Quantitative Assessment of Three-Dimensional Choroidal Vascularity and Choriocapillaris Flow Signal Voids in Myopic Patients Using SS-OCTA
Abstract
:1. Introduction
2. Methods
2.1. Participants
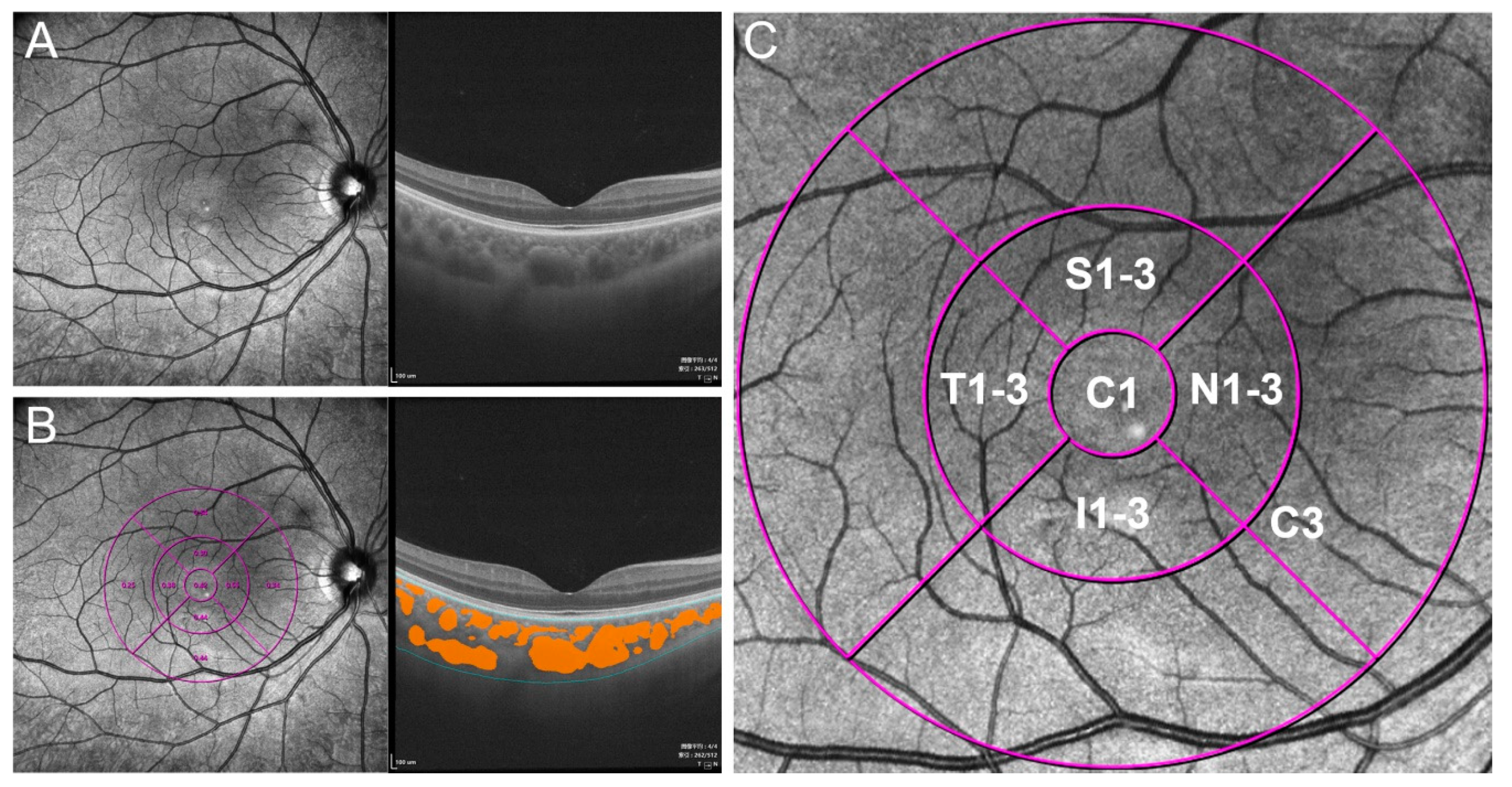
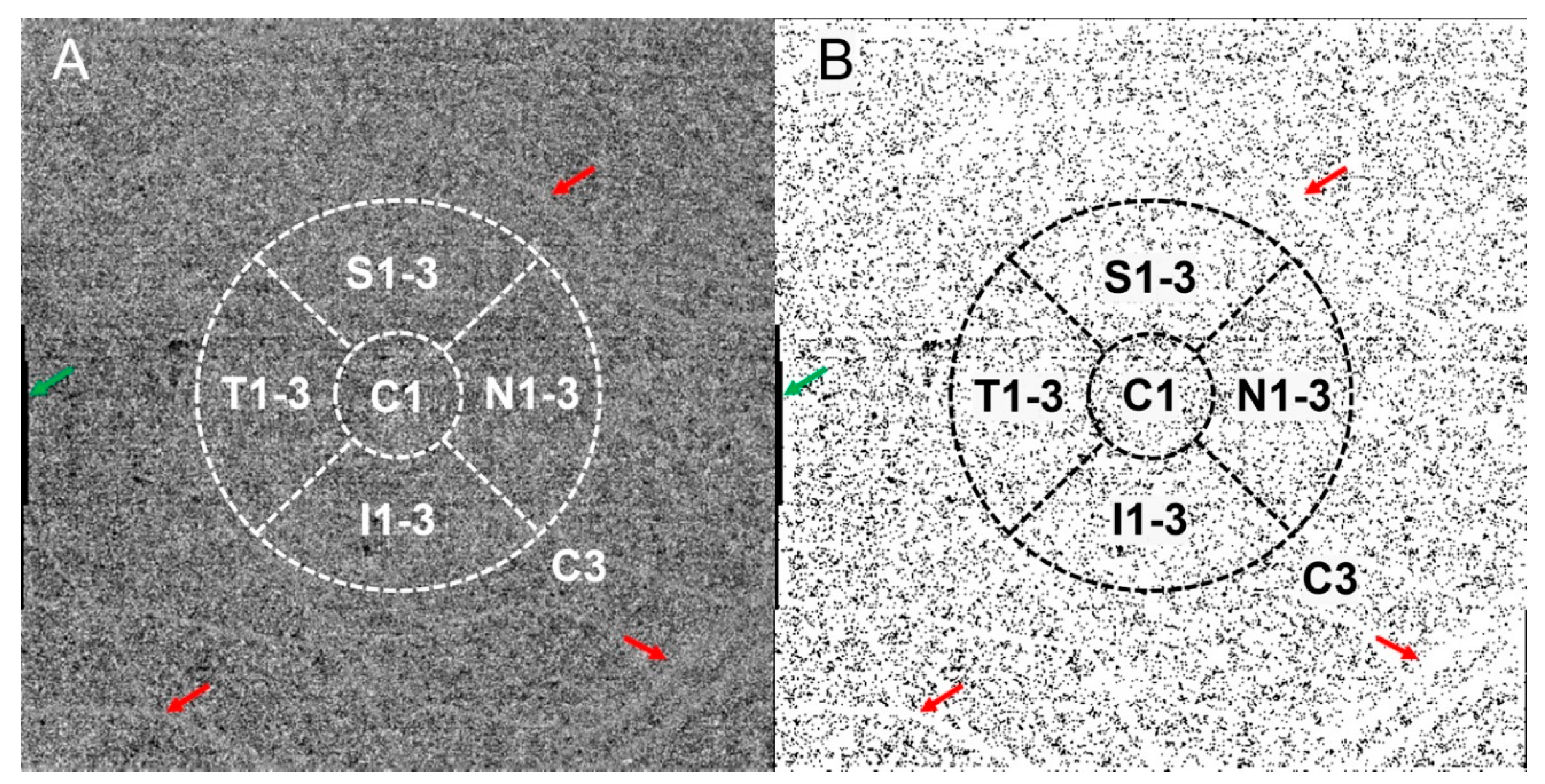
2.2. Image Acquisition and Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
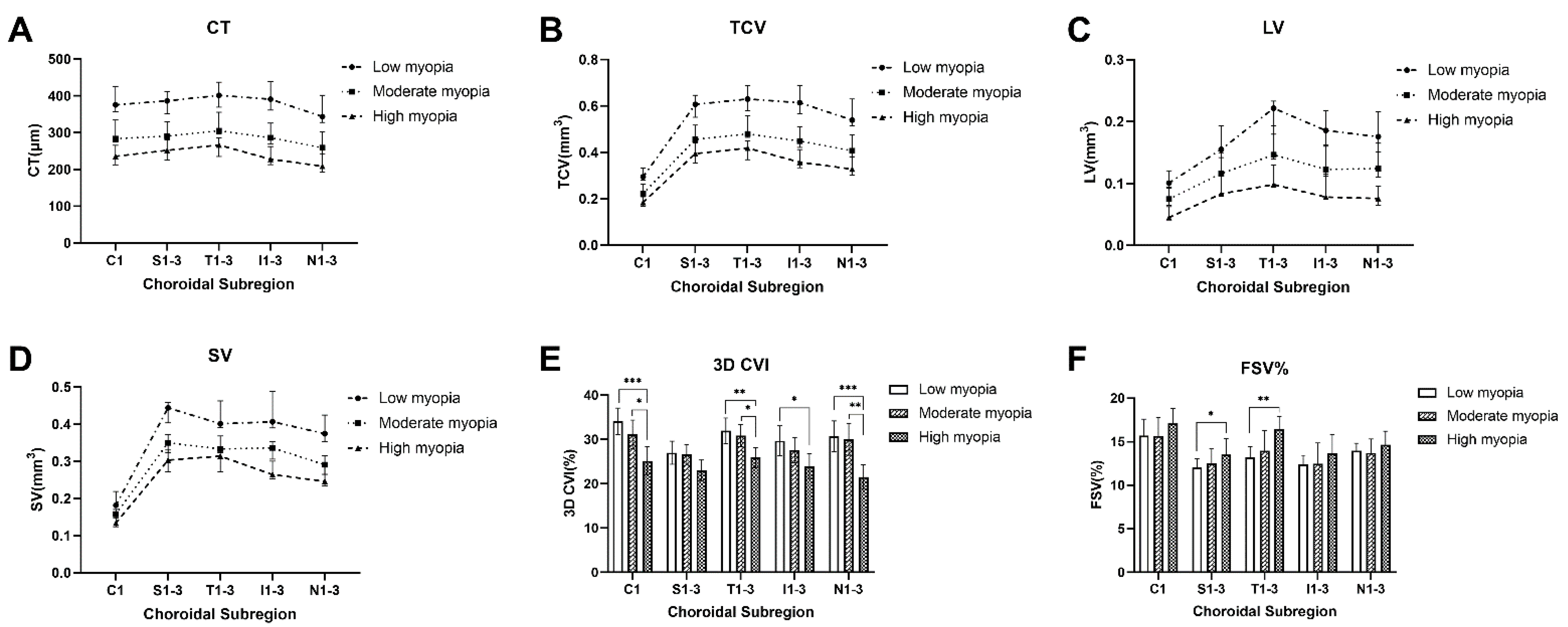
3.2. Global Analysis of Differences in Choroidal Vascularity and Choriocapillaris
3.3. Topographical Analysis of Differences in Choroidal Vascularity and Choriocapillaris
3.4. Correlation between Choroidal Parameters and Age and AL
3.5. Correlation between Choroidal Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, L.; Xu, Y.; Pang, Z.; Mu, G. The influence of the choroid on the onset and development of myopia: From perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 2021, 99, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Medrano, J.; Montero, J.A.; Flores-Moreno, I.; Arias, L.; García-Layana, A.; Ruiz-Moreno, J.M. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog. Retin. Eye Res. 2019, 69, 80–115. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moreno, I.; Ruiz-Medrano, J.; Duker, J.S.; Ruiz-Moreno, J.M. The relationship between retinal and choroidal thickness and visual acuity in highly myopic eyes. Br. J. Ophthalmol. 2013, 97, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, G.; Zhou, X.; Xu, R.; Wang, S.; Guan, Z.; Lu, J.; Srinivasalu, N.; Shen, M.; Jin, Z.; et al. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Investig. Opthalmol. Vis. Sci. 2019, 60, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhang, G.; Shen, M.; Xu, R.; Wang, P.; Guan, Z.; Xie, Z.; Jin, Z.; Chen, S.; Mao, X.; et al. Assessment of choroidal vascularity and choriocapillaris blood perfusion in anisomyopic adults by SS-OCT/OCTA. Investig. Opthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 2015, 159, 1123–1131.e1. [Google Scholar] [CrossRef]
- Yang, J.; Wang, E.; Yuan, M.; Chen, Y. Three-dimensional choroidal vascularity index in acute central serous chorioretinopathy using swept-source optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 258, 241–247. [Google Scholar] [CrossRef]
- Zhou, H.; Dai, Y.; Shi, Y.; Russell, J.F.; Lyu, C.; Noorikolouri, J.; Feuer, W.J.; Chu, Z.; Zhang, Q.; de Sisternes, L.; et al. Age-Related Changes in Choroidal Thickness and the Volume of Vessels and Stroma Using Swept-Source OCT and Fully Automated Algorithms. Ophthalmol. Retin. 2020, 4, 204–215. [Google Scholar] [CrossRef]
- Haarman, A.E.G.; Enthoven, C.A.; Tideman, J.W.L.; Tedja, M.S.; Verhoeven, V.J.M.; Klaver, C.C.W. The complications of myopia: A review and meta-analysis. Investig. Opthalmol. Vis. Sci. 2020, 61, 49. [Google Scholar] [CrossRef]
- Lee, S.W.; Yu, S.-Y.; Seo, K.H.; Kim, E.S.; Kwak, H.W. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy korean subjects. Retina 2014, 34, 385–393. [Google Scholar] [CrossRef]
- Sauvola, J.; Pietikäinen, M. Adaptive document image binarization. Pattern Recognit. 2000, 33, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, E.; Sarraf, D.; Freund, K.B.; Sadda, S.R. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res. 2018, 67, 30–55. [Google Scholar] [CrossRef]
- Jin, P.; Zou, H.; Zhu, J.; Xu, X.; Jin, J.; Chang, T.C.; Lu, L.; Yuan, H.; Sun, S.; Yan, B.; et al. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am. J. Ophthalmol. 2016, 168, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; He, X.; Zhang, B.; Deng, J.; Wang, J.; Lv, M.; Zhu, J.; Zou, H.; Xu, X. Changes in choroidal thickness varied by age and refraction in children and adolescents: A 1-year longitudinal study. Am. J. Ophthalmol. 2020, 213, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gupta, P.; Tan, K.-A.; Cheung, C.M.G.; Wong, T.-Y.; Cheng, C.-Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef]
- Gupta, P.; Thakku, S.G.; Saw, S.-M.; Tan, M.; Lim, E.; Tan, M.; Cheung, C.M.G.; Wong, T.-Y.; Cheng, C.-Y. Characterization of choroidal morphologic and vascular features in young men with high myopia using spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2017, 177, 27–33. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, C.; Wang, X.; Zhou, W.; Reinach, P.; Qu, J. Choroidal blood perfusion as a potential “rapid predictive index” for myopia development and progression. Eye Vis. 2021, 8, 1–5. [Google Scholar] [CrossRef]
- Read, S.A.; Alonso-Caneiro, D.; Vincent, S.J.; Collins, M. Longitudinal changes in choroidal thickness and eye growth in childhood. Investig. Opthalmol. Vis. Sci. 2015, 56, 3103. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Long, W.; Hu, Y.; Zhao, W.; Zhang, W.; Yang, X. Features of the choroidal structures in myopic children based on image binarization of optical coherence tomography. Investig. Opthalmol. Vis. Sci. 2020, 61, 18. [Google Scholar] [CrossRef] [Green Version]
- Alshareef, R.A.; Khuthaila, M.K.; Januwada, M.; Goud, A.; Ferrara, D.; Chhablani, J. Choroidal vascular analysis in myopic eyes: Evidence of foveal medium vessel layer thinning. Int. J. Retin. Vitr. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Investig. Opthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Zhang, Q.; Shi, Y.; Russell, J.F.; Motulsky, E.H.; Banta, J.T.; Chu, Z.; Zhou, H.; Patel, N.A.; de Sisternes, L.; et al. Age-dependent changes in the macular choriocapillaris of normal eyes imaged with swept-source optical coherence tomography angiography. Am. J. Ophthalmol. 2019, 200, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ji, Y.-S.; Tong, N.; Sarraf, D.; He, X.; Sun, X.; Xu, X.; Sadda, S.R. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, S.; Zhang, G.; Chen, Y.; Lei, Y.; Xiang, J.; Xu, R.; Qu, J.; Zhou, X. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Investig. Opthalmol. Vis. Sci. 2020, 61, 25. [Google Scholar] [CrossRef] [PubMed]
- Breher, K.; Terry, L.; Bower, T.; Wahl, S. Choroidal biomarkers: A repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Singh, S.R.; Invernizzi, A.; Cagini, C.; Goud, A.; Sahoo, N.K.; Cozzi, M.; Lupidi, M.; Chhablani, J. Wide-field choroidal thickness profile in healthy eyes. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Mohler, K.J.; Draxinger, W.; Klein, T.; Kolb, J.P.; Wieser, W.; Haritoglou, C.; Kampik, A.; Fujimoto, J.G.; Neubauer, A.S.; Huber, R.; et al. Combined 60° wide-field choroidal thickness maps and high-definition en face vasculature visualization using swept-source megahertz OCT at 1050 nm. Investig. Opthalmol. Vis. Sci. 2015, 56, 6284–6293. [Google Scholar] [CrossRef] [Green Version]
- Mendrinos, E.; Pournaras, C.J. Topographic variation of the choroidal watershed zone and its relationship to neovascularization in patients with age-related macular degeneration. Acta Ophthalmol. 2009, 87, 290–296. [Google Scholar] [CrossRef]
| Characteristic | Low Myopia (n = 32) | Moderate Myopia (n = 36) | High Myopia (n = 45) | p Value |
|---|---|---|---|---|
| Age, y, median (IQR) | 25.50 (8.00) | 24.50 (13.75) | 28.00 (10.00) | 0.253 * |
| Female, n (%) | 24 (66.67%) | 28 (62.22%) | 14 (46.7%) | 0.667 † |
| AL, mm, mean ± SD | 23.78 ± 0.92 | 25.30 ± 0.97 | 26.55 ± 1.19 | <0.001 ‡ |
| Spherical equivalent, D, median (IQR) | −1.50 (1.44) | −4.75 (1.25) | −7.00 (1.88) | <0.001 * |
| Age | AL | ||||||
|---|---|---|---|---|---|---|---|
| Variables | B | β Coefficient | p Value | B | β Coefficient | p Value | Adjusted R2 |
| CT_C1 (μm) | −0.251 | 0.024 | 0.762 | −42.265 | −0.599 | <0.001 | 0.365 |
| CT_C3 (μm) | −0.439 | −0.043 | 0.577 | −39.782 | −0.594 | <0.001 | 0.365 |
| TCV_C1 (mm3) | −0.000 | −0.024 | 0.762 | −0.033 | −0.599 | <0.001 | 0.365 |
| TCV_C3 (mm3) | −0.003 | −0.043 | 0.577 | −0.281 | −0.594 | <0.001 | 0.365 |
| LV_C1 (mm3) | −0.000 | −0.070 | 0.370 | −0.017 | −0.586 | <0.001 | 0.364 |
| LV_C3 (mm3) | −0.003 | −0.097 | 0.221 | −0.121 | −0.557 | <0.001 | 0.341 |
| SV_C1 (mm3) | −0.000 | 0.023 | 0.788 | −0.016 | −0.513 | <0.001 | 0.202 |
| SV_C3 (mm3) | 0.000 | 0.002 | 0.978 | −0.160 | −0.560 | <0.001 | 0.313 |
| 3D CVI_C1 (%) | −0.163 | −0.161 | 0.055 | −3.065 | −0.459 | <0.001 | 0.266 |
| 3D CVI_C3 (%) | −0.151 | −0.195 | 0.025 | −2.032 | −0.397 | <0.001 | 0.226 |
| FSV%_C1 (%) | 0.175 | 0.475 | <0.001 | 0.056 | 0.023 | 0.787 | 0.231 |
| FSV%_C3 (%) | 0.155 | 0.467 | <0.001 | 0.341 | 0.156 | 0.063 | 0.271 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Sun, G.; Duan, C.; Chen, Z.; Chen, C. Quantitative Assessment of Three-Dimensional Choroidal Vascularity and Choriocapillaris Flow Signal Voids in Myopic Patients Using SS-OCTA. Diagnostics 2021, 11, 1948. https://doi.org/10.3390/diagnostics11111948
Xu A, Sun G, Duan C, Chen Z, Chen C. Quantitative Assessment of Three-Dimensional Choroidal Vascularity and Choriocapillaris Flow Signal Voids in Myopic Patients Using SS-OCTA. Diagnostics. 2021; 11(11):1948. https://doi.org/10.3390/diagnostics11111948
Chicago/Turabian StyleXu, Amin, Gongpeng Sun, Chaoye Duan, Zhen Chen, and Changzheng Chen. 2021. "Quantitative Assessment of Three-Dimensional Choroidal Vascularity and Choriocapillaris Flow Signal Voids in Myopic Patients Using SS-OCTA" Diagnostics 11, no. 11: 1948. https://doi.org/10.3390/diagnostics11111948
APA StyleXu, A., Sun, G., Duan, C., Chen, Z., & Chen, C. (2021). Quantitative Assessment of Three-Dimensional Choroidal Vascularity and Choriocapillaris Flow Signal Voids in Myopic Patients Using SS-OCTA. Diagnostics, 11(11), 1948. https://doi.org/10.3390/diagnostics11111948






