Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Outcome Measures
2.4. Quality Assessment and Statistical Analysis
2.5. Publication Bias
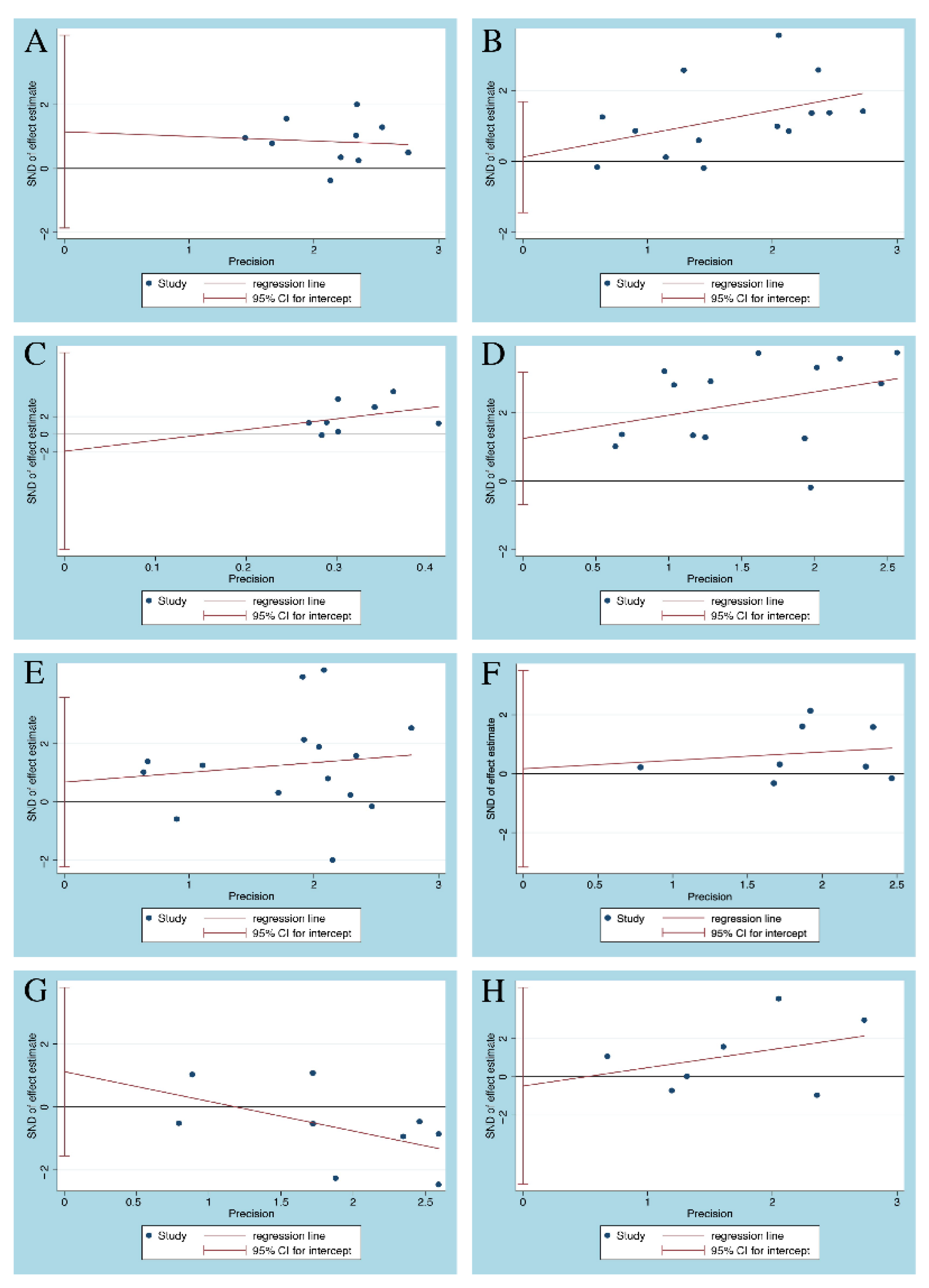
2.6. Sensitivity Analysis
3. Results
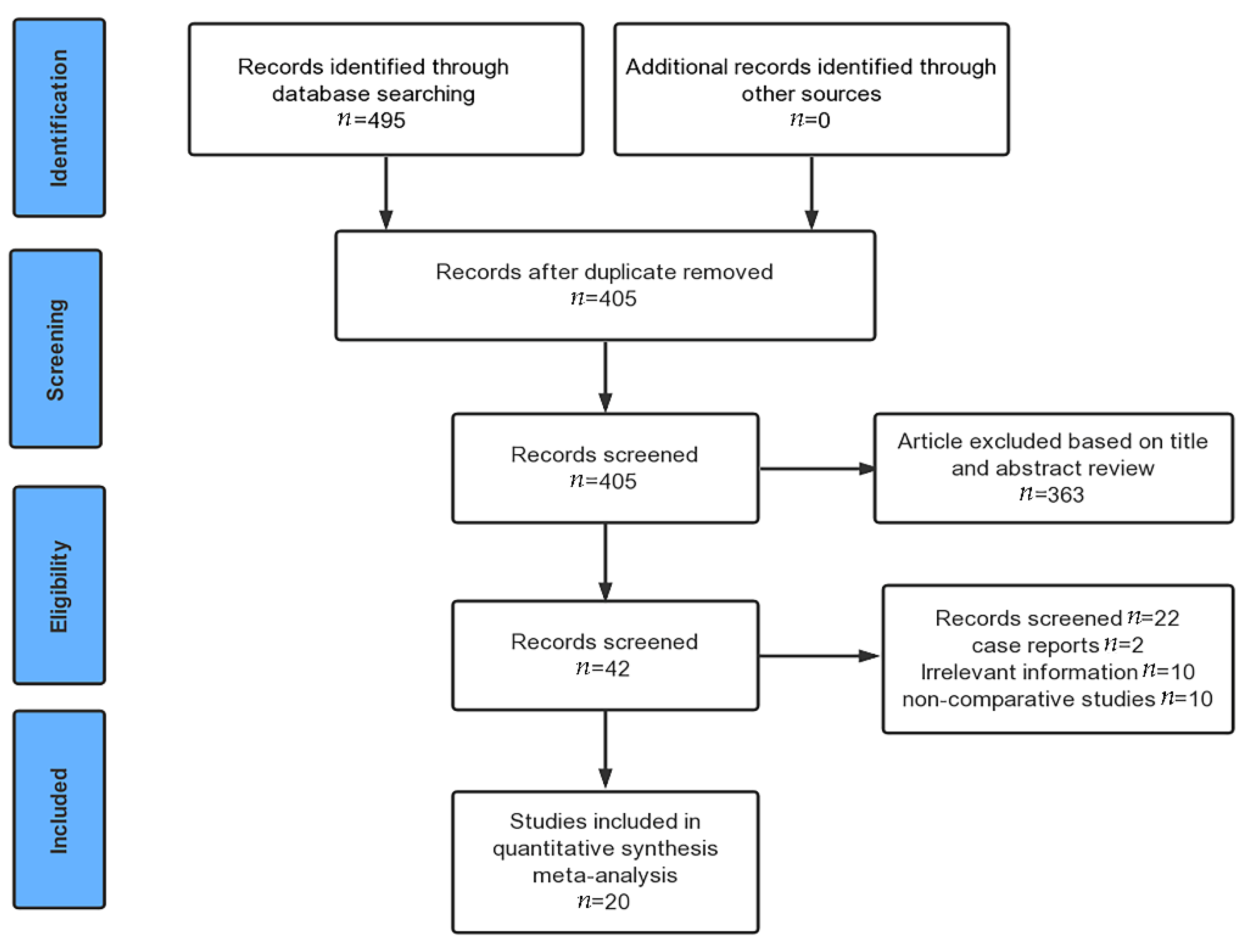
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Study Quality and Risk of Bias
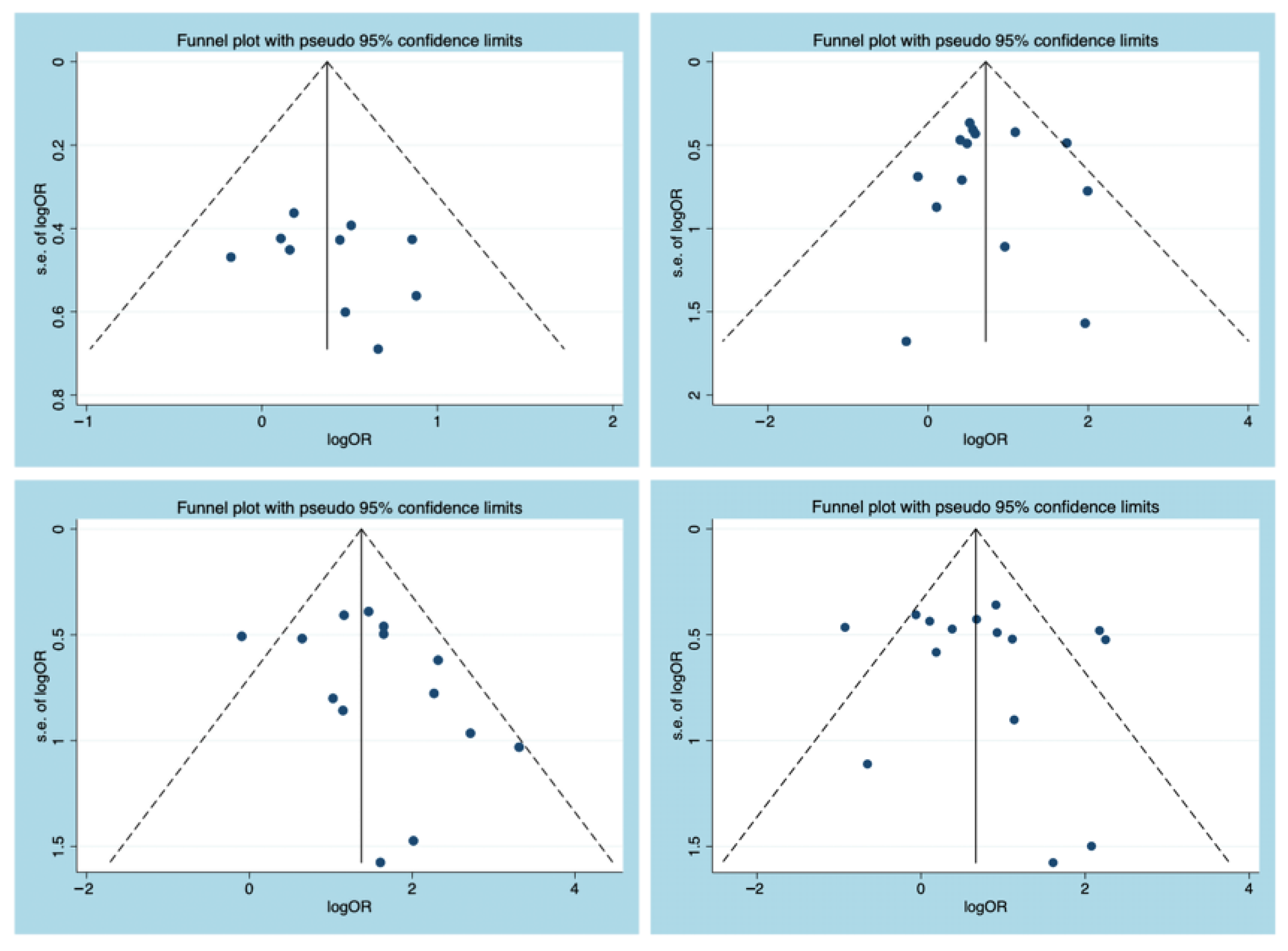
3.4. Publication Bias
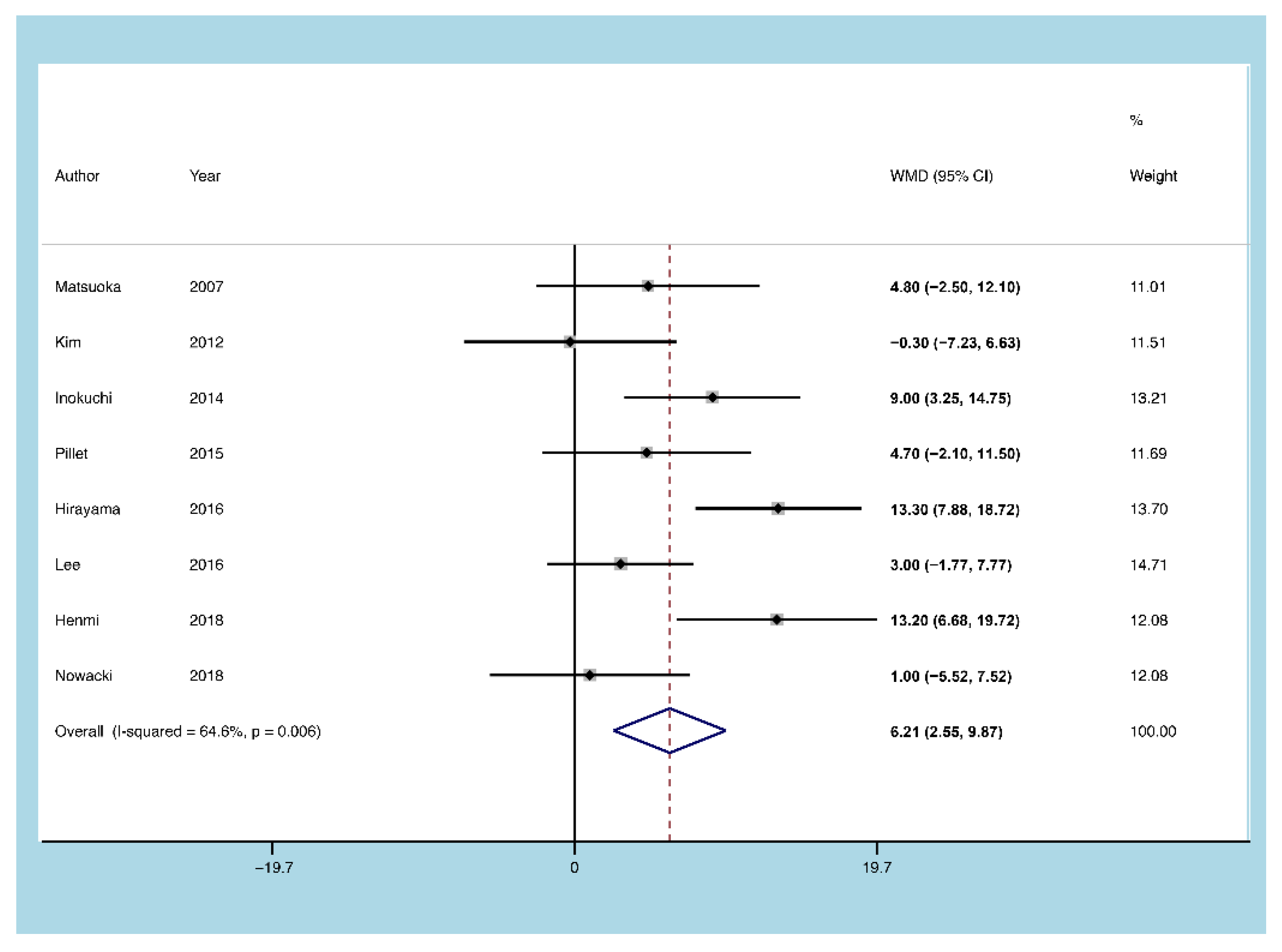
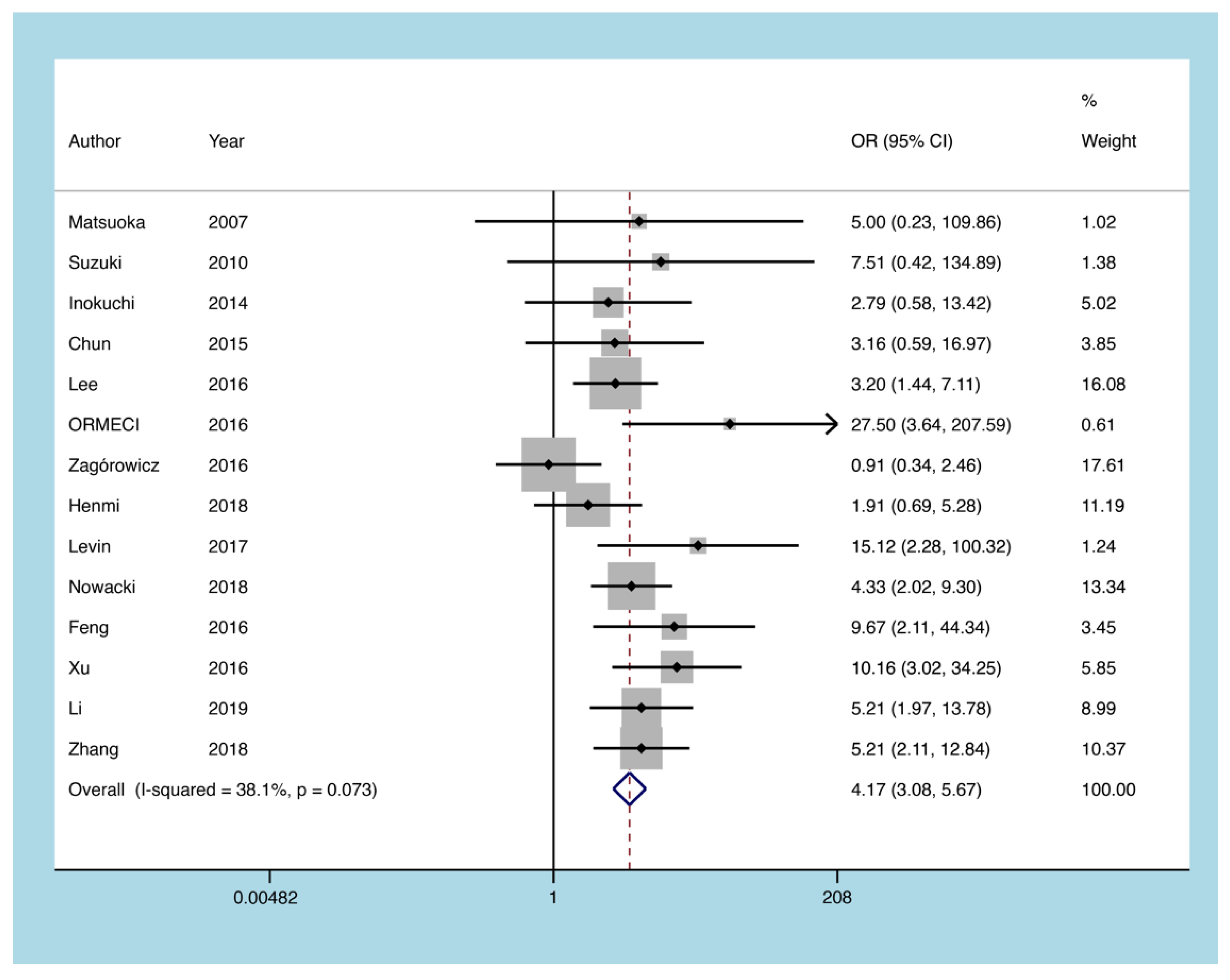
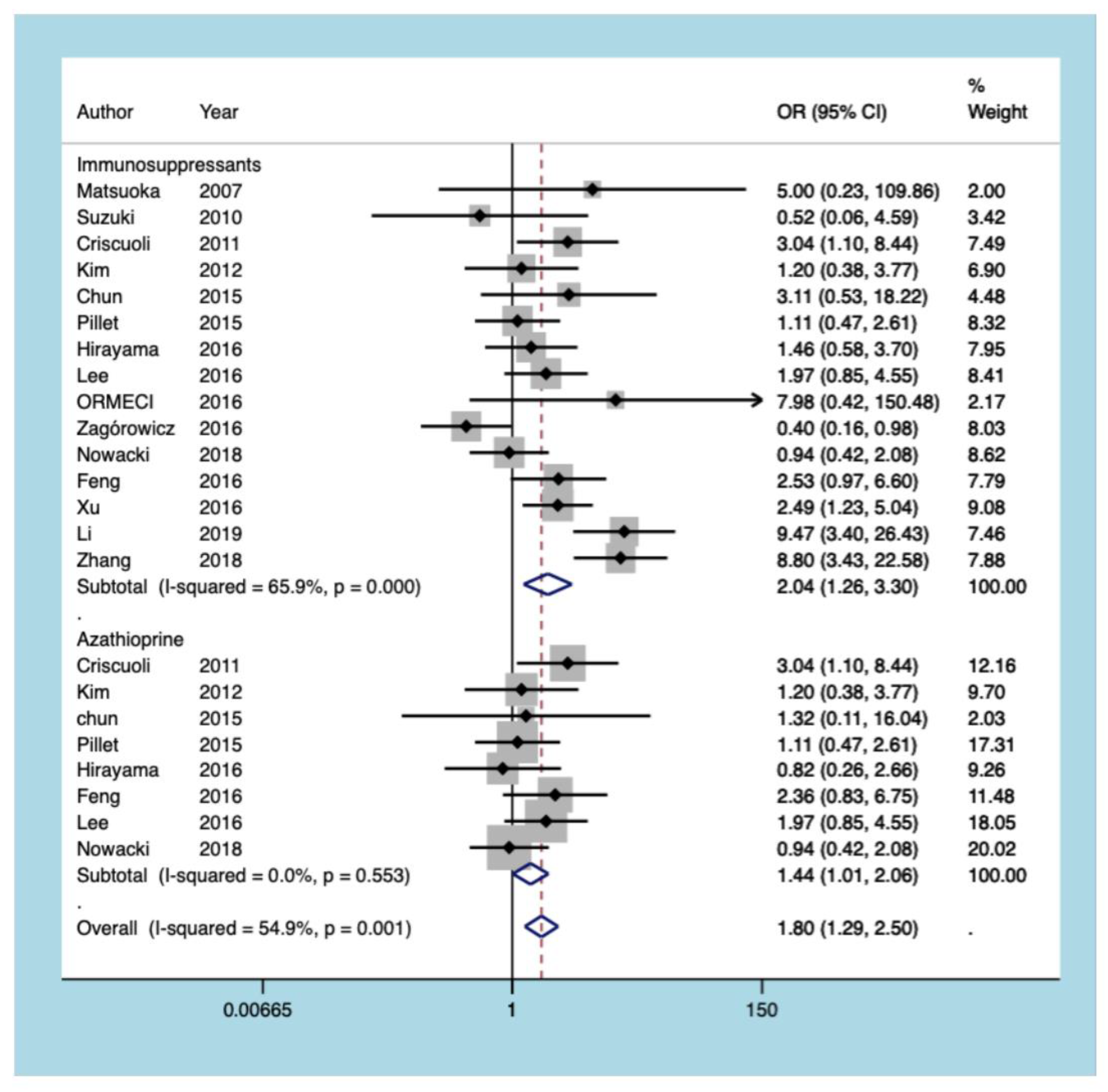
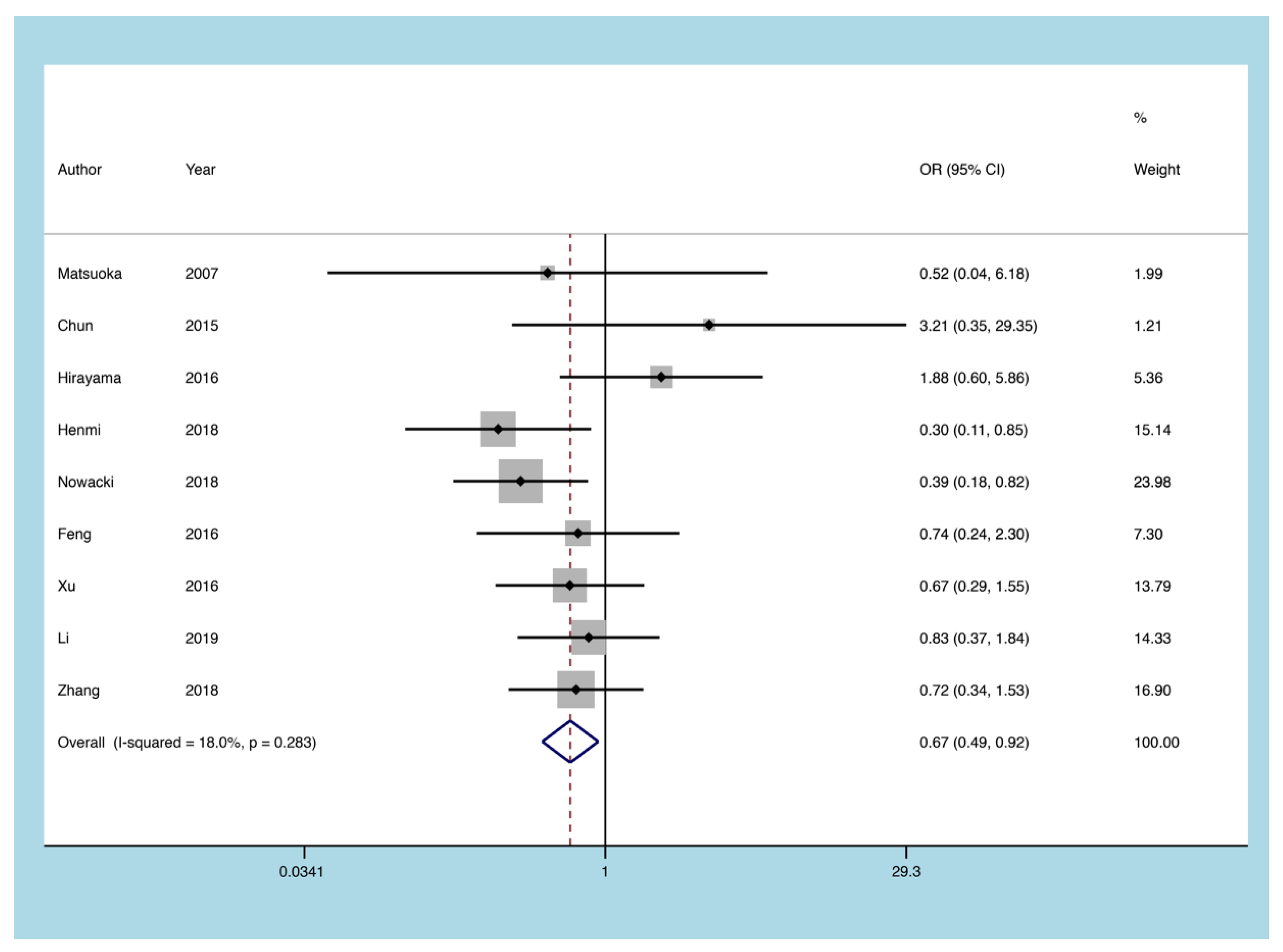
3.5. Outcomes of the Meta-Analysis
3.5.1. Severe UC
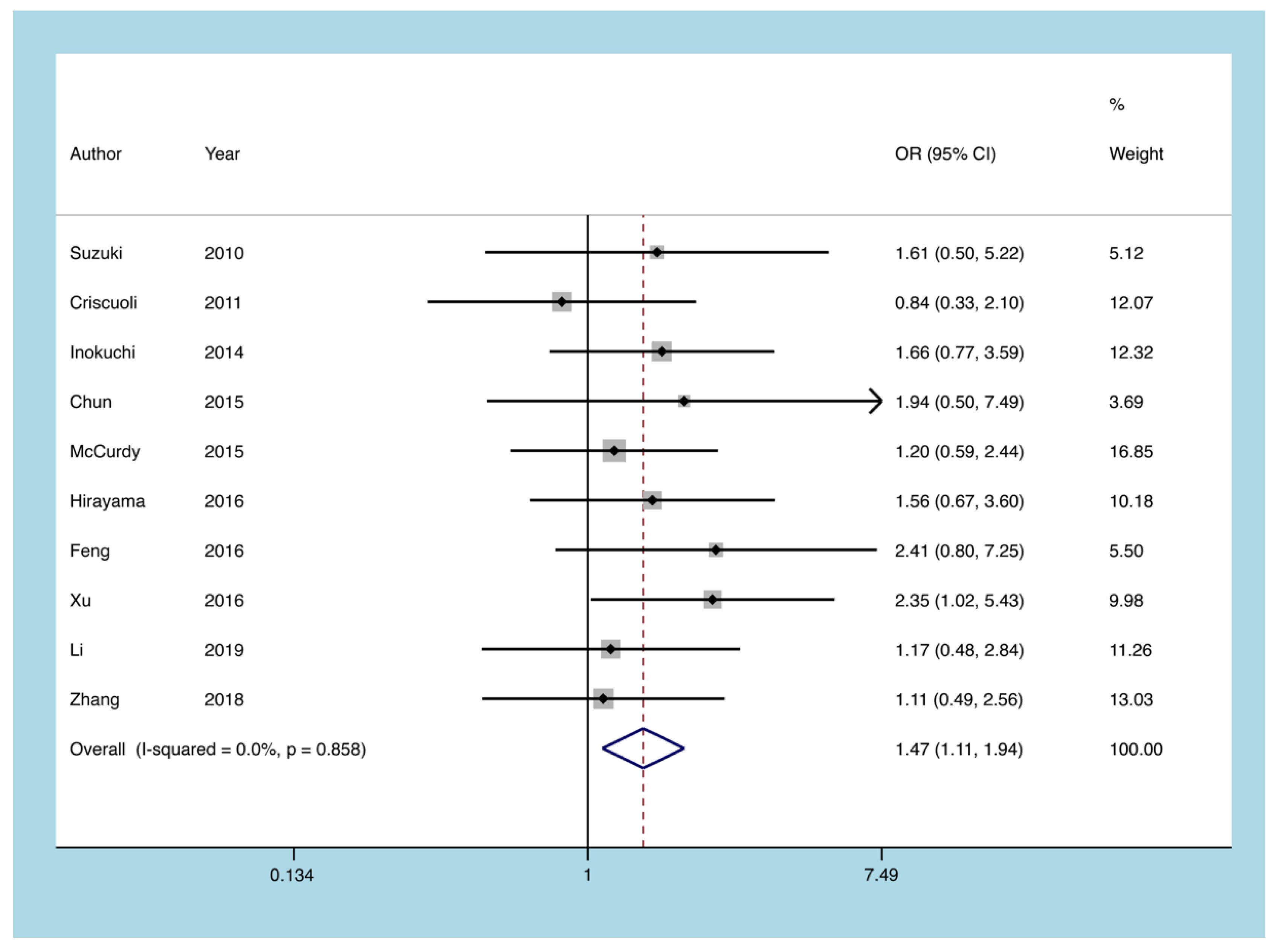
3.5.2. Pancolitis
3.5.3. Age of Onset
3.5.4. Glucocorticoids
3.5.5. Immunosuppressants
3.5.6. 5-ASA
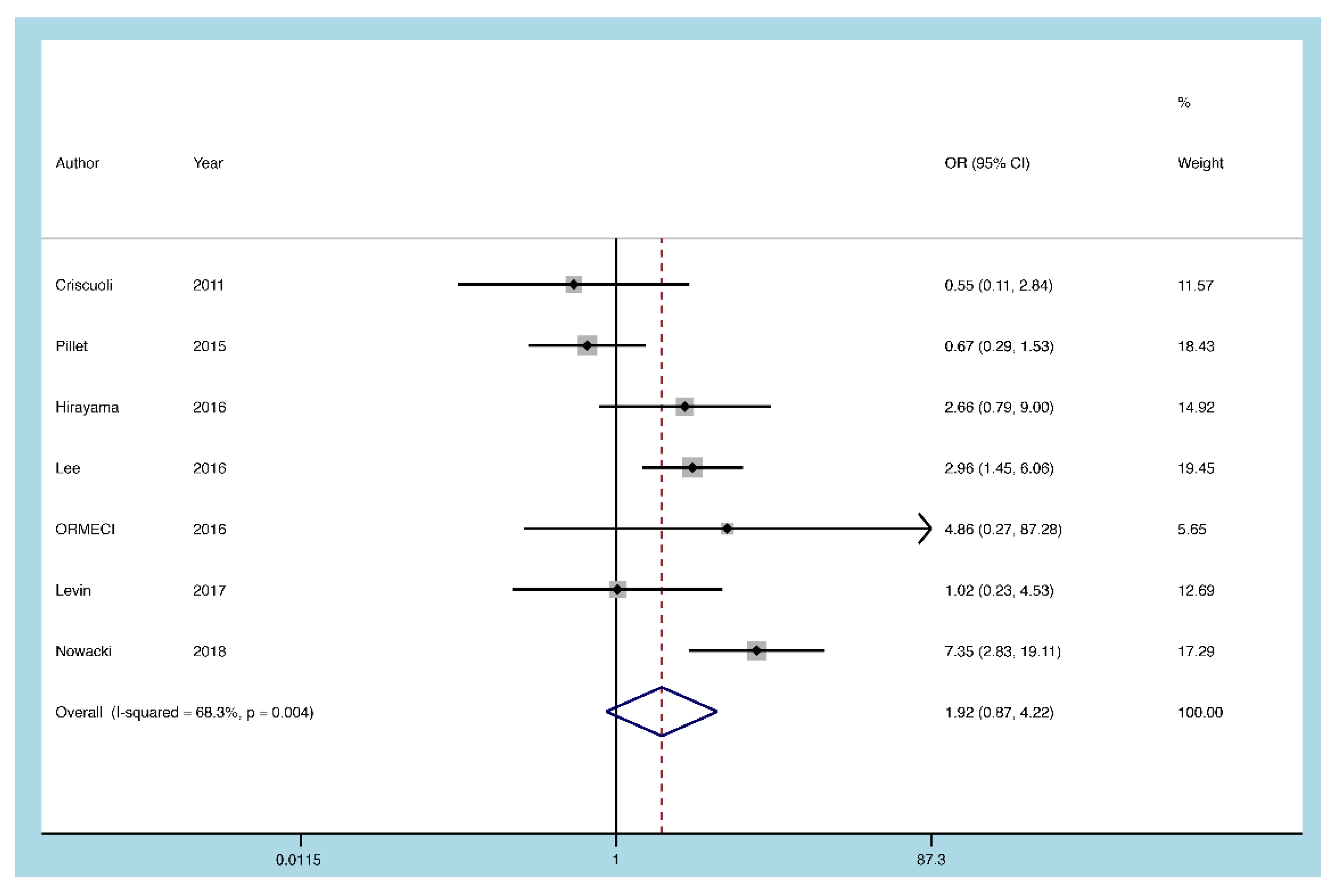
3.5.7. Infliximab
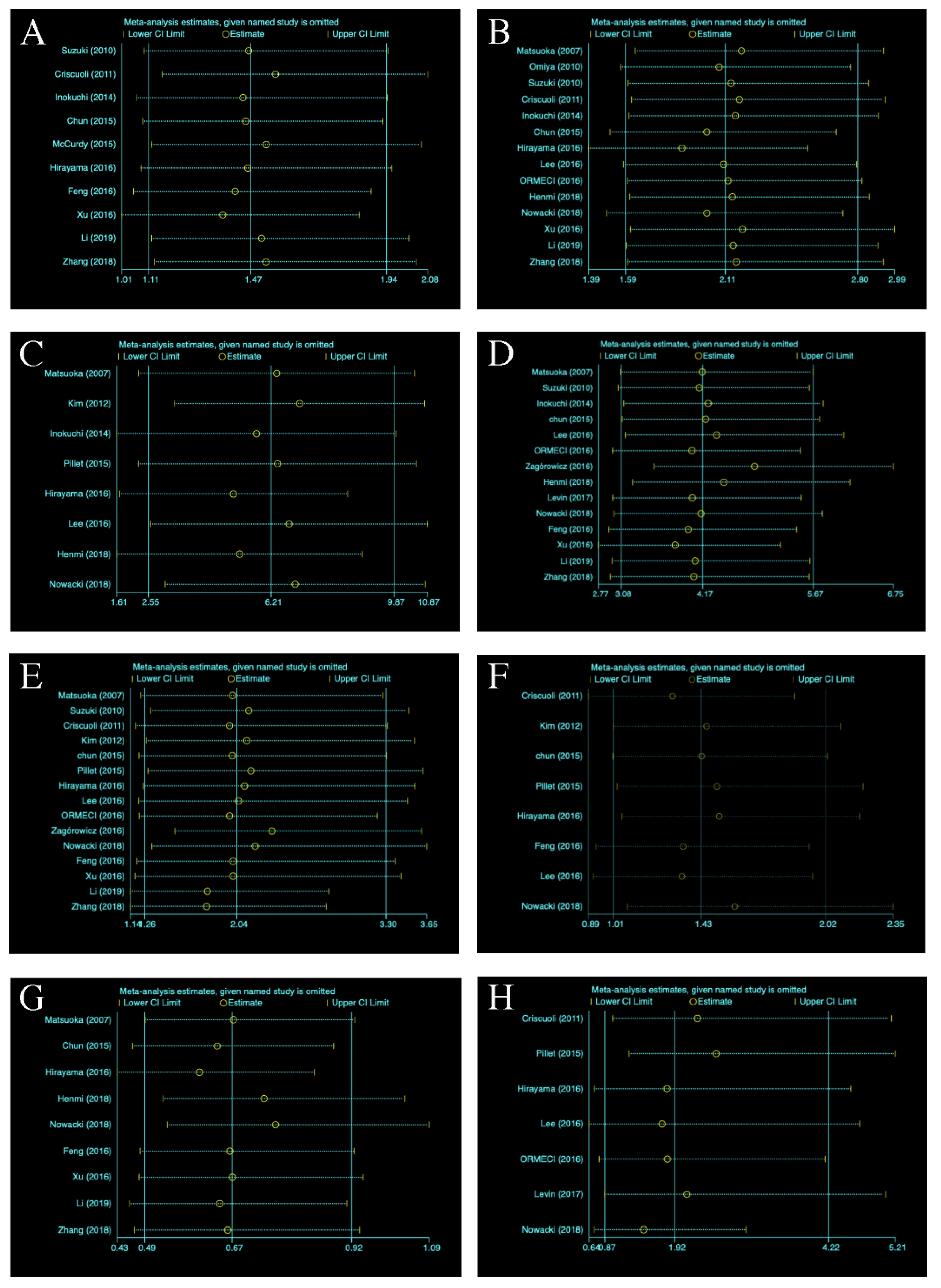
3.5.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Adams, S.M.; Bornemann, P.H. Ulcerative colitis. Am. Fam. Physician 2013, 87, 699–705. [Google Scholar]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.H.; Kim, S.H.; Kim, J.; Choi, J.; Lee, H.J.; Kim, W.S.; Lee, J.M.; Kwak, M.S.; Hwang, S.W.; et al. Risk Factors and Clinical Outcomes Associated with Cytomegalovirus Colitis in Patients with Acute Severe Ulcerative Colitis. Inflamm. Bowel. Dis. 2016, 22, 912–918. [Google Scholar] [CrossRef]
- Dowd, J.B.; Bosch, J.A.; Steptoe, A.; Jayabalasingham, B.; Lin, J.; Yolken, R.; Aiello, A.E. Persistent Herpesvirus Infections and Telomere Attrition Over 3 Years in the Whitehall II Cohort. J. Infect. Dis. 2017, 216, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, V.; Rizzuto, M.R.; Montalbano, L.; Gallo, E.; Cottone, M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J. Gastroenterol. 2011, 17, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Henmi, Y.; Kakimoto, K.; Inoue, T.; Nakazawa, K.; Kubota, M.; Hara, A.; Mikami, T.; Naka, Y.; Hirata, Y.; Hirata, Y.; et al. Cytomegalovirus infection in ulcerative colitis assessed by quantitative polymerase chain reaction: Risk factors and effects of immunosuppressants. J. Clin. Biochem. Nutr. 2018, 63, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Pozzetto, B.; Roblin, X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J. Gastroenterol. 2016, 22, 2030–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aggarwal, P.; Liu, X.; Lu, H.; Lian, L.; Wu, X.; Guo, S.; Aggarwal, N.; Lashner, B.; Shen, B. Antiviral Treatment for Colonic Cytomegalovirus Infection in Ulcerative Colitis Patients Significantly Improved Their Surgery Free Survival. J. Clin. Gastroenterol. 2018, 52, e27–e31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, Y.H.; Kim, J.S.; Cheon, J.H.; Ye, B.D.; Jung, S.A.; Park, Y.S.; Choi, C.H.; Jang, B.I.; Han, D.S.; et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: A prospective multicenter study. J. Clin. Gastroenterol. 2012, 46, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lee, C.K.; Kim, Y.-W.; Jeong, S.J.; Park, Y.M.; Oh, C.H.; Kim, J.-W.; Kim, H.J. True cytomegalovirus colitis is a poor prognostic indicator in patients with ulcerative colitis flares: The 10-year experience of an academic referral inflammatory bowel disease center. Scand. J. Gastroenterol. 2019, 54, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Racca, F.; Scudeller, L.; Piralla, A.; Formagnana, P.; Pozzi, L.; Betti, E.; Vanoli, A.; Riboni, R.; Kruzliak, P.; et al. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol. Res. 2016, 64, 191–203. [Google Scholar] [CrossRef]
- Shukla, T.; Singh, S.; Loftus, E.V., Jr.; Bruining, D.H.; McCurdy, J.D. Antiviral Therapy in Steroid-refractory Ulcerative Colitis with Cytomegalovirus: Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, A.; Li, L.; Liu, S.; Yang, F.; Yang, R. Meta-analysis of the Efficacy and Tolerability of Immune Checkpoint Inhibitors Combined with Chemotherapy in First-line Treatment of Small Cell Lung Cancer. Clin. Ther. 2021, 43, 582–593.e2. [Google Scholar] [CrossRef] [PubMed]
- Williams, Z.J.; Suzman, E.; Woynaroski, T.G. Prevalence of Decreased Sound Tolerance (Hyperacusis) in Individuals With Autism Spectrum Disorder: A Meta-Analysis. Ear Hear. 2021, 42, 1137–1150. [Google Scholar]
- Chun, J.; Lee, C.; Kwon, J.E.; Hwang, S.W.; Kim, S.G.; Kim, J.S.; Jung, H.C.; Im, J.P. Usefulness of the cytomegalovirus antigenemia assay in patients with ulcerative colitis. Intest. Res. 2015, 13, 50–59. [Google Scholar] [CrossRef][Green Version]
- Hirayama, Y.; Ando, T.; Hirooka, Y.; Watanabe, O.; Miyahara, R.; Nakamura, M.; Yamamura, T.; Goto, H. Characteristic endoscopic findings and risk factors for cytomegalovirus-associated colitis in patients with active ulcerative colitis. World J. Gastrointest. Endosc. 2016, 8, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, T.; Kato, J.; Hiraoka, S.; Suzuki, H.; Nakarai, A.; Hirakawa, T.; Akita, M.; Takahashi, S.; Harada, K.; Okada, H.; et al. Long-term follow-up of ulcerative colitis patients treated on the basis of their cytomegalovirus antigen status. World J. Gastroenterol. 2014, 20, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Yaari, S.; Stoff, R.; Caplan, O.; Wolf, D.G.; Israeli, E. Diagnosis of Cytomegalovirus Infection during Exacerbation of Ulcerative Colitis. Digestion 2017, 96, 142–148. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iwao, Y.; Mori, T.; Sakuraba, A.; Yajima, T.; Hisamatsu, T.; Okamoto, S.; Morohoshi, Y.; Izumiya, M.; Ichikawa, H.; et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am. J. Gastroenterol. 2007, 102, 331–337. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Jones, A.; Enders, F.T.; Killian, J.M.; Loftus, E.V., Jr.; Smyrk, T.C.; Bruining, D.H. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2015, 13, 131–137. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Bettenworth, D.; Meister, T.; Heidemann, J.; Lenze, F.; Schmidt, H.H.; Heinzow, H.S. Novel score predicts risk for cytomegalovirus infection in ulcerative colitis. J. Clin. Virol Off. Publ. Pan Am. Soc. Clin. Virol. 2018, 105, 103–108. [Google Scholar] [CrossRef]
- Omiya, M.; Matsushita, M.; Tanaka, T.; Kawamata, S.; Okazaki, K. The absence of large ulcer predicts latent cytomegalovirus infection in ulcerative colitis with positive mucosal viral assay. Intern. Med. 2010, 49, 2277–2282. [Google Scholar] [CrossRef]
- Ormeci, A.C.; Akyuz, F.; Baran, B.; Soyer, O.M.; Gokturk, S.; Onel, M.; Onel, D.; Agacfidan, A.; Demirci, M.; Yegen, G.; et al. Steroid-refractory inflammatory bowel disease is a risk factor for CMV infection. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 858–865. [Google Scholar] [PubMed]
- Pillet, S.; Jarlot, C.; Courault, M.; Del Tedesco, E.; Chardon, R.; Saint-Sardos, P.; Presles, E.; Phelip, J.M.; Berthelot, P.; Pozzetto, B.; et al. Infliximab Does Not Worsen Outcomes During Flare-ups Associated with Cytomegalovirus Infection in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 1580–1586. [Google Scholar] [CrossRef]
- Suzuki, H.; Kato, J.; Kuriyama, M.; Hiraoka, S.; Kuwaki, K.; Yamamoto, K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J. Gastroenterol. 2010, 16, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Zagorowicz, E.; Bugajski, M.; Wieszczy, P.; Pietrzak, A.; Magdziak, A.; Mroz, A. Cytomegalovirus Infection in Ulcerative Colitis is Related to Severe Inflammation and a High Count of Cytomegalovirus-positive Cells in Biopsy Is a Risk Factor for Colectomy. J. Crohn’s Colitis 2016, 10, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Yuhong, Z.; Xinpu, M. Clinical characteristics and risk factors of ulcerative colitis complicated with cytomegalovirus infection. Pract. Prev. Med. 2018, 3, 25. [Google Scholar]
- Tao, L.; Ping, Z. Clinical Characteristics and Risk Factors of Cytomegalovirus Infection in Patients with Ulcerative Colitis. J. Prev. Med. Chin. PLA 2019, 1, 37. [Google Scholar]
- Ting, F.; Minhu, C.; Yao, H.; Zhirong, Z.; Baili, C. Clinical features analysis of ulcerative colitis complicated with cytomegalovirus infection. Chin. J. Dig. 2016, 2, 78–85. [Google Scholar]
- Gang, X.; Linman, Y.; Xingyuan, H. Risk factors, clinical features and clinical outcome of cytomegalovirus infection in patients with ulcreative colitis. Chin. J. Gastroenterol. Hepatol. 2016, 10, 25. [Google Scholar]
- Uchino, M.; Matsuoka, H.; Bando, T.; Hirata, A.; Sasaki, H.; Hirose, K.; Takesue, Y.; Nakamura, S.; Tomita, N.; Ikeuchi, H. Clinical features and treatment of ulcerative colitis-related severe gastroduodenitis and enteritis with massive bleeding after colectomy. Int. J. Colorectal. Dis. 2014, 29, 239–245. [Google Scholar] [CrossRef]
- Gullett, J.C.; Nolte, F.S. Quantitative nucleic acid amplification methods for viral infections. Clin. Chem. 2015, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Fish, K.N.; Nelson, J.A. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Investig. 1997, 100, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Shimamura, M.; Musgrove, L.C.; Dennis, E.A.; Bimczok, D.; Novak, L.; Ballestas, M.; Fenton, A.; Dandekar, S.; Britt, W.J.; et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J. Immunol. 2014, 193, 5604–5612. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Fish, K.N.; Nelson, J.A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997, 91, 119–126. [Google Scholar] [CrossRef]
- Hahn, G.; Jores, R.; Mocarski, E.S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3937–3942. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C.; Nelson, J.Y. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host’s immune system. Intervirology 1999, 42, 314–321. [Google Scholar] [CrossRef]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late developmental plasticity in the T helper 17 lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Langner, C.; Magro, F.; Driessen, A.; Ensari, A.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Borralho Nunes, P.; Cathomas, G.; Fries, W.; et al. The histopathological approach to inflammatory bowel disease: A practice guide. Virchows Arch. 2014, 464, 511–527. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | Study Design | Study Group | Gender | Age * | Disease Duration (Years) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMV+ | CMV− | CMV+ (M/F) | CMV− (M/F) | CMV+ | CMV− | CMV+ | CMV− | ||||
| Matsuoka [25] | 2007 | Japan | Retrospective | 25 | 23 | 12/13 | 17/6 | 42 ± 14.5 | 37.2 ± 11.2 | 4.85 ± 3.85 | 5.35 ± 5.00 |
| Omiya [28] | 2010 | Japan | Prospective | 7 | 13 | NA | NA | NA | NA | NA | NA |
| Suzuki [31] | 2010 | Japan | Retrospective | 15 | 58 | 8/7 | 26/32 | NA | NA | 7.14 ± 7.41 | 4.12 ± 7.39 |
| Criscuoli [8] | 2011 | Italy | Prospective | 28 | 57 | 19/9 | 31/26 | NA | NA | NA | NA |
| Kim [12] | 2012 | Korea | Prospective | 31 | 41 | 20/11 | 28/13 | 43.1 ± 15.4 | 43.4 ± 14.1 | 2.4 ± 2.65 | 3.7 ± 4.94 |
| Inokuchi [23] | 2014 | Japan | Retrospective | 40 | 78 | 26/14 | 31/47 | 45 ± 14.2 | 36 ± 12.5 | 1.5 ± 0.60 | 4.6 ± 0.53 |
| Chun [21] | 2015 | Korea | Retrospective | 12 | 31 | 6/6 | 14/17 | NA | NA | NA | NA |
| McCurdy [26] | 2015 | USA | Retrospective | 45 | 139 | NA | NA | NA | NA | NA | NA |
| Pillet [30] | 2015 | France | Prospective | 39 | 70 | 28/11 | 44/26 | 51.2 ± 17.0 | 46.5 ± 18.0 | 6.59 ± 7.00 | 6.67 ± 6.75 |
| Hirayama [22] | 2016 | Japan | Retrospective | 34 | 115 | 19/15 | 64/51 | 42.3 ± 14.4 | 29.0 ± 14.4 | 4.6 ± 4.9 | 6.0 ± 7.4 |
| Lee [6] | 2016 | Korea | Retrospective | 50 | 99 | 30/20 | 50/49 | 45 ± 14.75 | 42 ± 10.33 | 2.2 ± 3.25 | 4.7 ± 4.83 |
| ORMECI [29] | 2016 | Turkey | Retrospective | 8 | 35 | 6/2 | 17/18 | NA | NA | NA | NA |
| Zagórowicz [32] | 2016 | Poland | Retrospective | 33 | 62 | 22/11 | 39/23 | NA | NA | NA | NA |
| Henmi [9] | 2018 | Japan | Retrospective | 26 | 60 | 15/11 | 38/22 | 55.0 ± 13.2 | 41.8 ± 16.2 | 5.95 ± 5.07 | 6.67 ± 7.45 |
| Levin [24] | 2017 | USA | Retrospective | 13 | 15 | 6/7 | 12/3 | NA | NA | NA | NA |
| Nowacki [27] | 2018 | Germany | Retrospective | 34 | 205 | 25/9 | 108/97 | 37 ± 17.8 | 36 ± 18.9 | 9.0 ± 8.4 | 4.4 ± 7.3 |
| Feng [35] | 2016 | China | Retrospective | 31 | 60 | 18/13 | 39/21 | NA | NA | NA | NA |
| Xu [36] | 2016 | China | Retrospective | 49 | 143 | 28/21 | 82/61 | NA | NA | NA | NA |
| Li [34] | 2019 | China | Retrospective | 34 | 91 | 21/13 | 49/42 | NA | NA | 3.92 ± 1.83 | 4.84 ± 2.31 |
| Zhang [33] | 2018 | China | Retrospective | 37 | 113 | 23/14 | 61/52 | NA | NA | 3.94 ± 1.95 | 4.86 ± 2.32 |
| Study | Year | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|---|
| Matsuoka | 2007 | 4 | 3 | 2 | 9 |
| Omiya | 2010 | 4 | 2 | 2 | 8 |
| Suzuki | 2010 | 4 | 2 | 3 | 9 |
| Criscuoli | 2011 | 4 | 2 | 2 | 8 |
| Kim | 2012 | 4 | 2 | 3 | 9 |
| Inokuchi | 2014 | 3 | 3 | 2 | 8 |
| Chun | 2015 | 4 | 2 | 3 | 9 |
| McCurdy | 2015 | 4 | 2 | 3 | 9 |
| Pillet | 2015 | 4 | 2 | 3 | 9 |
| Hirayama | 2016 | 3 | 2 | 3 | 8 |
| Lee | 2016 | 4 | 2 | 3 | 9 |
| ORMECI | 2016 | 4 | 2 | 2 | 8 |
| Zagórowicz | 2016 | 3 | 2 | 2 | 7 |
| Henmi | 2018 | 3 | 2 | 3 | 8 |
| Levin | 2017 | 4 | 2 | 3 | 9 |
| Nowacki | 2018 | 4 | 1 | 3 | 8 |
| Feng | 2016 | 4 | 1 | 3 | 8 |
| Xu | 2016 | 4 | 2 | 2 | 8 |
| Li | 2019 | 3 | 2 | 2 | 7 |
| Zhang | 2018 | 4 | 1 | 3 | 8 |
| Number of Studies | Std_Eff | t | p > |t| | 95% CI | ||
|---|---|---|---|---|---|---|
| Severe UC | 10 | Slope | −0.24 | 0.813 | −1.521 | 1.231 |
| Bias | 0.88 | 0.404 | −1.866 | 4.172 | ||
| Pancolitis | 14 | Slope | 1.66 | 0.123 | −0.206 | −1.442 |
| Bias | 0.17 | 0.868 | −1.442 | 1.686 | ||
| Age of onset | 8 | Slope | 0.87 | 0.419 | −22.170 | 9.180 |
| Bias | −0.42 | 0.690 | −12.965 | 9.180 | ||
| Glucocorticoids | 14 | Slope | 1.28 | 0.224 | −0.477 | 1.841 |
| Bias | 1.40 | 0.186 | −0.688 | 3.184 | ||
| Immunosuppressants | 15 | Slope | 0.48 | 0.642 | −1.186 | 1.854 |
| Bias | 0.50 | 0.623 | −2.391 | 3.598 | ||
| Azathioprine | 8 | Slope | 0.41 | 0.699 | −1.430 | 1.998 |
| Bias | 0.12 | 0.906 | −3.169 | 3.507 | ||
| 5-ASA | 9 | Slope | −1.66 | 0.140 | −2.285 | 0.399 |
| Bias | 0.99 | 0.354 | −1.554 | 3.803 | ||
| Infliximab | 7 | Slope | 0.87 | 0.423 | −1.860 | 3.770 |
| Bias | −0.24 | 0.821 | −5.632 | 4.675 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Wang, G.; Kong, D.; Li, G.; Wang, H.; Qin, H.; Wang, H. Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis. Diagnostics 2021, 11, 1952. https://doi.org/10.3390/diagnostics11111952
Qin Y, Wang G, Kong D, Li G, Wang H, Qin H, Wang H. Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis. Diagnostics. 2021; 11(11):1952. https://doi.org/10.3390/diagnostics11111952
Chicago/Turabian StyleQin, Yafei, Grace Wang, Dejun Kong, Guangming Li, Hongda Wang, Hong Qin, and Hao Wang. 2021. "Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis" Diagnostics 11, no. 11: 1952. https://doi.org/10.3390/diagnostics11111952
APA StyleQin, Y., Wang, G., Kong, D., Li, G., Wang, H., Qin, H., & Wang, H. (2021). Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis. Diagnostics, 11(11), 1952. https://doi.org/10.3390/diagnostics11111952





