Diagnostic Accuracy of Non-Invasive Imaging for Detection of Colonic Inflammation in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods, Types of Studies, and Participants
2.2. Index Tests and Target Conditions
2.3. Data Collection and Analysis
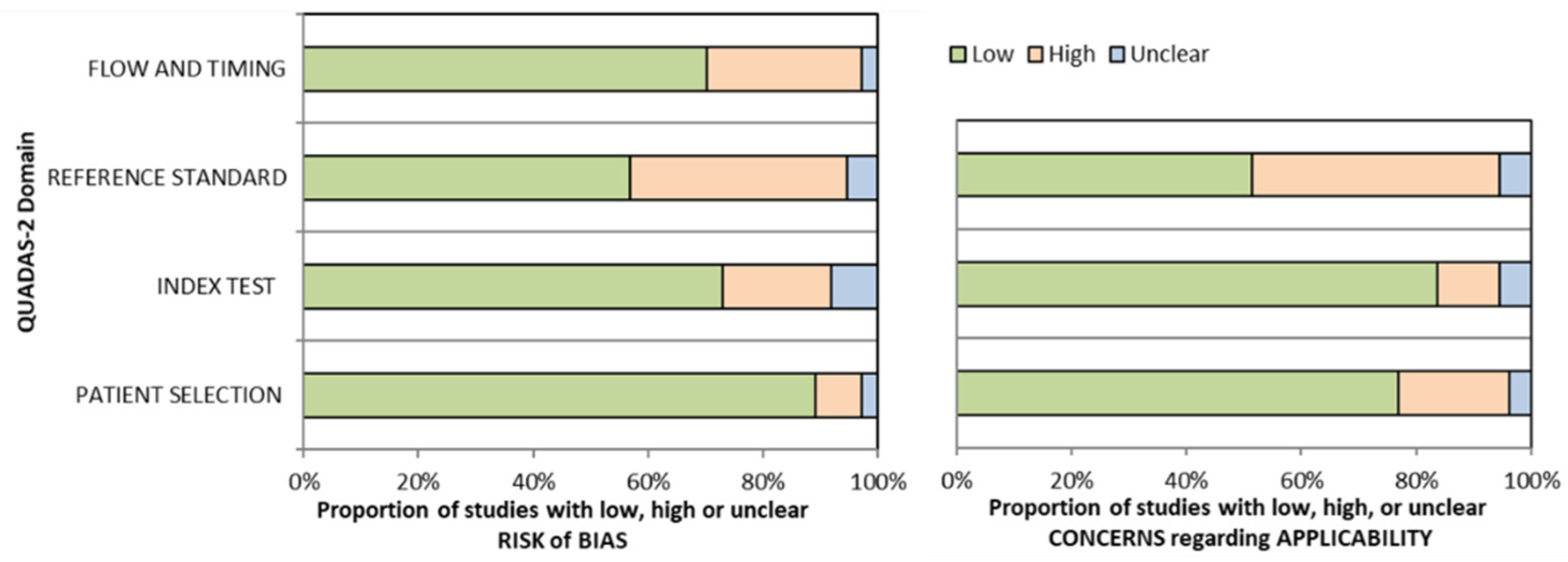
2.4. Risk of Bias Assessment
2.5. Statistical Analysis and Data Synthesis
3. Results
3.1. Results of the Search
3.2. Risk of Bias Assessment
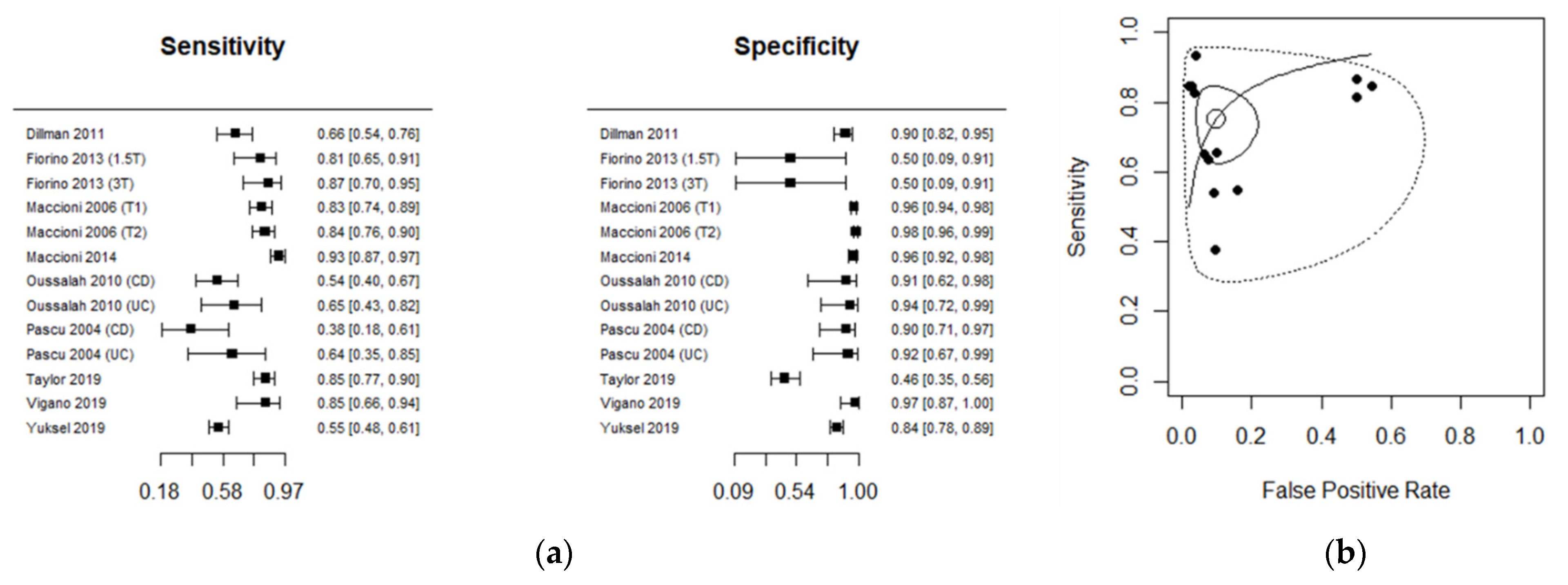
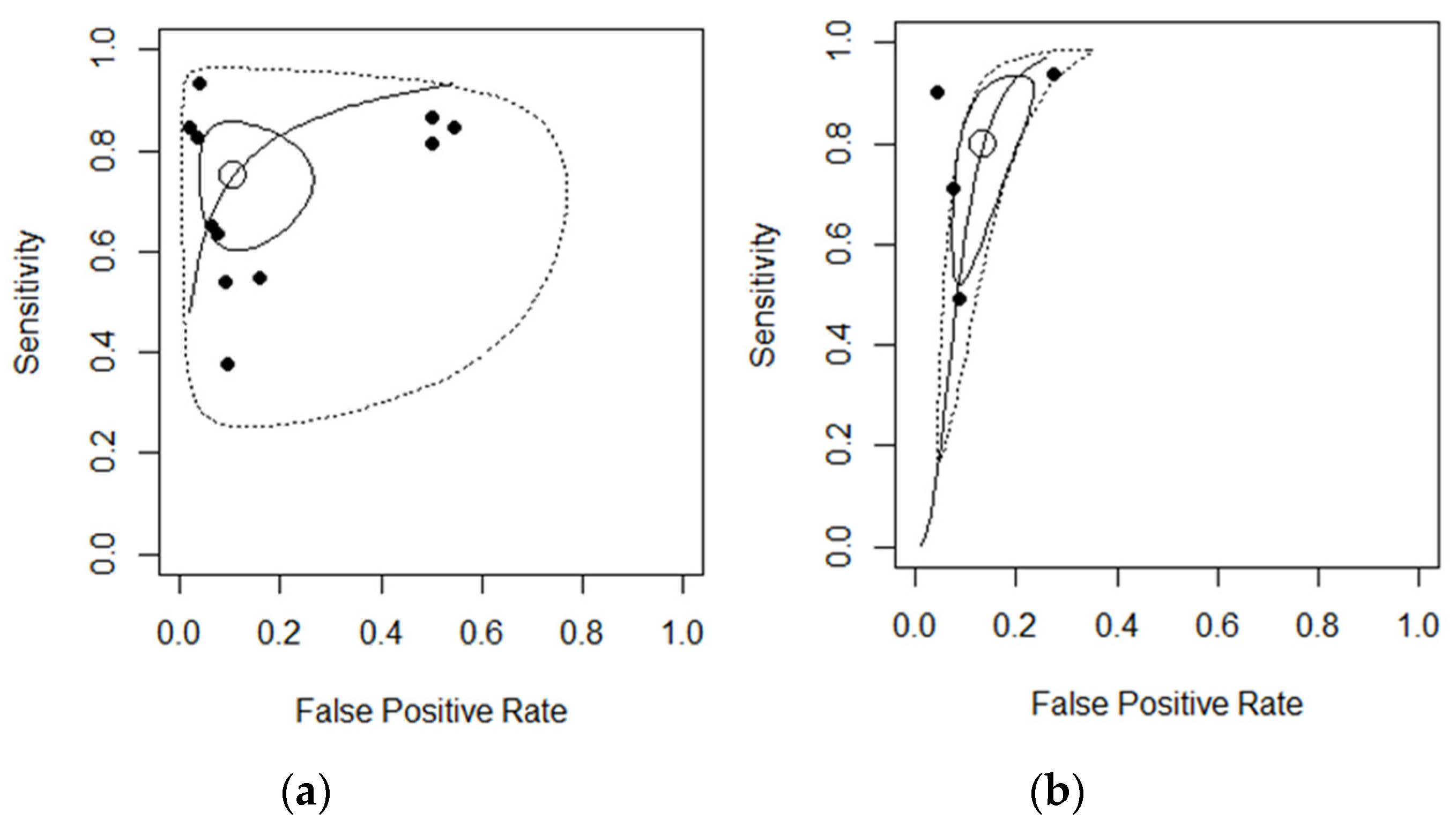
3.3. Diagnostic Accuracy for MRI
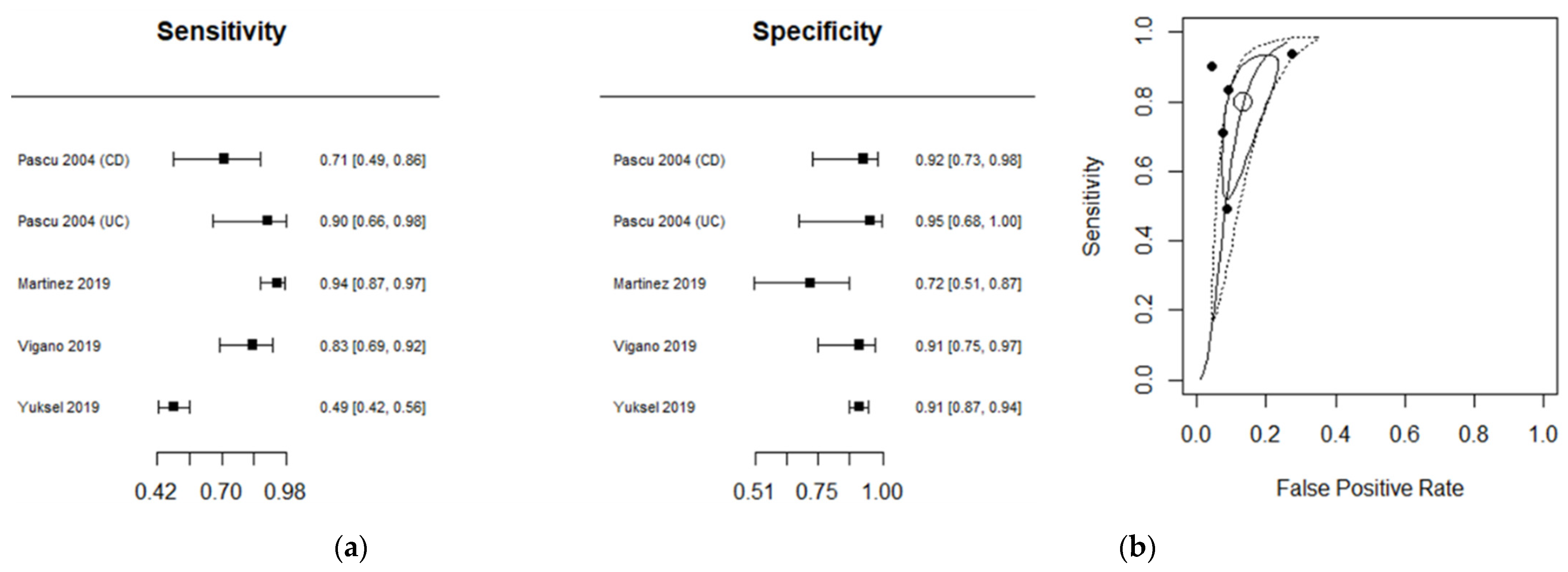
3.4. Diagnostic Accuracy for US
3.5. Between-Study Heterogeneity
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Lo, B.; Vester-Andersen, M.K.; Vind, I.; Prosberg, M.; Dubinsky, M.; Siegel, C.A.; Bendtsen, F.; Burisch, J. Changes in Disease Behaviour and Location in Patients with Crohn's Disease After Seven Years of Follow-Up: A Danish Population-based Inception Cohort. J. Crohn’s Colitis 2018, 12, 265–272. [Google Scholar] [CrossRef]
- Bouguen, G.; Levesque, B.G.; Feagan, B.G.; Kavanaugh, A.; Peyrin-Biroulet, L.; Colombel, J.F.; Hanauer, S.B.; Sandborn, W.J. Treat to target: A proposed new paradigm for the management of Crohn's disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1042–1050.e2. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2020, 160, 1570–1583. [Google Scholar] [CrossRef]
- Damore, L.J., II; Rantis, P.C.; Vernava, A.M., III; Longo, W.E. Colonoscopic perforations. Etiology, diagnosis, and management. Dis. Colon Rectum 1996, 39, 1308–1314. [Google Scholar] [CrossRef]
- Miles, A.; Bhatnagar, G.; Halligan, S.; Gupta, A.; Tolan, D.; Zealley, I.; Taylor, S.A.; on behalf of the METRIC Investigators. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: Patient acceptability and perceived burden. Eur. Radiol. 2019, 29, 1083–1093. [Google Scholar] [CrossRef]
- Ordas, I.; Rimola, J.; Rodriguez, S.; Gallego, M.; Ricart, E.; Panes, J. Imaging of the colon in inflammatory bowel disease: Ready for prime time? Curr. Drug Targets 2012, 13, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; Hamlin, P.J.; Hart, A.L.; Higginson, A.; Jacobs, I.; et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (METRIC): A multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 548–558. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; the QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Int. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Laurent, V.; Bruot, O.; Bressenot, A.; Bigard, M.A.; Regent, D.; Peyrin-Biroulet, L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010, 59, 1056–1065. [Google Scholar] [CrossRef]
- Pascu, M.; Roznowski, A.B.; Muller, H.P.; Adler, A.; Wiedemann, B.; Dignass, A.U. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm. Bowel Dis. 2004, 10, 373–382. [Google Scholar] [CrossRef]
- Yu, L.L.; Yang, H.S.; Zhang, B.T.; Lv, Z.W.; Wang, F.R.; Zhang, C.Y.; Chen, W.B.; Zhang, H.M. Diffusion-weighted magnetic resonance imaging without bowel preparation for detection of ulcerative colitis. World J. Gastroenterol. 2015, 21, 9785–9792. [Google Scholar] [CrossRef]
- Laurent, V.; Naudé, S.; Vuitton, L.; Zallot, C.; Baumann, C.; Girard-Gavanier, M.; Peyrin-Biroulet, L. Accuracy of Diffusion-weighted Magnetic Resonance Colonography in Assessing Mucosal Healing and the Treatment Response in Patients with Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 716–723. [Google Scholar] [CrossRef][Green Version]
- Fiorino, G.; Bonifacio, C.; Padrenostro, M.; Sposta, F.M.; Spinelli, A.; Malesci, A.; Balzarini, L.; Peyrin-Biroulet, L.; Danese, S. Comparison Between 1.5 and 3.0 Tesla Magnetic Resonance Enterography for the Assessment of Disease Activity and Complications in Ileo-Colonic Crohn's Disease. Dig. Dis. Sci. 2013, 58, 3246–3255. [Google Scholar] [CrossRef]
- Cansu, A.; Bekircavusoglu, S.; Oguz, S.; Bulut, E.; Fidan, S.; Thomas, M.A. Can diffusion weighted imaging be used as an alternative to contrast-enhanced imaging on magnetic resonance enterography for the assessment of active inflammation in Crohn disease? Medicine 2020, 99, e19202. [Google Scholar] [CrossRef] [PubMed]
- Limberg, B. Diagnosis of acute ulcerative colitis and colonic Crohn's disease by colonic sonography. J. Clin. Ultrasound 1989, 17, 25–31. [Google Scholar] [CrossRef]
- Koh, D.M.; Miao, Y.; Chinn, R.J.; Amin, Z.; Zeegen, R.; Westaby, D.; Healy, J.C. MR imaging evaluation of the activity of Crohn's disease. AJR Am. J. Roentgenol. 2001, 177, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Sakr, M.; Hamed, W.; Eltabbakh, M.; Askar, S.; Bassuny, A.; Elbaz, A.; Hussein, R. Comparative study between bowel ultrasound and magnetic resonance enterography among Egyptian inflammatory bowel disease patients. World J. Gastroenterol. 2020, 26, 5884–5895. [Google Scholar] [CrossRef] [PubMed]
- Haber, H.P.; Busch, A.; Ziebach, R.; Dette, S.; Ruck, P.; Stern, M. Ultrasonographic findings correspond to clinical, endoscopic, and histologic findings in inflammatory bowel disease and other enterocolitides. J. Ultrasound Med. 2002, 21, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Podgorska, J.; Pasicz, K.; Zagorowicz, E.; Mroz, A.; Golebiewski, B.; Kus, P.; Jasieniak, J.; Skrzynski, W.; Wieszczy, P.; Kukolowicz, P.; et al. Intravoxel incoherent motion MRI in evaluating inflammatory activity in ulcerative colitis: A pilot study. Acta Radiol. 2021, 62, 439–446. [Google Scholar] [CrossRef]
- Mostafa, M.E.; Mohamed, A.M.; Elbaser, E.S.S.A.; Zaher, T.I. Evaluation of colonic wall thickness in ulcerative colitis using trans abdominal ultrasound: Cross sectional study. Eur. J. Mol. Clin. Med. 2021, 8, 2764–2773. [Google Scholar]
- Yuksel, I.; Kilincalp, S.; Coskun, Y.; Akinci, H.; Hamamci, M.; Alkan, A. Diagnostic accuracy of intestinal ultrasound and magnetic resonance enterography for the detection of endoscopy-based disease activity in ileocolonic Crohn's disease. Eur. J. Gastroenterol. Hepatol. 2019, 31, 809–816. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Bonifacio, C.; Furfaro, F.; Gilardi, D.; Argollo, M.; Peyrin-Biroulet, L.; Danese, S. Comparative Accuracy of Bowel Ultrasound Versus Magnetic Resonance Enterography in Combination With Colonoscopy in Assessing Crohn's Disease and Guiding Clinical Decision-making. J. Crohn’s Colitis 2018, 12, 1280–1287. [Google Scholar] [CrossRef]
- Liu, C.; Ding, S.S.; Zhang, K.; Liu, L.N.; Guo, L.H.; Sun, L.P.; Zhang, Y.F.; Sun, X.M.; Ren, W.W.; Zhao, C.K.; et al. Correlation between ultrasound consolidated score and simple endoscopic score for determining the activity of Crohn’s disease. Br. J. Radiol. 2021, 93, 20190614. [Google Scholar] [CrossRef]
- Dillman, J.R.; Ladino-Torres, M.F.; Adler, J.; DeMatos-Malliard, V.; McHugh, J.B.; Khalatbari, S.; Strouse, P.J. Comparison of MR enterography and histopathology in the evaluation of pediatric Crohn disease. Pediatric Radiol. 2011, 41, 1552–1558. [Google Scholar] [CrossRef]
- Maccioni, F.; Al Ansari, N.; Mazzamurro, F.; Civitelli, F.; Viola, F.; Cucchiara, S.; Catalano, C. Detection of Crohn Disease Lesions of the Small and Large Bowel in Pediatric Patients: Diagnostic Value of MR Enterography Versus Reference Examinations. Am. J. Roentgenol. 2014, 203, W533–W542. [Google Scholar] [CrossRef]
- Maccioni, F.; Bruni, A.; Viscido, A.; Colaiacomo, M.C.; Cocco, A.; Montesani, C.; Caprilli, R.; Marini, M. MR imaging in patients with Crohn disease: Value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology 2006, 238, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Morris, S.; Quinn, L.; Tomini, F.; Miles, A.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; et al. Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: The METRIC diagnostic accuracy study. Health Technol. Assess. 2019, 23, 1–162. [Google Scholar] [CrossRef]
- Vigano, L.; Mineccia, M.; Bertolino, F.; Giraldi, F.; Rigazio, C.; Rocca, R.; Ferrero, A. Intraoperative ultrasonography in patients undergoing surgery for Crohn's disease. Prospective evaluation of an innovative approach to optimize staging and treatment planning. Updates Surg. 2019, 71, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jesus Martinez, M.; Ripolles, T.; Maria Paredes, J.; Moreno-Osset, E.; Manuel Pazos, J.; Blanc, E. Intravenous Contrast-Enhanced Ultrasound for Assessing and Grading Postoperative Recurrence of Crohn's Disease. Dig. Dis. Sci. 2019, 64, 1640–1650. [Google Scholar] [CrossRef]
- Boraschi, P.; Donati, F. MR colonography with a fecal tagging technique and water-based enema for the assessment of inflammatory bowel disease. Jpn. J. Radiol. 2016, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gaitini, D.; Kreitenberg, A.J.; Fischer, D.; Maza, I.; Chowers, Y. Color-Coded Duplex Sonography Compared to Multidetector Computed Tomography for the Diagnosis of Crohn Disease Relapse and Complications. J. Ultrasound Med. 2011, 30, 1691–1699. [Google Scholar] [CrossRef]
- Jesuratnam-Nielsen, K.; Logager, V.B.; Rezanavaz-Gheshlagh, B.; Munkholm, P.; Thomsen, H.S. Plain magnetic resonance imaging as an alternative in evaluating inflammation and bowel damage in inflammatory bowel disease—A prospective comparison with conventional magnetic resonance follow-through. Scand. J. Gastroenterol. 2015, 50, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Campari, A.; Napolitano, M.; Zuin, G.; Maestri, L.; Di Leo, G.; Sardanelli, F. Colonic inflammation in pediatric inflammatory bowel disease: Detection with magnetic resonance enterography. Pediatric Radiol. 2017, 47, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Dagia, C.; Ditchfield, M.; Kean, M.; Catto-Smith, T. Imaging for Crohn disease: Use of 3-T MRI in a paediatric setting. J. Med. Imaging Radiat. Oncol. 2008, 52, 480–488. [Google Scholar] [CrossRef]
- Schreyer, A.G.; Menzel, C.; Friedrich, C.; Poschenrieder, F.; Egger, L.; Dornia, C.; Schill, G.; Dendl, L.M.; Schacherer, D.; Girlich, C.; et al. Comparison of high-resolution ultrasound and MR-enterography in patients with inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Iijima, H.; Shinzaki, S.; Hiyama, S.; Yamaguchi, T.; Araki, M.; Iwatani, S.; Shiraishi, E.; Mukai, A.; Inoue, T.; et al. Usefulness of intestinal real-time virtual sonography in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2019, 34, 1743–1750. [Google Scholar] [CrossRef]
- Langhorst, J.; Kuhle, C.A.; Ajaj, W.; Nufer, M.; Barkhausen, J.; Michalsen, A.; Dobos, G.J.; Lauenstein, T.C. MR colonography without bowel purgation for the assessment of inflammatory bowel diseases: Diagnostic accuracy and patient acceptance. Inflamm. Bowel Dis. 2007, 13, 1001–1008. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- FDA; CDER. Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry; Draft Guidance; FDA: Rockville, MD, USA, 2016. [Google Scholar]
- European Medicines Agency. Guideline on the Development of New Medicinal Products for the Treatment of Crohn’s Disease; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Simon, E.G.; Wardle, R.; Thi, A.A.; Eldridge, J.; Samuel, S.; Moran, G.W. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn's disease?: A systematic review. Intest. Res. 2019, 17, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, R.; Novak, K.L.; Lebeuf-Taylor, E.; Wilson, S.R. Impact of Intestinal Ultrasound on Classification and Management of Crohn's Disease Patients with Inconclusive Colonoscopy. Can. J. Gastroenterol. Hepatol. 2016, 2016, 8745972. [Google Scholar] [CrossRef]
- Kim, S.J.; Ratchford, T.L.; Buchanan, P.M.; Patel, D.R.; Tao, T.Y.; Teckman, J.H.; Brown, J.J.; Farmakis, S.G. Diagnostic accuracy of non-contrast magnetic resonance enterography in detecting active bowel inflammation in pediatric patients with diagnosed or suspected inflammatory bowel disease to determine necessity of gadolinium-based contrast agents. Pediatric Radiol. 2019, 49, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Marciani, L.; Foley, S.; Totman, J.J.; Wright, J.; Bush, D.; Cox, E.F.; Campbell, E.; Spiller, R.C.; Gowland, P.A. Non-invasive quantification of small bowel water content by MRI: A validation study. Phys. Med. Biol. 2007, 52, 6909–6922. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Williams, H.G.; Hoad, C.L.; Alyami, A.; Ortori, C.A.; Grove, J.I.; Marciani, L.; Moran, G.W.; Spiller, R.C.; Menys, A.; et al. MR Measures of Small Bowel Wall T2 Are Associated with Increased Permeability. J. Magn. Reson. Imaging 2021, 53, 1422–1431. [Google Scholar] [CrossRef]
- Lenze, F.; Wessling, J.; Bremer, J.; Ullerich, H.; Spieker, T.; Weckesser, M.; Gonschorrek, S.; Kannengieber, K.; Rijcken, E.; Heidemann, J.; et al. Detection and differentiation of inflammatory versus fibromatous Crohn's disease strictures: Prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm. Bowel Dis. 2012, 18, 2252–2260. [Google Scholar] [CrossRef]
- Ponte, A.; Pinho, R.; Fernandes, S.; Rodrigues, A.; Alberto, L.; Silva, J.C.; Silva, J.; Rodrigues, J.; Sousa, M.; Silva, A.P.; et al. Impact of Histological and Endoscopic Remissions on Clinical Recurrence and Recurrence-free Time in Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 2238–2244. [Google Scholar] [CrossRef]
- O'Connor, J.P.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef]
- Barber, J.L.; Maclachlan, J.; Planche, K.; Furman, M.; Crespi, D.; Bab, N.; Beal, I. There is good agreement between MR enterography and bowel ultrasound with regards to disease location and activity in paediatric inflammatory bowel disease. Clinical Radiology 2017, 72, 590–597. [Google Scholar] [CrossRef]
- Johnson, K.T.; Hara, A.K.; Johnson, C.D. Evaluation of colitis: Usefulness of CT enterography technique. Emergency Radiology 2009, 16, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Thierry, M.L.; Rousseau, H.; Pouillon, L.; Girard-Gavanier, M.; Baumann, C.; Lopez, A.; Danese, S.; Laurent, V.; Peyrin-Biroulet, L. Accuracy of Diffusion-weighted Magnetic Resonance Imaging in Detecting Mucosal Healing and Treatment Response, and in Predicting Surgery, in Crohn's Disease. J Crohns Colitis 2018, 12, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Paparo, F.; Bacigalupo, L.; Garello, I.; Biscaldi, E.; Cimmino, M.A.; Marinaro, E.; Rollandi, G.A. Crohn's disease: Prevalence of intestinal and extraintestinal manifestations detected by computed tomography enterography with water enema. Abdom Imaging 2012, 37, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Ponorac, S.; Gosnak, R.D.; Urlep, D.; Kljucevsek, D. Contrast-enhanced ultrasonography in the evaluation of Crohn disease activity in children: Comparison with histopathology. Pediatric Radiology 2021, 51, 410–418. [Google Scholar] [CrossRef]

| PICO Framework | |
|---|---|
| Participants | Human (without any age limit) |
| Interventions | Non-invasive colonic imaging, such as MRI, CT, and US |
| Comparator | Colonoscopy or histology |
| Outcomes | Measuring colonic inflammation |
| Author | No. of Segments | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | MRI | Disease | Ref Standard |
|---|---|---|---|---|---|---|---|---|---|---|
| Dillman 2011 [28] | 149 | 44 | 8 | 23 | 74 | 0.65 [0.53; 0.76] | 0.90 [0.81; 0.95] | 1.5T | CD | Histopathology |
| Fiorino 2013 (1.5T) [17] | 34 | 26 | 1 | 6 | 1 | 0.81 [0.63; 0.92] | 0.50 [0.01; 0.98] | 1.5T | CD | Ileo-colonoscopy |
| Fiorino 2013 (3T) [17] | 32 | 26 | 1 | 4 | 1 | 0.86 [0.69; 0.96] | 0.50 [0.01; 0.98] | 3T | CD | Ileo-colonoscopy |
| Maccioni 2006 (T1) [30] | 413 | 85 | 11 | 18 | 299 | 0.82 [0.73; 0.89] | 0.96 [0.93; 0.98] | 1.5 T1 | CD | Ileo-colonoscopy |
| Maccioni 2006 (T2) [30] | 413 | 87 | 6 | 16 | 304 | 0.84 [0.76; 0.90] | 0.98 [0.95; 0.99] | 1.5 T2 | CD | Ileo-colonoscopy |
| Maccioni 2014 [29] | 300 | 112 | 7 | 8 | 173 | 0.93 [0.87; 0.97] | 0.96 [0.92; 0.98] | 1.5T | CD | Ileo-colonoscopy |
| Oussalah 2010 (CD) [13] | 61 | 27 | 1 | 23 | 10 | 0.54 [0.39; 0.68] | 0.90 [0.58; 0.99] | 1.5T | CD | Ileo-colonoscopy |
| Oussalah 2010 (UC) [13] | 36 | 13 | 1 | 7 | 15 | 0.65 [0.40; 0.84] | 0.93 [0.69; 0.99] | 1.5T | UC | Ileo-colonoscopy |
| Pascu 2004 (CD) [14] | 37 | 6 | 2 | 10 | 19 | 0.37 [0.15; 0.64] | 0.90 [0.69; 0.98] | 1.5T | CD | Ileo-colonoscopy |
| Pascu 2004 (UC) [14] | 24 | 7 | 1 | 4 | 12 | 0.63 [0.30; 0.89] | 0.92 [0.63; 0.99] | 1.5T | UC | Ileo-colonoscopy |
| Taylor 2019 [31] | 186 | 89 | 44 | 16 | 37 | 0.84 [0.76; 0.91] | 0.45 [0.34; 0.57] | 1.5T | CD | Ileo-colonoscopy |
| Vigano 2019 [32] | 65 | 22 | 1 | 4 | 38 | 0.84 [0.65; 0.95] | 0.97 [0.86; 0.99] | 1.5T | CD | Histopathology |
| Yuksel 2019 [25] | 426 | 123 | 32 | 101 | 170 | 0.54 [0.48; 0.61] | 0.84 [0.78; 0.88] | 1.5T | CD | Ileo-colonoscopy |
| Author | No. of Segments | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | IBD Type | Ref Standard |
|---|---|---|---|---|---|---|---|---|---|
| Pascu 2004 (CD) [14] | 37 | 13 | 1 | 5 | 18 | 0.7222 [0.4652; 0.9031] | 0.9474 [0.7397; 0.9987] | CD | Ileo-colonoscopy |
| Pascu 2004 (UC) [14] | 24 | 13 | 0 | 1 | 10 | 0.9286 [0.6613; 0.9982] | 1.0000 [0.6915; 1.0000] | UC | Ileo-colonoscopy |
| Jesus Martinez 2019 [33] | 108 | 84 | 5 | 5 | 14 | 0.9438 [0.8737; 0.9815] | 0.7368 [0.4880; 0.9085] | CD | Ileo-colonoscopy |
| Vigano 2019 [32] | 65 | 32 | 2 | 6 | 25 | 0.8421 [0.6875; 0.9398] | 0.9259 [0.7571; 0.9909] | CD | Histopathology |
| Yuksel 2019 [25] | 426 | 94 | 20 | 97 | 215 | 0.4921 [0.4192; 0.5653] | 0.9149 [0.8716; 0.9472] | CD | Ileo-colonoscopy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, M.T.; Stevenson, R.; Abdul-Aema, B.; Zou, G.; Jairath, V.; Radford, S.; Marciani, L.; Moran, G.W. Diagnostic Accuracy of Non-Invasive Imaging for Detection of Colonic Inflammation in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1926. https://doi.org/10.3390/diagnostics11101926
Alshammari MT, Stevenson R, Abdul-Aema B, Zou G, Jairath V, Radford S, Marciani L, Moran GW. Diagnostic Accuracy of Non-Invasive Imaging for Detection of Colonic Inflammation in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(10):1926. https://doi.org/10.3390/diagnostics11101926
Chicago/Turabian StyleAlshammari, Meshari T., Rebecca Stevenson, Buraq Abdul-Aema, Guangyong Zou, Vipul Jairath, Shellie Radford, Luca Marciani, and Gordon W. Moran. 2021. "Diagnostic Accuracy of Non-Invasive Imaging for Detection of Colonic Inflammation in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 10: 1926. https://doi.org/10.3390/diagnostics11101926
APA StyleAlshammari, M. T., Stevenson, R., Abdul-Aema, B., Zou, G., Jairath, V., Radford, S., Marciani, L., & Moran, G. W. (2021). Diagnostic Accuracy of Non-Invasive Imaging for Detection of Colonic Inflammation in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Diagnostics, 11(10), 1926. https://doi.org/10.3390/diagnostics11101926







