Analyzing the Estrogen Receptor Status of Liver Metastases with [18F]-FES-PET in Patients with Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. [18F]-FES-PET Imaging
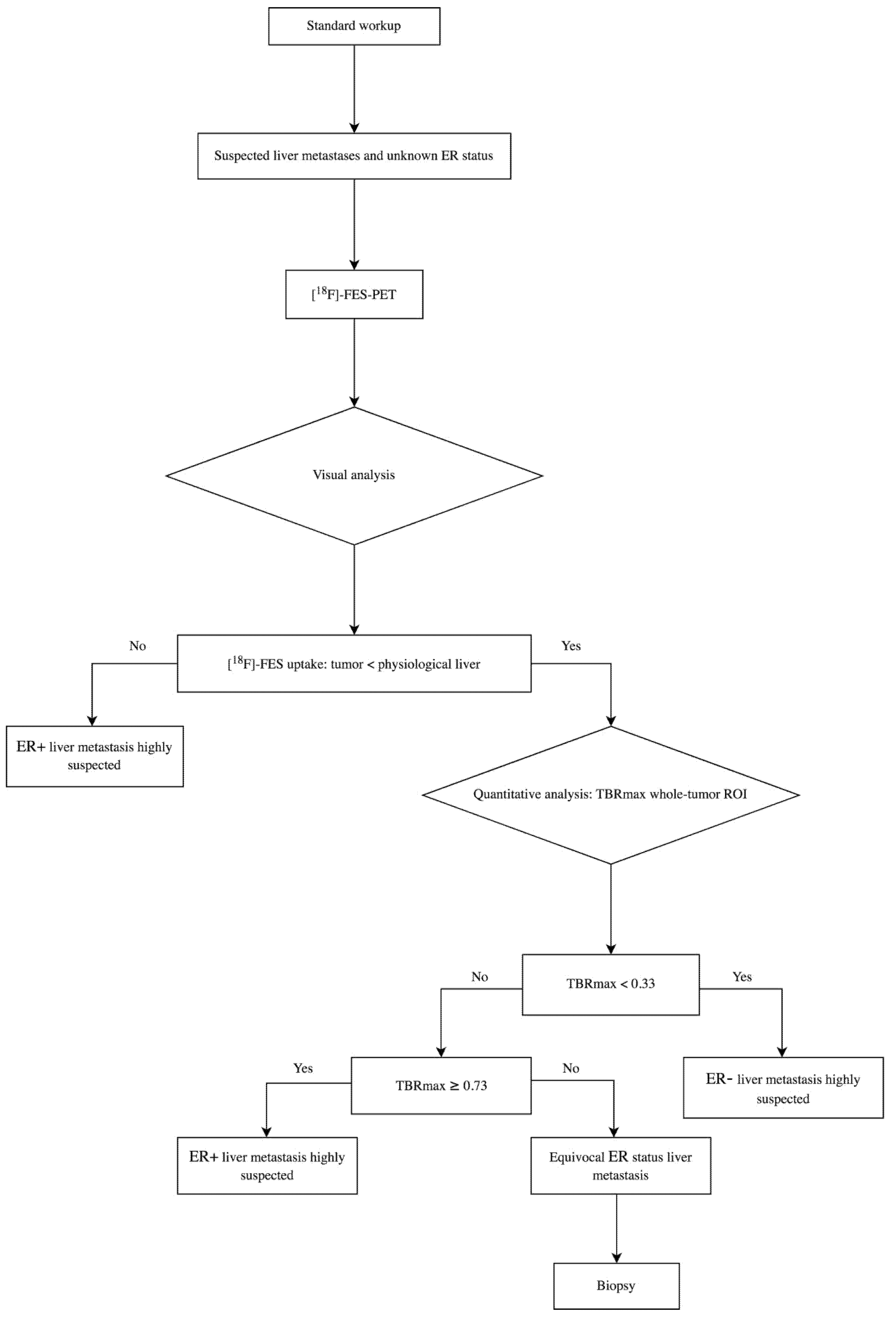
2.3. Image Analysis
2.4. Ultrasound-Guided Biopsy and Histopathological Examination
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Visual Assessment of [18F]-FES Uptake
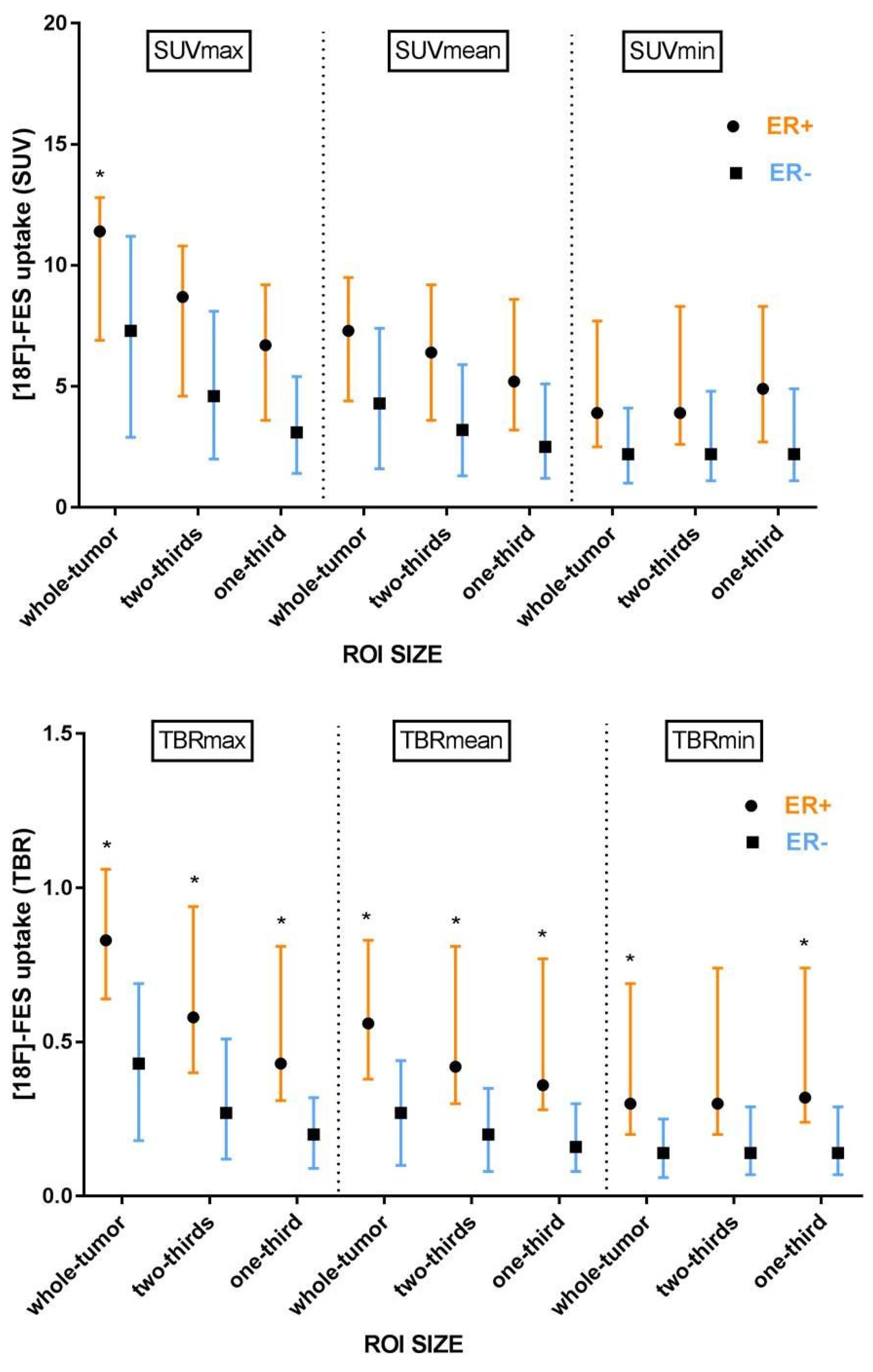
3.3. Quantitative Evaluation of [18F]-FES Uptake
3.4. The Effect of ROI Size
3.5. Background Correction
3.6. Lower and Upper Cut-Off Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | ROI Size | ER+ (n = 17) (Median [IQR]) | ER− (n = 6) (Median [IQR]) | p-Value |
|---|---|---|---|---|
| SUVmax | whole-tumor | 11.4 [6.9–12.8] | 7.3 [2.9–11.2] | 0.050 |
| SUVmean | whole-tumor | 7.3 [4.4–9.5] | 4.3 [1.6–7.4] | 0.093 |
| SUVmin | whole-tumor | 3.9 [2.5–7.7] | 2.2 [1.0–4.1] | 0.141 |
| SUVmax | two-thirds | 8.7 [4.6–10.8] | 4.6 [2.0–8.1] | 0.069 |
| SUVmean | two-thirds | 6.4 [3.6–9.2] | 3.2 [1.3–5.9] | 0.086 |
| SUVmin | two-thirds | 3.9 [2.6–8.3] | 2.2 [1.1–4.8] | 0.123 |
| SUVmax | one-third | 6.7 [3.6–9.2] | 3.1 [1.4–5.4] | 0.069 |
| SUVmean | one-third | 5.2 [3.2–8.6] | 2.5 [1.2–5.1] | 0.059 |
| SUVmin | one-third | 4.9 [2.7–8.3] | 2.2 [1.1–4.9] | 0.080 |
| TBRmax | whole-tumor | 0.83 [0.64–1.06] | 0.43 [0.18–0.69] | 0.008 |
| TBRmean | whole-tumor | 0.56 [0.38–0.83] | 0.27 [0.10–0.44] | 0.030 |
| TBRmin | whole-tumor | 0.30 [0.20–0.69] | 0.14 [0.06–0.25] | 0.050 |
| TBRmax | two-thirds | 0.58 [0.40–0.94] | 0.27 [0.12–0.51] | 0.036 |
| TBRmean | two-thirds | 0.42 [0.30–0.81] | 0.20 [0.08–0.35] | 0.021 |
| TBRmin | two-thirds | 0.30 [0.20–0.74] | 0.14 [0.07–0.29] | 0.069 |
| TBRmax | one-third | 0.43 [0.31–0.81] | 0.20 [0.09–0.32] | 0.017 |
| TBRmean | one-third | 0.36 [0.28–0.77] | 0.16 [0.08–0.30] | 0.014 |
| TBRmin | one-third | 0.32 [0.24–0.74] | 0.14 [0.07–0.29] | 0.030 |
| Parameter | ROI Size | AUC (95% CI) | Optimal Cut-Off ER+ | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| SUVmax | whole-tumor | 0.83 (0.63–1.00) | ≥4.0 | 100 | 60 |
| SUVmean | whole-tumor | 0.76 (0.50–1.00) | ≥2.2 | 94 | 60 |
| SUVmin | whole-tumor | 0.73 (0.50–0.97) | ≥1.9 | 83 | 60 |
| SUVmax | two-thirds | 0.78 (0.55–1.00) | ≥7.3 | 67 | 80 |
| SUVmean | two-thirds | 0.76 (0.51–1.00) | ≥1.7 | 94 | 60 |
| SUVmin | two-thirds | 0.73 (0.47–0.99) | ≥1.9 | 89 | 60 |
| SUVmax | one-third | 0.78 (0.56–1.00) | ≥2.4 | 89 | 60 |
| SUVmean | one-third | 0.78 (0.54–1.00) | ≥1.5 | 94 | 60 |
| SUVmin | one-third | 0.75 (0.53–0.96) | ≥3.3 | 65 | 67 |
| TBRmax | whole-tumor | 0.91 (0.78–1.00) | ≥0.69 | 78 | 100 |
| TBRmean | whole-tumor | 0.82 (0.63–1.00) | ≥0.45 | 61 | 100 |
| TBRmin | whole-tumor | 0.77 (0.55–0.99) | ≥0.13 | 83 | 60 |
| TBRmax | two-thirds | 0.82 (0.61–1.00) | ≥0.41 | 78 | 80 |
| TBRmean | two-thirds | 0.82 (0.62–1.00) | ≥0.33 | 72 | 80 |
| TBRmin | two-thirds | 0.74 (0.49–1.00) | ≥0.12 | 89 | 60 |
| TBRmax | one-third | 0.83 (0.66–1.00) | ≥0.32 | 72 | 80 |
| TBRmean | one-third | 0.83 (0.65–1.00) | ≥0.30 | 72 | 80 |
| TBRmin | one-third | 0.79 (0.58–1.00) | ≥0.13 | 89 | 60 |
| Parameter | ROI Size | Cut-Off ER− Sensitivity > 90% | Cut-Off ER+ Specificity > 90% | NPV * | PPV ‖ | Equivocal Metastases (%) ‡ |
|---|---|---|---|---|---|---|
| SUVmax | whole-tumor | <4.9 | ≥11.3 | 75 | 100 | 39 |
| SUVmean | whole-tumor | <2.2 | ≥7.7 | 75 | 100 | 48 |
| SUVmin | whole-tumor | <1.2 | ≥4.8 | 50 | 100 | 57 |
| SUVmax | two-thirds | <2.5 | ≥9.5 | 75 | 100 | 52 |
| SUVmean | two-thirds | <1.7 | ≥6.9 | 75 | 100 | 57 |
| SUVmin | two-thirds | <1.3 | ≥5.2 | 67 | 100 | 57 |
| SUVmax | one-third | <1.6 | ≥6.5 | 67 | 100 | 48 |
| SUVmean | one-third | <1.5 | ≥5.8 | 67 | 100 | 61 |
| SUVmin | one-third | <1.3 | ≥5.3 | 67 | 100 | 52 |
| TBRmax | whole-tumor | <0.33 | ≥0.69 | 75 | 100 | 22 |
| TBRmean | whole-tumor | <0.11 | ≥0.45 | 50 | 92 | 39 |
| TBRmin | whole-tumor | <0.07 | ≥0.29 | 50 | 100 | 52 |
| TBRmax | two-thirds | <0.15 | ≥0.65 | 75 | 100 | 48 |
| TBRmean | two-thirds | <0.10 | ≥0.42 | 75 | 100 | 44 |
| TBRmin | two-thirds | <0.08 | ≥0.37 | 67 | 100 | 57 |
| TBRmax | one-third | <0.10 | ≥0.39 | 50 | 100 | 35 |
| TBRmean | one-third | <0.09 | ≥0.34 | 60 | 91 | 30 |
| TBRmin | one-third | <0.08 | ≥0.32 | 67 | 100 | 35 |
| Parameter | ROI Size | AUC (95% CI) | Optimal Cut-Off ER+ | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| SUVmax | whole-tumor | 0.74 (0.49–0.99) | ≥4.0 | 100 | 50 |
| SUVmean | whole-tumor | 0.68 (0.41–0.95) | ≥2.2 | 93 | 50 |
| SUVmin | whole-tumor | 0.63 (0.36–0.90) | ≥1.9 | 79 | 50 |
| SUVmax | two-thirds | 0.71 (0.47–0.96) | ≥7.8 | 57 | 83 |
| SUVmean | two-thirds | 0.69 (0.42–0.96) | ≥1.7 | 93 | 50 |
| SUVmin | two-thirds | 0.66 (0.38– 0.92) | ≥1.9 | 86 | 50 |
| SUVmax | one-third | 0.70 (0.46–0.95) | ≥2.4 | 86 | 50 |
| SUVmean | one-third | 0.71 (0.47–0.96) | ≥1.5 | 93 | 50 |
| SUVmin | one-third | 0.69 (0.44–0.94) | ≥1.9 | 86 | 50 |
| TBRmax | whole-tumor | 0.85 (0.67–1.00) | ≥0.69 | 71 | 83 |
| TBRmean | whole-tumor | 0.76 (0.54–0.98) | ≥0.45 | 57 | 100 |
| TBRmin | whole-tumor | 0.73 (0.50–0.96) | ≥0.21 | 71 | 67 |
| TBRmax | two-thirds | 0.75 (0.52–0.98) | ≥0.41 | 71 | 67 |
| TBRmean | two-thirds | 0.79 (0.57–1.00) | ≥0.33 | 71 | 83 |
| TBRmin | two-thirds | 0.70 (0.45–0.96) | ≥0.21 | 71 | 67 |
| TBRmax | one-third | 0.80 (0.60– 0.99) | ≥0.32 | 71 | 83 |
| TBRmean | one-third | 0.81 (0.61–1.00) | ≥0.30 | 71 | 83 |
| TBRmin | one-third | 0.76 (0.54–0.98) | ≥0.21 | 79 | 67 |
References
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J. Natl Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Lopez, B.A.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, G.J.; Clark, A.S.; Schubert, E.K.; Mankoff, D.A. 18F-fluoroestradiol PET: Current status and potential future clinical applications. J. Nucl. Med. 2016, 57, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venema, C.M.; Apollonio, G.; Hospers, G.A.P.; Schröder, C.P.; Dierckx, R.A.J.O.; de Vries, E.F.J.; Glaudemans, A.W.J.M. Recommendations and technical aspects of 16α-[18F]Fluoro-17β-Estradiol PET to image the estrogen receptor in vivo. Clin. Nucl. Med. 2016, 41, 844–851. [Google Scholar] [CrossRef]
- van Kruchten, M.; Glaudemans, A.W.J.M.; de Vries, E.F.J.; Beets-Tan, R.G.H.; Schroder, C.P.; Dierckx, R.A.; de Vries, E.G.E.; Hospers, G.A.P. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J. Nucl. Med. 2012, 53, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.Y.; Ahn, S.H.; Kim, S.-B.; Han, S.; Lee, S.H.; Oh, S.J.; Lee, S.J.; Kim, H.J.; Ko, B.S.; Lee, J.W.; et al. Diagnostic accuracy and safety of 16alpha-[18F]fluoro-17beta-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: A prospective cohort study. Lancet Oncol. 2019, 20, 546–555. [Google Scholar] [CrossRef]
- Eisses, B.; Angus, L.; van der Vegt, B.; Sieuwerts, A.M.; Kraan, J.; Martens, J.W.M.; Glaudemans, A.W.J.M.; Brouwers, A.H.; Emmering, J.; Oyen, W.; et al. Non-invasive estrogen receptor assessment by [18F]-fluorestradiol (FES)-PET or circulating tumor cells predicts receptor status in patients with metastatic breast cancer. Cancer Res. 2018, 79, 4. [Google Scholar]
- Boers, J.; Venema, C.M.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Kwee, T.C.; Schuuring, E.; Martens, J.W.M.; Elias, S.G.; Hospers, G.A.P.; Schröder, G.P.; et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur. J. Cancer 2020, 126, 11–20. [Google Scholar] [CrossRef] [PubMed]
- van Es, S.C.; van der Vegt, B.; Bensch, F.; Gerritse, S.; van Helden, E.J.; Boon, E.; Angus, L.; Overbosch, J.; Menke-van der Houven van Oordt, C.W.; Verheul, H.M.; et al. Decalcification of breast cancer bone metastases with EDTA does not affect ER, PR, and HER2 results. Am. J. Surg Pathol. 2019, 43, 1355–1360. [Google Scholar] [CrossRef]
- van Es, S.C.; Velleman, T.; Elias, S.G.; Bensch, F.; Brouwers, A.H.; Glaudemans, A.W.J.M.; Kwee, T.C.; Marleen Woltman-van Iersel, M.; Maduro, J.H.; Oosting, S.F.; et al. Assessment of bone lesions with 18F-FDG-PET compared to technetium bone scintigraphy leads to clinically relevant differences in metastatic breast cancer management. J. Nucl. Med. 2021, 62, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Brouwers, A.; Glaudemans, A.; de Jong, J.; de Vries, E.; van der Graaf, W.; Boon, E.; Oyen, W.; de Geus-Oei, L.-F.; Visser, E.; et al. IMPACT: Imaging Patients for Cancer Drug Selection-Metastatic Breast Cancer (MBC); OT3-2-01; Annual CTRC-AACR San Antonio Breast Cancer Symposium: San Antonio, TX, USA, 2014. [Google Scholar]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Vetter, T.R.; Schober, P.; Mascha, E.J. Diagnostic testing and decision-making: Beauty is not just in the eye of the beholder. Anesth Analg. 2018, 127, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Datta, A.; Choudhury, P.; Dsouza, M.; Batra, U.; Mishra, A. Can 18F-fluoroestradiol positron emission tomography become a new imaging standard in the estrogen receptor-positive breast cancer patient: A prospective comparative study with 18F-fluorodeoxyglucose positron emission tomography? World J. Nucl. Med. 2017, 16, 133–139. [Google Scholar] [CrossRef]
- Moek, K.L.; Waaijer, S.J.H.; Kok, I.C.; Suurs, F.V.; Brouwers, A.H.; Menke-van der Houven van Oordt, C.W.; van Oordt, H.; Wind’, T.T.; Gietema, J.A.; Schröder, G.P.; et al. 89Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor uptake. Clin. Cancer Res. 2019, 25, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18F-fluoro-17β-estradiol positron emission tomography: Meta-analysis and recommendations for integration into clinical applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef]
- Boellaard, R.; Krak, N.C.; Hoekstra, O.S.; Lammertsma, A.A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: A simulation study. J. Nucl. Med. 2004, 45, 1519–1527. [Google Scholar]
- Nienhuis, H.H.; van Kruchten, M.; Elias, S.G.; Glaudemans, A.W.J.M.; de Vries, E.F.J.; Bongaerts, A.H.H.; Schröder, C.P.; de Vries, E.G.E.; Hospers, G.A.P. 18F-fluoroestradiol tumor uptake is heterogeneous and influenced by site of metastasis in breast cancer patients. J. Nucl. Med. 2018, 59, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Boers, J.; Giatagana, K.; Schröder, C.P.; Hospers, G.A.P.; de Vries, E.F.J.; Glaudemans, A.W.J.M. Image quality and interpretation of [18F]-FES-PET: Is there any effect of food intake? Diagnostics 2020, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.M.; Kurland, B.F.; Link, J.M.; Schubert, E.K.; Stekhova, S.; Linden, H.M.; Mankoff, D.A. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl. Med. Biol. 2011, 38, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kruchten, M.; de Vries, E.G.E.; Brown, M.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Schröder, C.P.; Hospers, G.A.P. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013, 14, e465–e475. [Google Scholar] [CrossRef]
- Paquette, M.; Lavallee, E.; Phoenix, S.; Ouellet, R.; Senta, H.; van Lier, J.E.; Guérin, B. Lecomte, R.; Turcotte, E. Improved estrogen receptor assessment by PET using the novel radiotracer 18F-4FMFES in estrogen receptor-positive breast cancer patients: An ongoing phase II clinical trial. J. Nucl. Med. 2018, 59, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonson, S.D.; Bonasera, T.A.; Dehdashti, F.; Cristel, M.E.; Katzenellenbogen, J.A.; Welch, M.J. Comparative breast tumor imaging and comparative in vitro metabolism of 16α-[18F]fluoroestradiol-17β and 16β-[18F]fluoromoxestrol in isolated hepatocytes. Nucl. Med. Biol. 1999, 26, 123–130. [Google Scholar] [CrossRef]
- Sulkes, A.; Fuks, Z.; Gordon, A.; Gross, J. Sex hormone binding globulin (SHBG) in breast cancer: A correlation with obesity but not with estrogen receptor status. Eur. J. Cancer Clin. Oncol. 1984, 20, 19–23. [Google Scholar] [CrossRef]
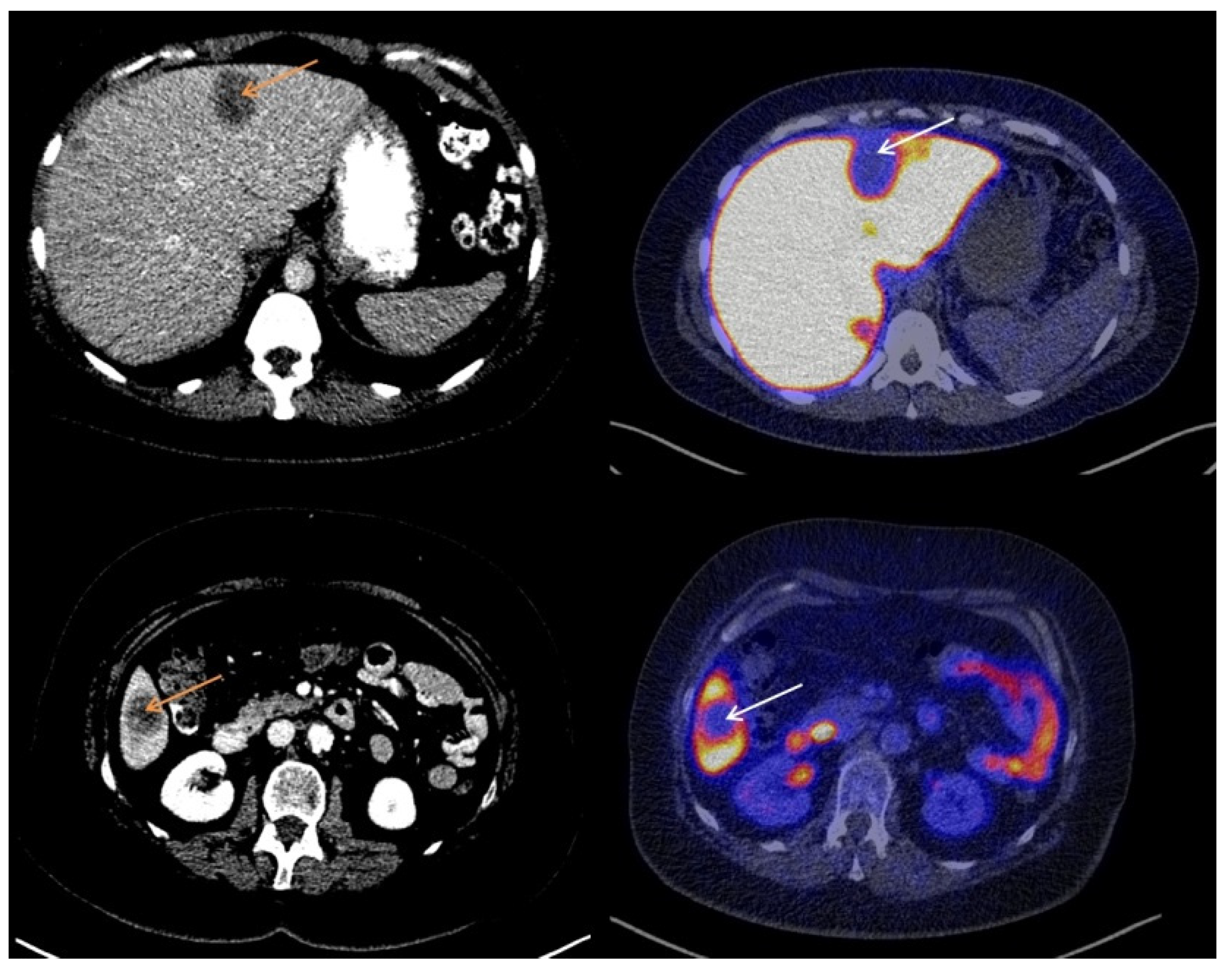
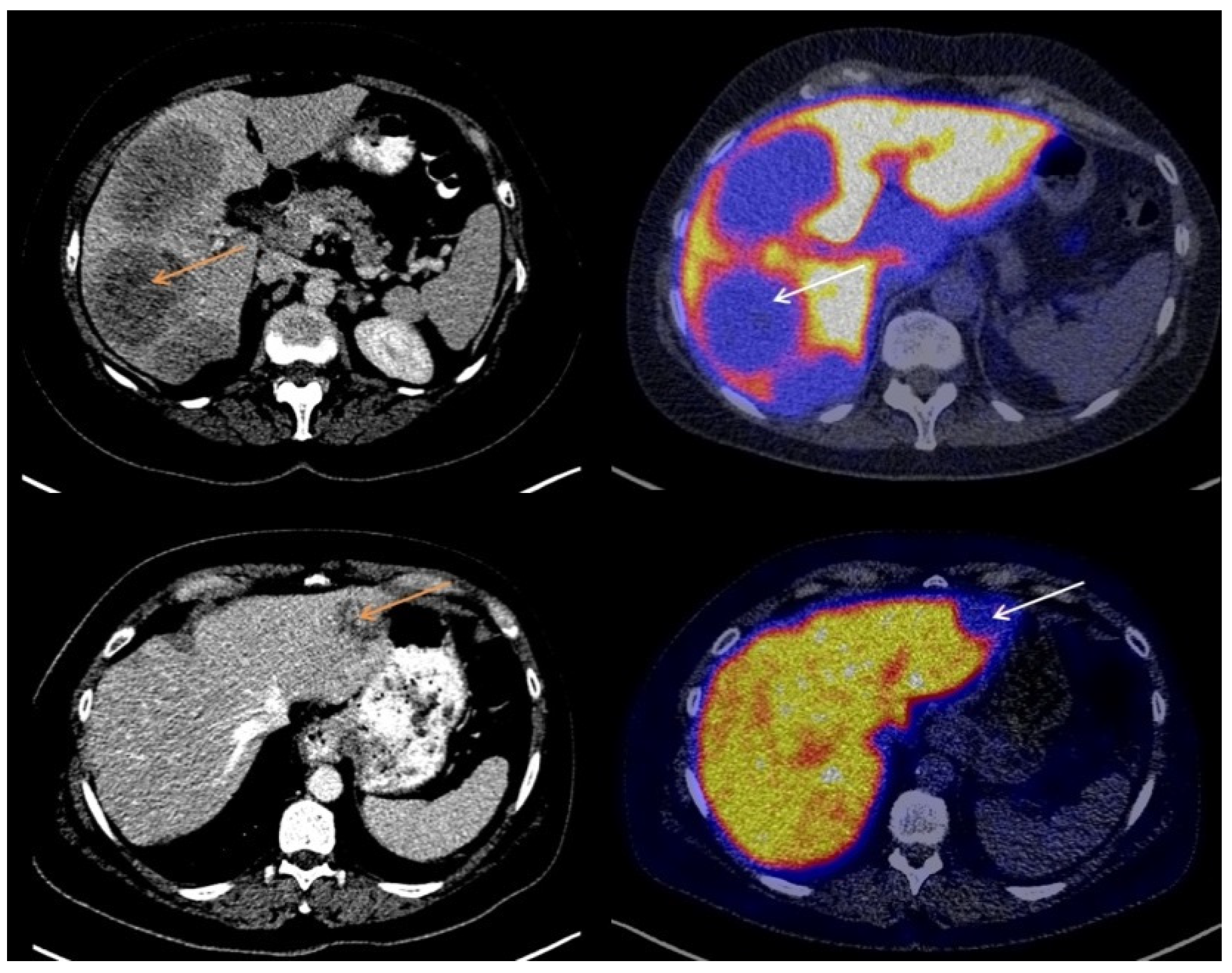
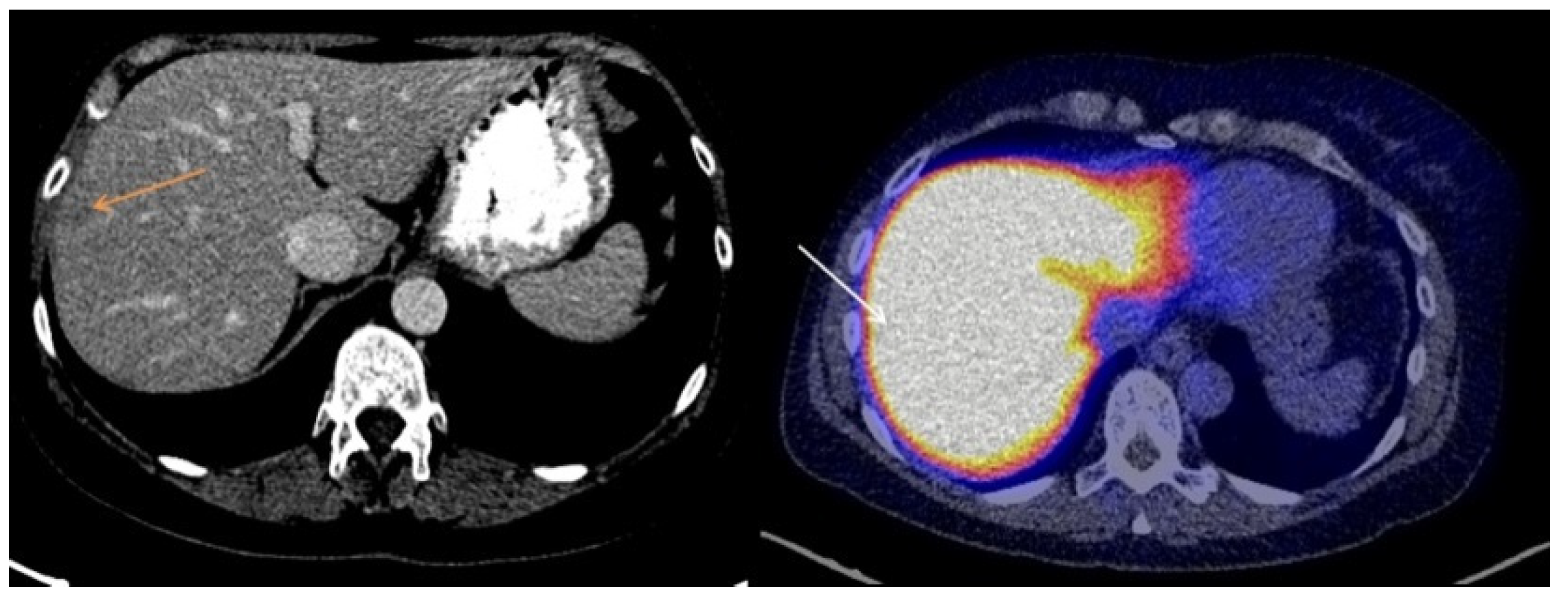
| Total (n = 23) | ER+ (n = 17) | ER− (n = 6) | |
|---|---|---|---|
| Age, years (mean ± SD) | 59 ± 9 | 59 ± 9 | 60 ± 12 |
| Previous palliative systemic treatment | |||
| Yes | 5 (22%) | 5 (29%) | 0 |
| No | 18 (78%) | 12 (71%) | 6 (100%) |
| Tumor diameter on CT, mm (median; min-max range) | |||
| Longest | 29 [13–76] | 28 [13–67] | 32 [22–76] |
| Smallest | 23 [12–62] | 22 [12–62] | 25 [15–42] |
| Parameter | ROI Size | AUC (95% CI) | Optimal Cut-Off ER+ | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| SUVmax | whole-tumor | 0.78 (0.55–1.00) | ≥11.4 | 53 | 100 |
| SUVmean | whole-tumor | 0.74 (0.51–0.97) | ≥2.2 | 94 | 50 |
| SUVmin | whole-tumor | 0.71 (0.48–0.93) | ≥3.9 | 53 | 83 |
| SUVmax | two-thirds | 0.76 (0.54–0.97) | ≥7.8 | 65 | 83 |
| SUVmean | two-thirds | 0.74 (0.51–0.97) | ≥5.7 | 59 | 83 |
| SUVmin | two-thirds | 0.72 (0.48–0.95) | ≥1.9 | 88 | 50 |
| SUVmax | one-third | 0.76 (0.55–0.97) | ≥5.4 | 59 | 83 |
| SUVmean | one-third | 0.77 (0.55–0.98) | ≥5.0 | 59 | 83 |
| SUVmin | one-third | 0.75 (0.53–0.96) | ≥3.3 | 65 | 67 |
| TBRmax | whole-tumor | 0.87 (0.72–1.00) | ≥0.69 | 77 | 83 |
| TBRmean | whole-tumor | 0.80 (0.62–0.99) | ≥0.45 | 65 | 100 |
| TBRmin | whole-tumor | 0.78 (0.58–0.97) | ≥0.21 | 77 | 67 |
| TBRmax | two-thirds | 0.79 (0.60–0.99) | ≥0.52 | 65 | 83 |
| TBRmean | two-thirds | 0.82 (0.64–1.00) | ≥0.33 | 77 | 83 |
| TBRmin | two-thirds | 0.76 (0.54–0.97) | ≥0.21 | 77 | 67 |
| TBRmax | one-third | 0.83 (0.67–1.00) | ≥0.32 | 77 | 83 |
| TBRmean | one-third | 0.84 (0.68–1.00) | ≥0.30 | 77 | 83 |
| TBRmin | one-third | 0.80 (0.62–0.99) | ≥0.21 | 82 | 67 |
| Parameter | ROI Size | Cut-Off ER− Sensitivity >90% | Cut-Off ER+ Specificity >90% | NPV * | PPV ‖ | Equivocal Metastases (%) ‡ |
|---|---|---|---|---|---|---|
| SUVmax | whole-tumor | <4.9 | ≥11.4 | 75 | 90 | 39 |
| SUVmean | whole-tumor | <2.2 | ≥7.7 | 75 | 100 | 48 |
| SUVmin | whole-tumor | <1.2 | ≥4.8 | 67 | 89 | 48 |
| SUVmax | two-thirds | <2.5 | ≥9.5 | 75 | 100 | 52 |
| SUVmean | two-thirds | <1.7 | ≥6.9 | 75 | 100 | 57 |
| SUVmin | two-thirds | <1.3 | ≥5.2 | 67 | 100 | 57 |
| SUVmax | one-third | <1.6 | ≥6.5 | 67 | 100 | 48 |
| SUVmean | one-third | <1.5 | ≥5.8 | 67 | 100 | 52 |
| SUVmin | one-third | <1.3 | ≥5.3 | 67 | 100 | 52 |
| TBRmax | whole-tumor | <0.33 | ≥0.73 | 75 | 100 | 30 |
| TBRmean | whole-tumor | <0.14 | ≥0.45 | 75 | 92 | 30 |
| TBRmin | whole-tumor | <0.07 | ≥0.29 | 50 | 100 | 52 |
| TBRmax | two-thirds | <0.15 | ≥0.65 | 75 | 100 | 48 |
| TBRmean | two-thirds | <0.10 | ≥0.42 | 67 | 90 | 43 |
| TBRmin | two-thirds | <0.08 | ≥0.37 | 67 | 100 | 57 |
| TBRmax | one-third | <0.10 | ≥0.39 | 67 | 100 | 39 |
| TBRmean | one-third | <0.09 | ≥0.34 | 50 | 91 | 43 |
| TBRmin | one-third | <0.08 | ≥0.32 | 67 | 100 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boers, J.; Loudini, N.; de Haas, R.J.; Willemsen, A.T.M.; van der Vegt, B.; de Vries, E.G.E.; Hospers, G.A.P.; Schröder, C.P.; Glaudemans, A.W.J.M.; de Vries, E.F.J. Analyzing the Estrogen Receptor Status of Liver Metastases with [18F]-FES-PET in Patients with Breast Cancer. Diagnostics 2021, 11, 2019. https://doi.org/10.3390/diagnostics11112019
Boers J, Loudini N, de Haas RJ, Willemsen ATM, van der Vegt B, de Vries EGE, Hospers GAP, Schröder CP, Glaudemans AWJM, de Vries EFJ. Analyzing the Estrogen Receptor Status of Liver Metastases with [18F]-FES-PET in Patients with Breast Cancer. Diagnostics. 2021; 11(11):2019. https://doi.org/10.3390/diagnostics11112019
Chicago/Turabian StyleBoers, Jorianne, Naila Loudini, Robbert J. de Haas, Antoon T. M. Willemsen, Bert van der Vegt, Elisabeth G. E. de Vries, Geke A. P. Hospers, Carolina P. Schröder, Andor W. J. M. Glaudemans, and Erik F. J. de Vries. 2021. "Analyzing the Estrogen Receptor Status of Liver Metastases with [18F]-FES-PET in Patients with Breast Cancer" Diagnostics 11, no. 11: 2019. https://doi.org/10.3390/diagnostics11112019
APA StyleBoers, J., Loudini, N., de Haas, R. J., Willemsen, A. T. M., van der Vegt, B., de Vries, E. G. E., Hospers, G. A. P., Schröder, C. P., Glaudemans, A. W. J. M., & de Vries, E. F. J. (2021). Analyzing the Estrogen Receptor Status of Liver Metastases with [18F]-FES-PET in Patients with Breast Cancer. Diagnostics, 11(11), 2019. https://doi.org/10.3390/diagnostics11112019









