Duodenal Pseudomelanosis: A Literature Review
Abstract
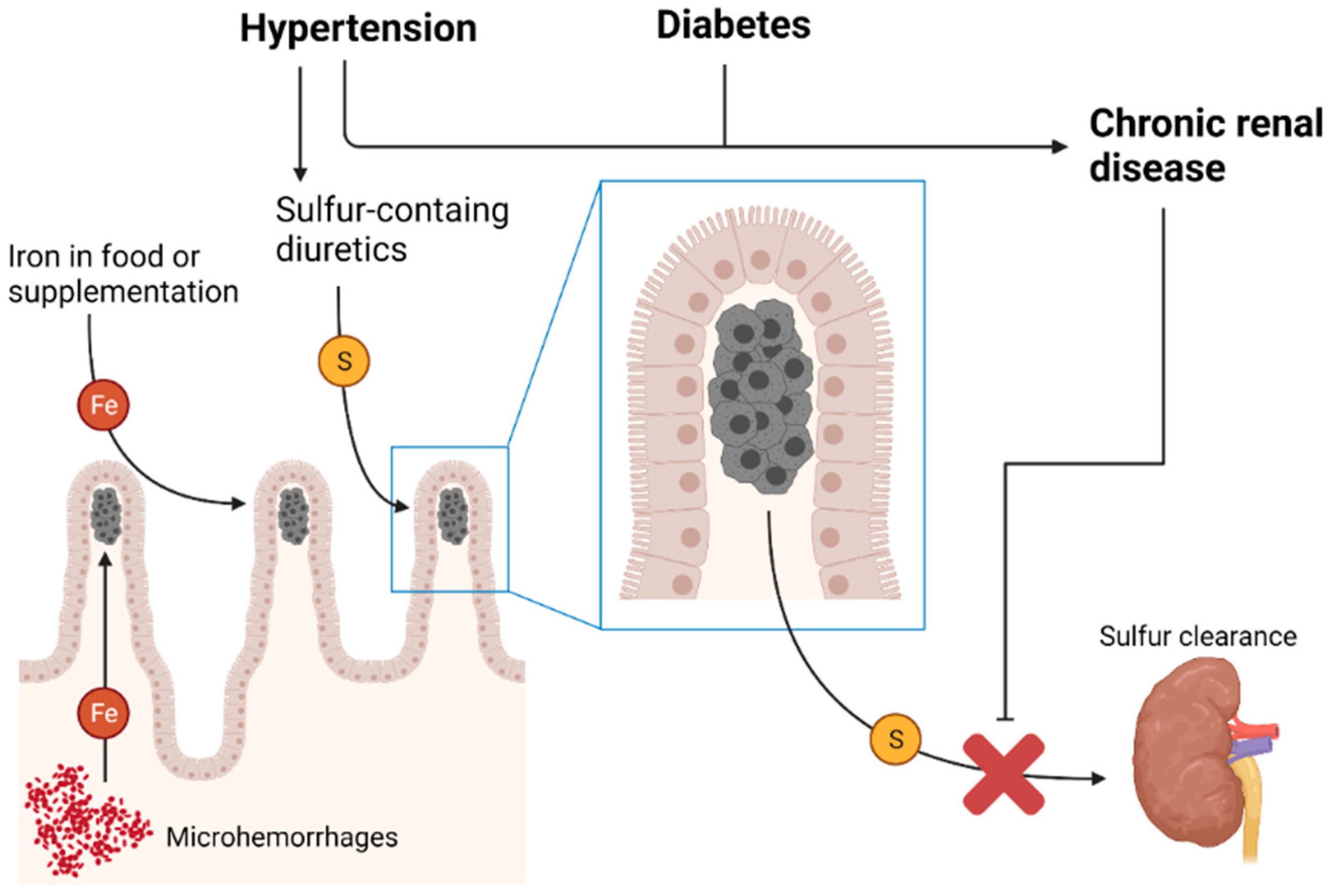
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsai, Y.-N.; Tsai, J.-W.; Tai, C.-M. Magnifying endoscopy for pseudomelanosis duodeni. J. Gastroenterol. Hepatol. 2016, 31, 520. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choung, R.S.; Kwon, B.S.; Hyun, J.J.; Jung, S.W.; Koo, J.S.; Yim, H.J.; Lee, S.W.; Choi, J.H. Small Bowel Pseudomelanosis Associated with Oral Iron Therapy. J. Korean Med. Sci. 2013, 28, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Napthali, K. Pseudomelanosis Duodeni. N. Engl. J. Med. 2020, 383, e113. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, P.; Saleem, S.A.; Riaz, S.; Pendela, V.S.; Vasigh, M.; Heisig, D. Black speckled duodenal mucosa. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxfordshire, UK, 2020; Volume 33, pp. 237–238. [Google Scholar] [CrossRef]
- Jeung, J.; Ashour, S.; Fuller, L. Iron pill-induced duodenitis: A distinct pattern of duodenal mucosal injury in a patient with a duodenal mass. Pathol. -Res. Pract. 2020, 216, 152916. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Jetly-Shridhar, R.; De Felice, K. A Case of Pseudomelanosis Duodeni: Striking Endoscopic Features with Subtle but Characteristic Pathologic Findings. Int. J. Surg. Pathol. 2019, 27, 765–766. [Google Scholar] [CrossRef]
- Tang, S.-J.; Zhang, S.; Grunes, D.E. Gastric and duodenal pseudomelanosis: A new insight into its pathogenesis. VideoGIE 2019, 4, 467–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutaria, A.; Bhutani, M.S. An Alarming but Benign Appearance, A Case of Pseudomelanosis Duodeni. Clin. Gastroenterol. Hepatol. 2018, 16, A32. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, T.; Caughey, M.E.; Gaduputi, V. Rare finding of concomitant pseudomelanosis of stomach and duodenum; case report and literature review. Gastroenterol. Hepatol. Bed Bench 2018, 11, 86. [Google Scholar] [PubMed]
- Shimamura, Y.; Akram, H.; Winer, S.; Marcon, N. Pseudomelanosis Duodeni and Duodenal Polyp. Intern. Med. 2018, 57, 1049–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundi, I.; Pankaj, R.; Chhabra, M.; Banerjee, A.K. Pseudomelanosis Duodeni. Int. J. Surg. Pathol. 2017, 25, 165. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, M.; Oka, S.; Kanzaki, H.; Tanaka, T.; Kawano, S.; Kawahara, Y.; Okada, H. Pseudomelanosis duodeni:a case report. Nihon Shokakibyo Gakkai Zasshi 2017, 114, 1264–1268. [Google Scholar]
- AbdelWareth, A.; Molyneux, A.; Madhotra, R.; Ishaq, S.; Rostami, K. Small bowel pigmentation. Gastroenterol. Hepatol. Bed Bench 2016, 9, 343–344. [Google Scholar] [PubMed]
- Coelho, R.; Ribeiro, A.; Silva, R.; Rios, E.; Silva, M.; Macedo, G. Pseudomelanosis duodeni: Is there a common denominator? Rev. Española De Enferm. Dig. 2016, 108, 658–659. [Google Scholar]
- Kothadia, J.P.; Kaminski, M.; Giashuddin, S. Duodenal siderosis: A rare clinical finding in a patient with duodenal inflammation. Ann. Gastroenterol. 2016, 29, 379. [Google Scholar] [CrossRef] [PubMed]
- Cochet, A.E.; Cunningham, J.R.; Butler, S.L. Pseudomelanosis duodeni and Strongyloides stercoralis. Am. J. Gastroenterol. 2015, 110, 1651. [Google Scholar] [CrossRef] [PubMed]
- Sathyamurthy, A.; Chela, H.; Arif, Z.; Holly, J.; Arif, M. Pseudomelanosis Duodeni. ACG Case Rep. J. 2015, 2, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Siderits, R.; Hazra, A.; Mikhail, N.; Chiaffarano, J.; Lou, W.; Fyfe, B. Endoscopically identified pseudomelanosis duodeni: Striking yet harmless. Gastrointest. Endosc. 2014, 80, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Schuerle, T.; Aoun, E.; Clarke, K. Pseudomelanosis duodeni in a postrenal transplant patient. BMJ Case Rep. 2013, 2013, bcr2013200466. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.S.; Shah, D.K.; Khot, A.A. Pseudomelanosis Duodeni of Undetermined Etiology. Gastroenterol. Res. 2012, 5, 171–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Magalhães Costa, M.H.D.M.; Pegado, M.G.F.; Vargas, C.; Castro, M.E.C.; Madi, K.; Nunes, T.; Zaltman, C. Pseudomelanosis duodeni associated with chronic renal failure. World J. Gastroenterol. 2012, 18, 1414–1416. [Google Scholar] [CrossRef]
- Felipe-Silva, A.; De Campos, F.P.F.; Da Silva, J.G.N. Duodenal pseudomelanosis (pseudomelanosis duodeni): A rare endoscopic finding. Autops. Case Rep. 2011, 1, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kakati, B.; Krishna, S.; Sharma, S.; Rego, R. Pseudomelanosis duodeni: A rare finding from upper endoscopy. Dig. Endosc. 2011, 23, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Yun, L. Gastrointestinal: Pseudomelanosis duodeni. J. Gastroenterol. Hepatol. 2010, 25, 427. [Google Scholar] [CrossRef]
- Monajemzadeh, M.; Tayari, N.; Najafi, M.; Madani, A.; Mahjoub, F.; Esfahani, S.T.; Ataei, N.; Ashtiani, M.T.H.; Mohseni, P.; Shams, S. Pseudomelanosis duodeni in a child with chronic renal failure. Saudi J. Kidney Dis. Transplant. 2008, 19, 645–646. [Google Scholar]
- Giusto, D.; Jakate, S. Pseudomelanosis duodeni: Associated with multiple clinical conditions and unpredictable iron stainability-a case series. Endoscopy 2008, 40, 165–167. [Google Scholar] [CrossRef]
- Cantu, J.A.; Adler, D. Pseudomelanosis Duodeni. Endoscopy 2005, 37, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorland, W.A.N. Dorland’s Illustrated Medical Dictionary; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Rana, N.K.; Minhas, U.; Mahl, T. A Rare Case of Duodenal Melanosis: Case Report. Cureus 2020, 12, e10475. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.C.V.D.; Diniz, L.M.; Lucas, E.A.; Capeli, R.L.D.A. Diffuse cutaneous melanosis: Rare complication of metastatic melanoma. An. Bras. De Dermatol. 2017, 92, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Pasick, L.J.; Paknezhad, H.; Sataloff, R.T. Laryngeal Melanosis. Ear Nose Throat J. 2019, 98, 70–71. [Google Scholar] [CrossRef]
- Nesheiwat, Z.; Al Nasser, Y. Melanosis coli. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yabuki, K.; Shiba, E.; Harada, H.; Uchihashi, K.; Matsuyama, A.; Haratake, J.; Hisaoka, M. Lanthanum deposition in the gastrointestinal mucosa and regional lymph nodes in dialysis patients: Analysis of surgically excised specimens and review of the literature. Pathol. Res. Pract. 2016, 212, 919–926. [Google Scholar] [CrossRef]
- Bhangale, N.P.; Abraham, P.; Joshi, A.; Desai, D. Clofazimine-related duodenal pigmentation. Indian J. Gastroenterol. 2021, 40, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, S.C.; Fermoyle, S. An unusual cause of small-intestine mucosal pigmentation. Gastrointest. Endosc. 2011, 73, 1285–1286. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.; Swartz, D.; Tydd, T.; Leong, R.W. Gastrointestinal: Eosinophilic enteritis manifesting as brown-pigmented duodenal ulcers. J. Gastroenterol. Hepatol. 2009, 24, 1892. [Google Scholar] [CrossRef]
- Voudoukis, E.; Mpitouli, A.; Giannakopoulou, K.; Velegraki, M.; Datseri, G.; Bachlitzanaki, M.; Kazamias, G.; Fahouridi, A.; Mastorakis, E.; Vardas, E.; et al. Disseminated metastatic cutaneous melanoma to pancreas and upper gastrointestinal tract diagnosed by endoscopic ultrasound: An unusual case. Clin. J. Gastroenterol. 2019, 13, 134–138. [Google Scholar] [CrossRef]
- Houissa, F.; Bouzaidi, S.; Mouelhi, L.; Ben Rejeb, M.; Moussa, A.; Mekki, H.; Dabbeche, R.; Trabelsi, S.; Said, Y.; Salem, M.; et al. Diffuse primary malignant melanoma of the upper gastrointestinal tract. Gastroentérologie Clin. Et Biol. 2010, 34, 85–87. [Google Scholar] [CrossRef]


| Study | Endoscopy Reason | Patient’s Gender | Patient’s Age | Patient’s History | Medications | Special Studies | Other Relevant Info | |
|---|---|---|---|---|---|---|---|---|
| Cook D, Napthali K, 2020 [3] | Dysphagia | M | 83 | Chronic kidney disease, hypertension, type 2 diabetes mellitus | Hydralazine, irbesartan, lercanidipine, and metoprolol | No | ||
| Kudaravalli P et al., 2020 [4] | Gastrointestinal bleeding | F | 83 | Hypertension, chronic kidney disease, hypothyroidism, atrial fibrillation | Aspirin, furosemide, metoprolol, levothyroxine, and warfarin | Yes (not published) | ||
| Jeung J et al., 2020 [5] | Early satiety, weight loss | F | 55 | Iron deficiency anemia | Ferrous sulfate | Prussian blue | Pigmentation not evident at endoscopy, Brunner gland cyst, active inflammation, erosion, gastric foveolar metaplasia, reactive mucin loss | |
| Nakanishi Y et al., 2019 [6] | Possible Crohn’s disease | M | 72 | Proctocolectomy with end ileostomy, chronic kidney disease, hypertension, diabetes, coronary artery disease, gout, arthritis | Not stated | Prussian blue | ||
| Tang SJ et al., 2019 [7] | Not specified | Not stated | Not stated | Iron pill–induced mucositis | Iron supplementation | Prussian blue | Concomitant gastric pseudomelanosis | |
| Sutaria A, Bhutani MS, 2018 [8] | Epigastric pain | F | 77 | Hypertension, hyperlipidemia, diabetes mellitus, hypothyroidism | Not stated | No | ||
| Sunkara T et al., 2018 [9] | Determination of bleeding risk | F | 83 | Atrial fibrillation, chronic obstructive pulmonary disease, pulmonary hypertension, chronic heart failure, non-Hodgkin’s lymphoma | Rivaroxaban (discontinued) | No | Concomitant pseudomelanosis gastri | |
| Shimamura Y et al., 2018 [10] | Gastrointestinal bleeding | M | 68 | Not stated | Not stated | Prussian blue | ||
| Mundi I et al., 2017 [11] | Suspect celiac disease | M | 61 | Chronic renal failure | Not stated | Prussian blue | ||
| Iwamuro M et al., 2017 [12] | Screening | M | 83 | Hypertension, chronic kidney disease, chronic heart failure, and chronic myeloid leukemia | Ferrous citrate, furosemide, spironolactone, tolvaptan, bisoprolol, nicorandil, warfarin, nilotinib, febuxostat, esomeprazole, digestive enzyme complex, ambroxol, carbocysteine, potassium L-aspartate | X-ray spectroscopy, elemental mapping | ||
| Abdelwareth A et al., 2016 [13] | Dyspepsia | M | 73 | COPD, ischemic heart disease, Iron deficiency anaemia, colonic polyp | Esmoeprazole, ranitidine, atorvastain, losartan, inhalers, and ferrous sulfate | Prussian blue | ||
| Coelho R et al., 2016 [14] | Dysphagia | F | 76 | Diabetes mellitus, chronic renal failure, iron deficiency anemia | Ferrous sulfate | Prussian blue | Schatzki’s ring | |
| Kothadia JP et, 2016 [15] | Anemia, weight loss | M | 84 | Not stated | Not stated | Prussian blue | Duodenal polyp, duodenitis | |
| Tsai YN et al., 2016 [1] | Acid reflux | M | 63 | Hypertension | Hydralazine | No | ||
| Cochet AE et al., 2015 [16] | Diarrhea | M | Not stated | Not stated | Not stated | No | Strongyloides stercoralis infection | |
| Sathyamurthy A et al., 2015 [17] | Intractable nausea and vomiting | M | 55 | Coronary artery disease, chronic kidney disease stage 4, diabetes mellitus, hypertension, iron deficiency anemia | Antihypertensives (not specified) | Prussian blue | ||
| Siderits R et al., 2014 [18] | Abdominal pain | F | 80 | Hypertension | Hydralazine, laxatives (senna) | Prussian blue | ||
| Kim SJ et al., 2013 [2] | 1 | Not specified | F | 73 | Diabetes mellitus, hypertension | Iron sulfide, antidiabetics, loop diuretics, angiotensin II receptor blockers, calcium channel blockers (not specified) | Prussian blue | |
| 2 | Not specified | M | 71 | Diabetes mellitus, hypertension, chronic renal failure | Iron sulfide, antidiabetics, calcium channel blockers, potassium binders (not specified) | Prussian blue | ||
| 3 | Not specified | F | 70 | Hypertension, chronic renal failure | Iron sulfide, loop diuretics, angiotensin II receptor blockers, beta-blockers, statins (not specified) | Prussian blue | ||
| 4 | Not specified | F | 34 | Chronic renal failure | Iron sulfide, loop diuretics, statins, potassium binders (not specified) | Prussian blue | ||
| 5 | Not specified | F | 69 | Diabetes mellitus, hypertension, chronic renal failure | Iron sulfide, antidiabetics, loop diuretics, angiotensin II receptor blockers, calcium channel blockers, beta-blockers, potassium binders (not specified) | Prussian blue | ||
| 6 | Not specified | F | 55 | Hypertension, chronic renal failure | Iron sulfide, loop diuretics, statins (not specified) | Prussian blue | ||
| Schuerle T et al., 2013 [19] | Nausea, vomiting and diarrhoea | F | 54 | Diabetes mellitus type 2, end-stage renal disease, kidney transplant, hypertension, anaemia, hypothyroidism | Insulin, pantoprazole, hydralazine, losartan, diltiazem, paroxetine, thyroxine, atorvastatin, cyclosporine, mycophenolate mofetil, trimethoprim/sulfamethoxazole | Fontana-Masson: positive; PAS: negative; Prussian blue: negative | ||
| Jain SS et al., 2012 [20] | Epigastric pain | F | 48 | None | Proton pump inhibitor (not specified) | Prussian blue: positive; Fontana Masson: negative | ||
| de Magalhães Costa MH et al., 2012 [21] | 1 | Melena, anemia | F | 66 | Diabetes mellitus, hypertension, chronic renal failure | Angiotensin-converting enzyme inhibitors (not specified), furosemide, ferrous sulfate, folic acid and insulin | Fontana-Masson: positive (interpreted as iron) | |
| 2 | Acid reflux | F | 37 | Hypertensive nephropathy, renal transplantation | Furosemide, propranolol, hydralazine, ferrous sulfate | Not stated | ||
| 3 | Anemia | F | 70 | Diabetes, hypertension, chronic renal failure, nephrolitiasis, nephrectomy | Calcium channel blockers (not specified), propranolol, α-methyldopa, furosemide, glibenclamide | Not stated | ||
| 4 | Epigastric pain, nausea, vomiting | F | 30 | Diabetes, renal and pancreatic transplantation | Insulin, tracolimus, mycophenolate and corticosteroids (not specified) | Not stated | ||
| Felipe-Silva A et al., 2011 [22] | Suspect upper gastrointestinal bleeding | F | 86 | Hypertension, ischemic strokes, left hemiplegia, central VII nerve palsy | Hydralazine, captopril, hydrochlorthiazide, aspirin | Prussian blue: negative; Fontana-Masson: negative | Distal esophagitis, gastritis and gastric angiodysplasia | |
| Kakati B et al., 2011 [23] | Anemia | F | 65 | End-stage renal disease, diabetes mellitus, hypertension, hypothyroidism | Zolpidem, aspirin, clonidine, hydralazine, insulin, levothyroxine, and metoprolol | Not specified | ||
| Yun L, 2010 [24] | Melena | F | 67 | End stage renal disease, coronary artery disease, diabetes, hypertension, hyperlipidemia, anemia, hypothyroidism | Aspirin, clopidogrel, hydralazine, frusemide, benazepril, atorvastatin, ezetimibe, levothyroxine, and iron | No | ||
| Monajemzadeh M et al., 2008 [25] | Abdominal discomfort | F | 8 | Polycystic kidney disease, nephrotic syndrome, endstage renal disease, kidney transplant | Hydralazine, clonidine, amilodipine, oral iron supplements, phenytoin | Prussian blue: positive; Fontana Masson: positive | ||
| Giusto D, Jakate S, 2008 [26] | 1 | Not specified | F | 86 | Hypertension | Not specified | Prussian blue: positive | |
| 2 | Not specified | F | 65 | Hypertension, end stage renal disease | Not specified | Prussian blue: partial | ||
| 3 | Not specified | F | 63 | Hypertension, diabetes | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 4 | Not specified | F | 68 | Hypertension | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 5 | Not specified | M | 60 | Hypertension, end stage renal disease | Not specified | Prussian blue: negative | ||
| 6 | Not specified | F | 63 | Hypertension, diabetes | Not specified | Prussian blue: positive | No evidence of mucosal pigmentation at endoscopy | |
| 7 | Not specified | F | 66 | Hypertension, end stage renal disease, diabetes | Not specified | Prussian blue: partial | ||
| 8 | Not specified | M | 70 | Hypertension, end stage renal disease | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 9 | Not specified | F | 34 | Hypertension, end stage renal disease | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 10 | Not specified | M | 52 | Hypertension, end stage renal disease, diabetes | Not specified | Prussian blue: partial | ||
| 11 | Not specified | M | 59 | Hypertension, diabetes | Not specified | Prussian blue: negative | No evidence of mucosal pigmentation at endoscopy | |
| 12 | Not specified | F | 53 | Hypertension, end stage renal disease | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 13 | Not specified | M | 65 | Hypertension | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| 14 | Not specified | M | 52 | Hypertension, end stage renal disease | Not specified | Prussian blue: negative | No evidence of mucosal pigmentation at endoscopy | |
| 15 | Not specified | F | 49 | Hypertension, end stage renal disease | Not specified | Prussian blue: partial | ||
| 16 | Not specified | F | 58 | Hypertension | Not specified | Prussian blue: positive | ||
| 17 | Not specified | F | 67 | Hypertension, end stage renal disease, diabetes | Not specified | Prussian blue: partial | No evidence of mucosal pigmentation at endoscopy | |
| Cantu JA, Adler DG, 2005 [27] | Jaundice after cholecystectomy | M | 49 | Chronic renal insufficiency, hypertension | Hydralazine | Not stated | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, G.; D’Ercole, M.; Ferrero, S.; Croci, G.A. Duodenal Pseudomelanosis: A Literature Review. Diagnostics 2021, 11, 1974. https://doi.org/10.3390/diagnostics11111974
Lopez G, D’Ercole M, Ferrero S, Croci GA. Duodenal Pseudomelanosis: A Literature Review. Diagnostics. 2021; 11(11):1974. https://doi.org/10.3390/diagnostics11111974
Chicago/Turabian StyleLopez, Gianluca, Marianna D’Ercole, Stefano Ferrero, and Giorgio Alberto Croci. 2021. "Duodenal Pseudomelanosis: A Literature Review" Diagnostics 11, no. 11: 1974. https://doi.org/10.3390/diagnostics11111974
APA StyleLopez, G., D’Ercole, M., Ferrero, S., & Croci, G. A. (2021). Duodenal Pseudomelanosis: A Literature Review. Diagnostics, 11(11), 1974. https://doi.org/10.3390/diagnostics11111974






