Diagnostic Performance of [18F]FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Procedures
2.3. Histopathology
2.4. Patient-Based Analysis
2.5. Lesional Analysis: Qualitative and Semi-Quantitative FDG PET Readings
2.6. Clinical Implications on Treatment Plan
2.7. Statistical Analysis
3. Results
3.1. Patients
3.2. Patient-Based Analysis
3.3. Lesional Analysis
3.4. Correlation between FDG PET Parameters and Histopathology
3.5. Implications for the Plan
4. Discussion
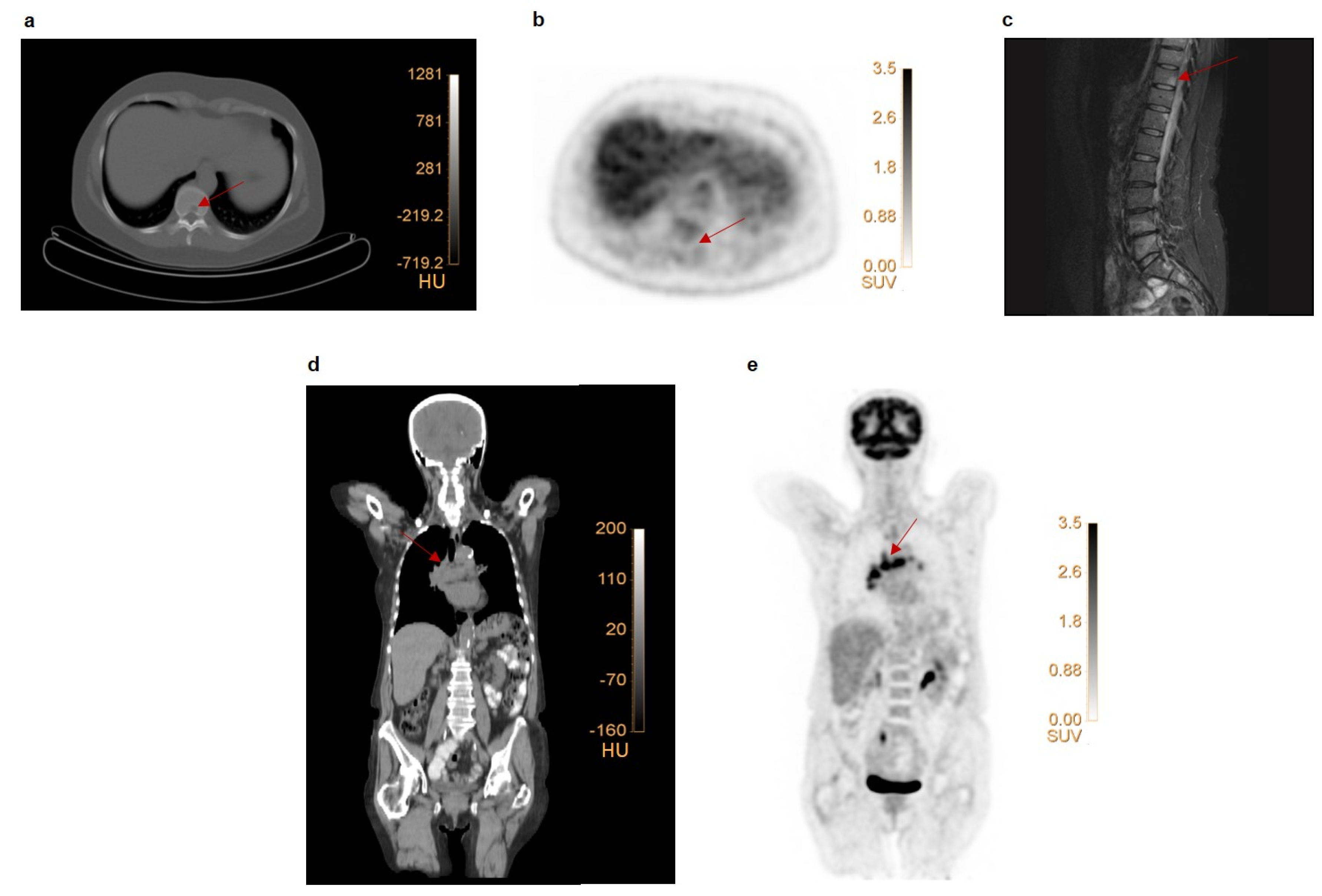
4.1. TNM Lesion Detection
4.2. Association between FDG PET Parameters and Histopathology
4.3. Limitations
4.4. Scientific Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Vondeling, G.T.; Menezes, G.L.; Dvortsin, E.P.; Jansman, F.G.A.; Konings, I.R.; Postma, M.J.; Rozenbaum, M.H. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer 2018, 18, 262. [Google Scholar] [CrossRef]
- Breast Cancer Dutch Guideline. Available online: https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html (accessed on 11 October 2020).
- Kwapisz, D. Oligometastatic breast cancer. Breast Cancer 2019, 26, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.E.; Houssami, N. Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. Breast 2012, 21, 112–123. [Google Scholar] [CrossRef]
- Segaert, I.; Mottaghy, F.; Ceyssens, S.; De Wever, W.; Stroobants, S.; Van Ongeval, C.; Van Limbergen, E.; Wildiers, H.; Paridaens, R.; Vergote, I.; et al. Additional Value of PET-CT in Staging of Clinical Stage IIB and III Breast Cancer. Breast J. 2010, 16, 617–624. [Google Scholar] [CrossRef]
- Elfgen, C.; Schmid, S.M.; Tausch, C.J.; Montagna, G.; Güth, U. Radiological Staging for Distant Metastases in Breast Cancer Patients with Confirmed Local and/or Locoregional Recurrence: How Useful are Current Guideline Recommendations? Ann. Surg. Oncol. 2019, 26, 3455–3461. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group; McGale, P.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; Whelan, T.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Breast Cancer (Version 8.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 11 October 2021).
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.-L.; Hindié, E.; Mankoff, D. 18F-FDG PET/CT for Staging and Restaging of Breast Cancer. J. Nucl. Med. 2016, 57, 17S–26S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Wang, L.; Jiang, X.; She, W.; He, L.; Hu, G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: A systematic review and meta-analysis. Nucl. Med. Commun. 2016, 37, 1180–1188. [Google Scholar] [CrossRef]
- Gil-Rendo, A.; Martínez-Regueira, F.; Zornoza, G.; Garcia-Velloso, M.J.; Beorlegui, C.; Rodriguez-Spiteri, N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. J. Br. Surg. 2009, 96, 166–170. [Google Scholar] [CrossRef]
- Crippa, F.; Seregni, E.; Agresti, R.; Chiesa, C.; Pascali, C.; Bogni, A.; Decise, D.; De Sanctis, V.; Greco, M.; Daidone, M.G.; et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: A preliminary observation. Eur. J. Nucl. Med. Mol. Imaging 1998, 25, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Espie, M.; Giachetti, S.; Hindie, E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology 2013, 266, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Groheux, D.; Giacchetti, S.; Moretti, J.-L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; De Roquancourt, A.; Hamy, A.-S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.Y.; Kim, S.H.; Choi, B.B.; Sung, M.S. Associations between the standardized uptake value of 18F-FDG PET/CT and the prognostic factors of invasive lobular carcinoma: In comparison with invasive ductal carcinoma. World J. Surg. Oncol. 2015, 13, 113. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Chauhan, A.; Zhuang, H.; Chandra, P.; Schnall, M.; Alavi, A. Clinicopathologic factors associated with false negative FDG–PET in primary breast cancer. Breast Cancer Res. Treat. 2006, 98, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Van der Hoeven, J.J.; Van der Wall, E.; Van der Groep, P.; Van Diest, P.J.; Comans, E.F.; Semenza, G.L.; Hoekstra, O.S.; Lammertsma, A.A.; Molthoff, C.F.M. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J. Clin. Oncol. 2002, 20, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Chen, W.; Tchou, J.; Mavi, A.; Cermik, T.; Czerniecki, B.; Schnall, M.; Alavi, A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: A potentially useful method for disease characterization. Cancer 2008, 112, 995–1000. [Google Scholar]
- Mavi, A.; Cermik, T.F.; Urhan, M.; Puskulcu, H.; Basu, S.; Jian, Q.Y.; Zhuang, H.; Czerniecki, B.; Alavi, A. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J. Nucl. Med. 2007, 48, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.R.; Port, E.; Gonen, M.; Doane, A.; Yeung, H.; Gerald, W.; Cook, J.B.; Larson, S. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: Microarray and immunohistochemical analysis. J. Nucl. Med. 2010, 51, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological types of breast cancer: How special are they? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Lemarignier, C.; Martineau, A.; Teixeira, L.; Vercellino, L.; Espié, M.; Merlet, P.; Groheux, D. Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1145–1154. [Google Scholar] [CrossRef]
- Groheux, D.; Martineau, A.; Teixeira, L.; Espié, M.; De Cremoux, P.; Bertheau, P.; Merlet, P.; Lemarignier, C. 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: Comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017, 19, 3. [Google Scholar] [CrossRef] [Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Boellaard, R. Quantitative oncology molecular analysis suite: ACCURATE. J. Nucl. Med. 2018, 59, 1753. [Google Scholar]
- Vogsen, M.; Jensen, J.D.; Christensen, I.Y.; Gerke, O.; Jylling, A.M.B.; Larsen, L.B.; Braad, P.-E.; Søe, K.L.; Bille, C.; Ewertz, M.; et al. FDG-PET/CT in high-risk primary breast cancer—a prospective study of stage migration and clinical impact. Breast Cancer Res. Treat. 2021, 185, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, J.Y. Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2021, 46, 271–282. [Google Scholar] [CrossRef]
- Heusner, T.A.; Kuemmel, S.; Umutlu, L.; Koeninger, A.; Freudenberg, L.S.; Hauth, E.A.; Kimmig, K.R.; Forsting, M.; Bockisch, A.; Antoch, G. Breast Cancer Staging in a Single Session: Whole-Body PET/CT Mammography. J. Nucl. Med. 2008, 49, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, D.; Duch, J.; Paredes, P.; Velasco, M.; Muñoz, M.; Santamaría, G.; Fontanillas, M.; Pons, F. Preoperative Staging of Large Primary Breast Cancer with [18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Compared with Conventional Imaging Procedures. J. Clin. Oncol. 2008, 26, 4746–4751. [Google Scholar] [CrossRef]
- Veronesi, U.; De Cicco, C.; Galimberti, V.; Fernandez, J.; Rotmensz, N.; Viale, G.; Spano, G.; Luini, A.; Intra, M.; Berrettini, A.; et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann. Oncol. 2007, 18, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Alberini, J.-L.; Lerebours, F.; Wartski, M.; Fourme, E.; Le Stanc, E.; Gontier, E.; Madar, O.; Cherel, P.; Pecking, A.P. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer 2009, 115, 5038–5047. [Google Scholar] [CrossRef] [PubMed]
- Carkaci, S.; Macapinlac, H.A.; Cristofanilli, M.; Mawlawi, O.; Rohren, E.; Angulo, A.M.G.; Dawood, S.; Resetkova, E.; Le-Petross, H.T.; Yang, W.-T. Retrospective Study of 18F-FDG PET/CT in the Diagnosis of Inflammatory Breast Cancer: Preliminary Data. J. Nucl. Med. 2009, 50, 231–238. [Google Scholar] [CrossRef] [Green Version]
- van der Hoeven, J.J.; Krak, N.C.; Hoekstra, O.S.; Comans, E.F.; Boom, R.P.; Van Geldere, D.; van der Wall, E.; Buter, J.; Pinedo, H.M.; Teule, G.J.J.; et al. 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography in staging of locally advanced breast cancer. J. Clin. Oncol. 2004, 22, 1253–1259. [Google Scholar] [CrossRef]
- Cook, G.J.; Azad, G.K.; Goh, V. Imaging Bone Metastases in Breast Cancer: Staging and Response Assessment. J. Nucl. Med. 2016, 57, 27S–33S. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.M.; Kim, H.J.; Jung, S.J.; Lim, H.S.; Lee, S.W.; Cho, S.H.; Jang, Y.-J.; Lee, H.J.; Kim, G.C.; Jung, J.H.; et al. Incidental Breast Lesions Identified by18F-FDG PET/CT: Which Clinical Variables Differentiate between Benign and Malignant Breast Lesions? J. Breast Cancer 2015, 18, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Pencharz, D.; Nathan, M.; Wagner, T.L. Evidence based management of incidental focal uptake of fluorodeoxyglucose on PET-CT. Br. J. Radiol. 2017, 91, 20170774. [Google Scholar] [CrossRef]
- Avril, N.; Menzel, M.; Dose, J.; Schelling, M.; Weber, W.; Jänicke, F.; Nathrath, W.; Schwaiger, M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. J. Nucl. Med. 2001, 42, 9–16. [Google Scholar] [PubMed]
- Buck, A.; Schirrmeister, H.; Kühn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18F-fluoro-17β-estradiol positron emission tomography: Meta-analysis and recommendations for integration into clinical applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef] [PubMed]

| N (%) or Median (Range) | |
| Age at diagnosis (y) | 49 (28–94) |
| Clinical stage at presentation | |
| IIB | 36 (48.6) |
| III | 35 (47.3) |
| Locoregional recurrence | 3 (4.1) |
| Histological subtype * | |
| Ductal | 57 (77.0) |
| Lobular | 17 (22.7) |
| Micropapillary | 1 (1.3) |
| Grade | |
| 1 | 7 (9.5) |
| 2 | 67 (91.5) |
| ER receptor | |
| Positive | 74 (100.0) |
| PR receptor | |
| Negative | 13 (17.6) |
| Positive | 61 (82.4) |
| HER2neu receptor | |
| Negative | 65 (87.8) |
| Positive | 9 (12.2) |
| Treatment received | |
| Neo-adjuvant therapy | |
| (after FDG PET imaging) | |
| yes | 58 (78.4) |
| - chemotherapy | - 53 (91.4) |
| - endocrine therapy | - 5 (8.6) |
| no ** | 16 (21.6) |
| Surgery | |
| - yes | 65 (87.8) |
| - before FDG PET imaging | - 4 (6.2) |
| - after FDG PET imaging | - 61 (93.8) |
| - no | 9 (12.2) |
| Adjuvant therapy | |
| - yes | 69 (93.2) |
| - no | 2 (2.7) |
| - unknown | 3 (4.1) |
| (A) Clinical Stage | (B) [18F]FDG PET Stage | (C) Final Stage Baseline | |||
|---|---|---|---|---|---|
| Local Recurrence | IIB | III | IV | ||
| Local recurrence | Local recurrence | 2 | 0 | 0 | 0 |
| IIB | 0 | 0 | 0 | 0 | |
| III | 0 | 0 | 0 | 0 | |
| IV | 0 | 0 | 0 | 1 | |
| IIB | Local recurrence/stage I/IIA | 0 | 0 | 0 | 0 |
| IIB | 0 | 21 | 0 | 0 | |
| III | 0 | 3 | 0 | 0 | |
| IV | 0 | 4 | 0 | 1 | |
| No lesions visible on scan | 0 | 1 | 0 | 0 | |
| III | Local recurrence/stage I/IIA | 0 | 0 | 0 | 0 |
| IIB | 0 | 0 | 2 | 0 | |
| III | 0 | 0 | 24 | 0 | |
| IV | 0 | 0 | 6 | 5 | |
 : Correctly identified by FDG PET,
: Correctly identified by FDG PET,  : Incorrectly downstaged by FDG PET,
: Incorrectly downstaged by FDG PET,  : Correctly upstaged by FDG PET,
: Correctly upstaged by FDG PET,  : Incorrectly upstaged by FDG PET.
: Incorrectly upstaged by FDG PET.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, R.; Mammatas, L.H.; Aras, T.; Vogel, W.V.; van de Brug, T.; Oprea-Lager, D.E.; Verheul, H.M.W.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Diagnostic Performance of [18F]FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer. Diagnostics 2021, 11, 1954. https://doi.org/10.3390/diagnostics11111954
Iqbal R, Mammatas LH, Aras T, Vogel WV, van de Brug T, Oprea-Lager DE, Verheul HMW, Hoekstra OS, Boellaard R, Menke-van der Houven van Oordt CW. Diagnostic Performance of [18F]FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer. Diagnostics. 2021; 11(11):1954. https://doi.org/10.3390/diagnostics11111954
Chicago/Turabian StyleIqbal, Ramsha, Lemonitsa H. Mammatas, Tuba Aras, Wouter V. Vogel, Tim van de Brug, Daniela E. Oprea-Lager, Henk M. W. Verheul, Otto S. Hoekstra, Ronald Boellaard, and Catharina W. Menke-van der Houven van Oordt. 2021. "Diagnostic Performance of [18F]FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer" Diagnostics 11, no. 11: 1954. https://doi.org/10.3390/diagnostics11111954
APA StyleIqbal, R., Mammatas, L. H., Aras, T., Vogel, W. V., van de Brug, T., Oprea-Lager, D. E., Verheul, H. M. W., Hoekstra, O. S., Boellaard, R., & Menke-van der Houven van Oordt, C. W. (2021). Diagnostic Performance of [18F]FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer. Diagnostics, 11(11), 1954. https://doi.org/10.3390/diagnostics11111954






