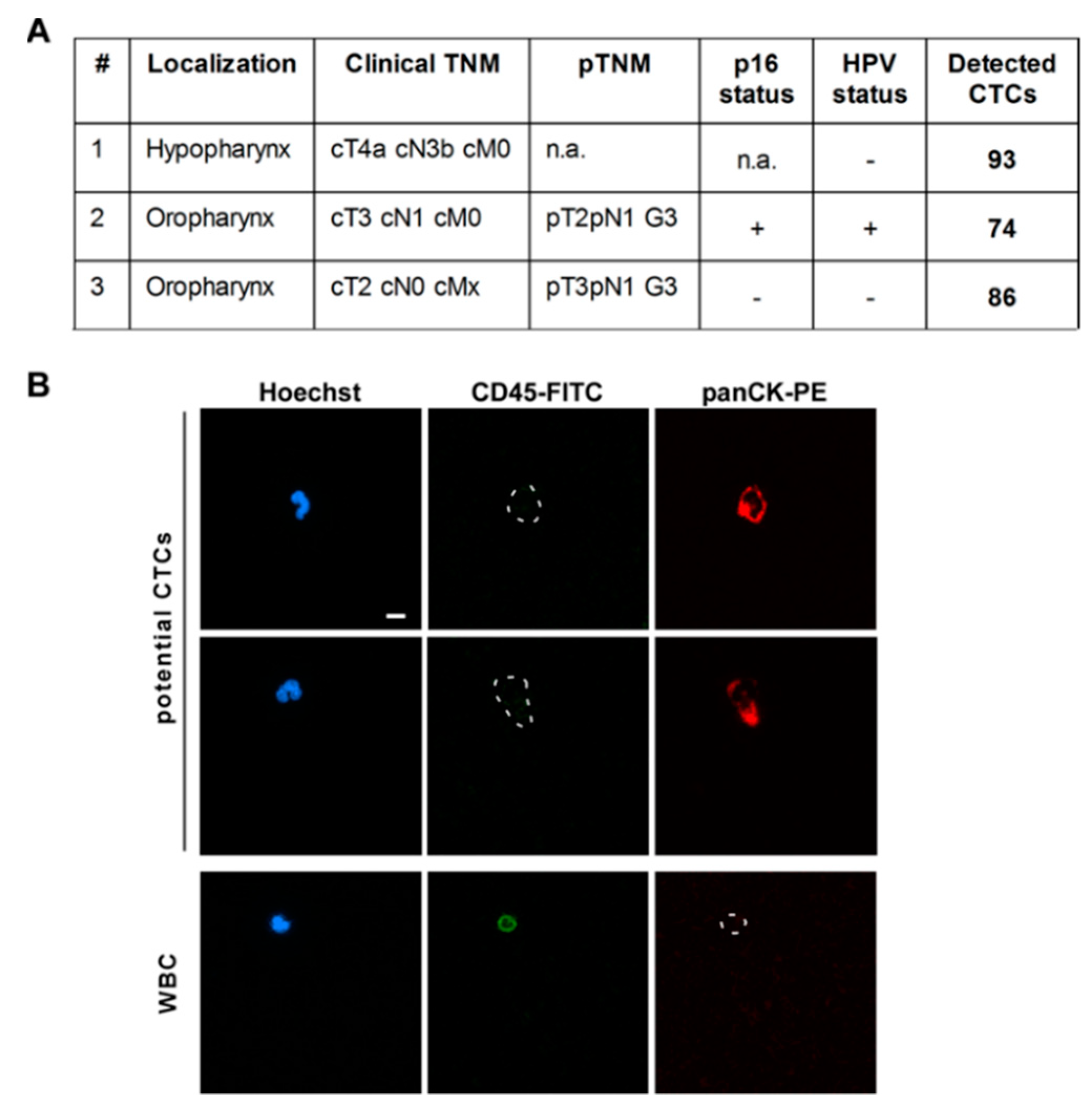
3.4. CTC Screening Using Blood of HNSCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunzel, J.; Gribko, A.; Lu, Q.; Stauber, R.H.; Wunsch, D. Nanomedical detection and downstream analysis of circulating tumor cells in head and neck patients. Biol. Chem. 2019, 400, 1465–1479. [Google Scholar] [CrossRef] [PubMed]
- Gül, D.; Habtemichael, N.; Dietrich, D.; Dietrich, J.; Gößwein, D.; Khamis, A.; Deuss, E.; Künzel, J.; Schneider, G.; Strieth, S.; et al. Identification of cytokeratin24 as a tumor suppressor for the management of head and neck cancer. Biol. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Siemer, S.; Fauth, T.; Scholz, P.; Al-Zamel, Y.; Khamis, A.; Gül, D.; Freudelsperger, L.; Wollenberg, B.; Becker, S.; Stauber, R.H.; et al. Profiling Cisplatin Resistance in Head and Neck Cancer: A Critical Role of the VRAC Ion Channel for Chemoresistance. Cancers 2021, 13, 4831. [Google Scholar] [CrossRef]
- Beltz, A.; Gosswein, D.; Zimmer, S.; Limburg, I.; Wunsch, D.; Gribko, A.; Deichelbohrer, M.; Hagemann, J.; Stauber, R.H.; Kunzel, J. Staging of oropharyngeal squamous cell carcinoma of the head and neck: Prognostic features and power of the 8th edition of the UICC staging manual. Eur. J. Surg. Oncol. 2019, 45, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Kiweler, N.; Wunsch, D.; Wirth, M.; Mahendrarajah, N.; Schneider, G.; Stauber, R.H.; Brenner, W.; Butter, F.; Kramer, O.H. Histone deacetylase inhibitors dysregulate DNA repair proteins and antagonize metastasis-associated processes. J. Cancer Res. Clin. Oncol. 2020, 146, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yu, X.; Xu, G.; Liu, S. Metastasis: An early event in cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Nam, J.-S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Australas. Med. J. 1869, 14, 146–147. [Google Scholar]
- Murray, N.P.; Albarran, V.; Perez, G.; Villalon, R.; Ruiz, A. Secondary Circulating Tumor Cells (CTCs) but not Primary CTCs are Associated with the Clinico-Pathological Parameters in Chilean Patients With Colo-Rectal Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4745–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendel, M.; Bazhenova, L.; Boshuizen, R.; Kolatkar, A.; Honnatti, M.; Cho, E.H.; Marrinucci, D.; Sandhu, A.; Perricone, A.; Thistlethwaite, P.; et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: A glimpse into lung cancer biology. Phys. Biol. 2012, 9, 016005. [Google Scholar] [CrossRef] [Green Version]
- Engel, H.; Kleespies, C.; Friedrich, J.; Breidenbach, M.; Kallenborn, A.; Schondorf, T.; Kolhagen, H.; Mallmann, P. Detection of circulating tumour cells in patients with breast or ovarian cancer by molecular cytogenetics. Br. J. Cancer 1999, 81, 1165–1173. [Google Scholar] [CrossRef]
- Jaeger, B.A.; Jueckstock, J.; Andergassen, U.; Salmen, J.; Schochter, F.; Fink, V.; Alunni-Fabbroni, M.; Rezai, M.; Beck, T.; Beckmann, M.W.; et al. Evaluation of two different analytical methods for circulating tumor cell detection in peripheral blood of patients with primary breast cancer. Biomed. Res. Int. 2014, 2014, 491459. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Hoon, D.; Ambrosi, A.; Nitti, D.; Rossi, C.R. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin. Cancer Res. 2006, 12, 4605–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wang, L.; Zhang, W.; Liu, F.; Zhang, Y.; Jiang, B.; Wang, J.; Yuan, H. Circulating Tumor Cells Correlate With Prognosis in Head and Neck Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2021, 20, 1533033821990037. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Jovanovic, L.; Nelson, C.; Punyadeera, C. Circulating tumour cells in metastatic head and neck cancers. Int. J. Cancer 2015, 136, 2515–2523. [Google Scholar] [CrossRef]
- Muller, V.; Stahmann, N.; Riethdorf, S.; Rau, T.; Zabel, T.; Goetz, A.; Janicke, F.; Pantel, K. Circulating tumor cells in breast cancer: Correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin. Cancer Res. 2005, 11, 3678–3685. [Google Scholar] [CrossRef] [Green Version]
- Weller, P.; Nel, I.; Hassenkamp, P.; Gauler, T.; Schlueter, A.; Lang, S.; Dountsop, P.; Hoffmann, A.C.; Lehnerdt, G. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC). PLoS ONE 2014, 9, e113706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantel, K.; Alix-Panabieres, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Pérez-Barrios, C.; Nieto-Alcolado, I.; Torrente, M.; Jiménez-Sánchez, C.; Calvo, V.; Gutierrez-Sanz, L.; Palka, M.; Donoso-Navarro, E.; Provencio, M.; Romero, A. Comparison of methods for circulating cell-free DNA isolation using blood from cancer patients: Impact on biomarker testing. Transl. Lung Cancer Res. 2016, 5, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofanilli, M.; Broglio, K.R.; Guarneri, V.; Jackson, S.; Fritsche, H.A.; Islam, R.; Dawood, S.; Reuben, J.M.; Kau, S.W.; Lara, J.M.; et al. Circulating tumor cells in metastatic breast cancer: Biologic staging beyond tumor burden. Clin. Breast Cancer 2007, 7, 471–479. [Google Scholar] [CrossRef]
- Tinhofer, I.; Staudte, S. Circulating tumor cells as biomarkers in head and neck cancer: Recent advances and future outlook. Expert Rev. Mol. Diagn. 2018, 18, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Mitra, A.; Brownlee, Z.; Xia, X.; Bellister, S.; Overman, M.J.; Kopetz, S.; Ellis, L.M.; Meng, Q.H.; Li, S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. 2015, 21, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisbeck, M.; Richter, L.; Helou, M.J.; Arlinghaus, S.; Anton, B.; van Dommelen, I.; Nitzsche, M.; Baßler, M.; Kappes, B.; Friedrich, O.; et al. Hybrid integration of scalable mechanical and magnetophoretic focusing for magnetic flow cytometry. Biosens. Bioelectron. 2018, 109, 98–108. [Google Scholar] [CrossRef]
- Gribko, A.; Kunzel, J.; Wunsch, D.; Lu, Q.; Nagel, S.M.; Knauer, S.K.; Stauber, R.H.; Ding, G.B. Is small smarter? Nanomaterial-based detection and elimination of circulating tumor cells: Current knowledge and perspectives. Int. J. Nanomed. 2019, 14, 4187–4209. [Google Scholar] [CrossRef] [Green Version]
- Siemer, S.; Wunsch, D.; Khamis, A.; Lu, Q.; Scherberich, A.; Filippi, M.; Krafft, M.P.; Hagemann, J.; Weiss, C.; Ding, G.B.; et al. Nano Meets Micro-Translational Nanotechnology in Medicine: Nano-Based Applications for Early Tumor Detection and Therapy. Nanomaterials 2020, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Rauscher, H.; Sokull-Kluttgen, B.; Stamm, H. The European Commission’s recommendation on the definition of nanomaterial makes an impact. Nanotoxicology 2013, 7, 1195–1197. [Google Scholar] [CrossRef]
- Shashni, B.; Ariyasu, S.; Takeda, R.; Suzuki, T.; Shiina, S.; Akimoto, K.; Maeda, T.; Aikawa, N.; Abe, R.; Osaki, T.; et al. Size-Based Differentiation of Cancer and Normal Cells by a Particle Size Analyzer Assisted by a Cell-Recognition PC Software. Biol. Pharm. Bull. 2018, 41, 487–503. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.C.; Lowes, L.E.; Szeto, C.C.; Basmaji, J.; Dhaliwal, S.; Chapeskie, C.; Todorovic, B.; Read, N.; Venkatesan, V.; Hammond, A.; et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck 2012, 34, 1440–1444. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Muller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Janicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riethdorf, S.; O’Flaherty, L.; Hille, C.; Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug Deliv. Rev. 2018, 125, 102–121. [Google Scholar] [CrossRef]
- Obayashi, K.; Akatsuka, J.; Endo, Y.; Takeda, H.; Hayashi, T.; Toyama, Y.; Suzuki, Y.; Hamasaki, T.; Kimura, G.; Ohnaga, T.; et al. Initial detection of circulating tumor cells from metastatic prostate cancer patients with a novel small device. Prostate Int. 2019, 7, 131–138. [Google Scholar] [CrossRef]
- Boukamp, P.; Tilgen, W.; Dzarlieva, R.T.; Breitkreutz, D.; Haag, D.; Riehl, R.K.; Bohnert, A.; Fusenig, N.E. Phenotypic and genotypic characteristics of a cell line from a squamous cell carcinoma of human skin. J. Natl. Cancer Inst. 1982, 68, 415–427. [Google Scholar]
- Welkoborsky, H.J.; Jacob, R.; Riazimand, S.H.; Bernauer, H.S.; Mann, W.J. Molecular biologic characteristics of seven new cell lines of squamous cell carcinomas of the head and neck and comparison to fresh tumor tissue. Oncology 2003, 65, 60–71. [Google Scholar] [CrossRef]
- Gribko, A.; Hahlbrock, A.; Strieth, S.; Becker, S.; Hagemann, J.; Deichelbohrer, M.; Hildebrandt, A.; Habtemichael, N.; Wunsch, D. Disease-relevant signalling-pathways in head and neck cancer: Taspase1’s proteolytic activity fine-tunes TFIIA function. Sci. Rep. 2017, 7, 14937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goesswein, D.; Habtemichael, N.; Gerhold-Ay, A.; Mazur, J.; Wunsch, D.; Knauer, S.K.; Kunzel, J.; Matthias, C.; Strieth, S.; Stauber, R.H. Expressional analysis of disease-relevant signalling-pathways in primary tumours and metastasis of head and neck cancers. Sci. Rep. 2018, 8, 7326. [Google Scholar] [CrossRef]
- Wünsch, D.; Hahlbrock, A.; Heiselmayer, C.; Backer, S.; Heun, P.; Goesswein, D.; Stocker, W.; Schirmeister, T.; Schneider, G.; Kramer, O.H.; et al. Fly versus man: Evolutionary impairment of nucleolar targeting affects the degradome of Drosophila’s Taspase1. FASEB J. 2015, 29, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Deuss, E.; Gosswein, D.; Gul, D.; Zimmer, S.; Foersch, S.; Eger, C.S.; Limburg, I.; Stauber, R.H.; Kunzel, J. Growth Factor Receptor Expression in Oropharyngeal Squamous Cell Cancer: Her1-4 and c-Met in Conjunction with the Clinical Features and Human Papillomavirus (p16) Status. Cancers 2020, 12, 3358. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Goike, H.; Panaretakis, K.W.; Einarsson, R. Clinical utility of cytokeratins as tumor markers. Clin. Biochem. 2004, 37, 529–540. [Google Scholar] [CrossRef]
- Foddai, A.; Elliott, C.T.; Grant, I.R. Maximizing capture efficiency and specificity of magnetic separation for Mycobacterium avium subsp. paratuberculosis cells. Appl. Environ. Microbiol. 2010, 76, 7550–7558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foddai, A.C.G.; Grant, I.R. A novel one-day phage-based test for rapid detection and enumeration of viable Mycobacterium avium subsp. paratuberculosis in cows’ milk. Appl. Microbiol. Biotechnol. 2020, 104, 9399–9412. [Google Scholar] [CrossRef] [PubMed]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wunsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, C.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2018, 192, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Gellrich, D.; Siemer, S.; Reichel, C.A.; Eckrich, J.; Dietrich, D.; Knauer, S.K.; Stauber, R.H.; Strieth, S. TNF-alpha-Inhibition Improves the Biocompatibility of Porous Polyethylene Implants In Vivo. Tissue Eng. Regen. Med. 2021, 18, 297–303. [Google Scholar] [CrossRef]
- Westmeier, D.; Siemer, S.; Vallet, C.; Steinmann, J.; Docter, D.; Buer, J.; Knauer, S.K.; Stauber, R.H. Boosting nanotoxicity to combat multidrug-resistant bacteria in pathophysiological environments. Nanoscale Adv. 2020, 2, 5428–5440. [Google Scholar] [CrossRef]
- Stauber, R.H.; Siemer, S.; Becker, S.; Ding, G.B.; Strieth, S.; Knauer, S.K. Small Meets Smaller: Effects of Nanomaterials on Microbial Biology, Pathology, and Ecology. ACS Nano 2018, 12, 6351–6359. [Google Scholar] [CrossRef]
- Stauber, R.H.; Westmeier, D.; Wandrey, M.; Becker, S.; Docter, D.; Ding, G.B.; Thines, E.; Knauer, S.K.; Siemer, S. Mechanisms of nanotoxicity—Biomolecule coronas protect pathological fungi against nanoparticle-based eradication. Nanotoxicology 2020, 14, 1157–1174. [Google Scholar] [CrossRef]
- Sieuwerts, A.M.; Kraan, J.; Bolt-de Vries, J.; van der Spoel, P.; Mostert, B.; Martens, J.W.; Gratama, J.W.; Sleijfer, S.; Foekens, J.A. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res. Treat. 2009, 118, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Chudziak, J.; Burt, D.J.; Mohan, S.; Rothwell, D.G.; Mesquita, B.; Antonello, J.; Dalby, S.; Ayub, M.; Priest, L.; Carter, L.; et al. Clinical evaluation of a novel microfluidic device for epitope-independent enrichment of circulating tumour cells in patients with small cell lung cancer. Analyst 2016, 141, 669–678. [Google Scholar] [CrossRef]
- Ciccioli, M.; Bravo-Santano, N.; Davis, A.; Lewis, J.; Malcolm, R.; Pailhes-Jimenez, A.S. Mesenchymal markers: The new avenue for circulating tumor cells detection. In Proceedings of the ANGLE plc AACR 2021, Virtual Meeting, 9–14 April 2021. [Google Scholar]
- Grover, P.K.; Cummins, A.G.; Price, T.J.; Roberts-Thomson, I.C.; Hardingham, J.E. Circulating tumour cells: The evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann. Oncol. 2014, 25, 1506–1516. [Google Scholar] [CrossRef]
- Grisanti, S.; Almici, C.; Consoli, F.; Buglione, M.; Verardi, R.; Bolzoni-Villaret, A.; Bianchetti, A.; Ciccarese, C.; Mangoni, M.; Ferrari, L.; et al. Circulating tumor cells in patients with recurrent or metastatic head and neck carcinoma: Prognostic and predictive significance. PLoS ONE 2014, 9, e103918. [Google Scholar] [CrossRef]
- Menarini Silicon Biosystems Inc. Available online: https://documents.cellsearchctc.com/pdf/e631600006/e631600006_EN.pdf (accessed on 10 October 2021).
| Beads | Size | Surface Functionalization |
|---|---|---|
| Dynabeads MyOne Streptavidin T1 | 1 µm | Tosyl-activated, hydrophobic |
| Dynabeads MyOne Streptavidin C1 | 1 µm | Carboxylic acid, hydrophilic |
| Dynabeads M-270 Streptavidin | 2.8 µm | Carboxylic acid, hydrophilic |
| Dynabeads M-280 Streptavidin | 2.8 µm | Tosyl-activated, hydrophobic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribko, A.; Stiefel, J.; Liebetanz, L.; Nagel, S.M.; Künzel, J.; Wandrey, M.; Hagemann, J.; Stauber, R.H.; Freese, C.; Gül, D. IsoMAG—An Automated System for the Immunomagnetic Isolation of Squamous Cell Carcinoma-Derived Circulating Tumor Cells. Diagnostics 2021, 11, 2040. https://doi.org/10.3390/diagnostics11112040
Gribko A, Stiefel J, Liebetanz L, Nagel SM, Künzel J, Wandrey M, Hagemann J, Stauber RH, Freese C, Gül D. IsoMAG—An Automated System for the Immunomagnetic Isolation of Squamous Cell Carcinoma-Derived Circulating Tumor Cells. Diagnostics. 2021; 11(11):2040. https://doi.org/10.3390/diagnostics11112040
Chicago/Turabian StyleGribko, Alena, Janis Stiefel, Lana Liebetanz, Sophie Madeleine Nagel, Julian Künzel, Madita Wandrey, Jan Hagemann, Roland H. Stauber, Christian Freese, and Désirée Gül. 2021. "IsoMAG—An Automated System for the Immunomagnetic Isolation of Squamous Cell Carcinoma-Derived Circulating Tumor Cells" Diagnostics 11, no. 11: 2040. https://doi.org/10.3390/diagnostics11112040
APA StyleGribko, A., Stiefel, J., Liebetanz, L., Nagel, S. M., Künzel, J., Wandrey, M., Hagemann, J., Stauber, R. H., Freese, C., & Gül, D. (2021). IsoMAG—An Automated System for the Immunomagnetic Isolation of Squamous Cell Carcinoma-Derived Circulating Tumor Cells. Diagnostics, 11(11), 2040. https://doi.org/10.3390/diagnostics11112040







