A Promising and Challenging Approach: Radiologists’ Perspective on Deep Learning and Artificial Intelligence for Fighting COVID-19
Abstract
:1. Introduction
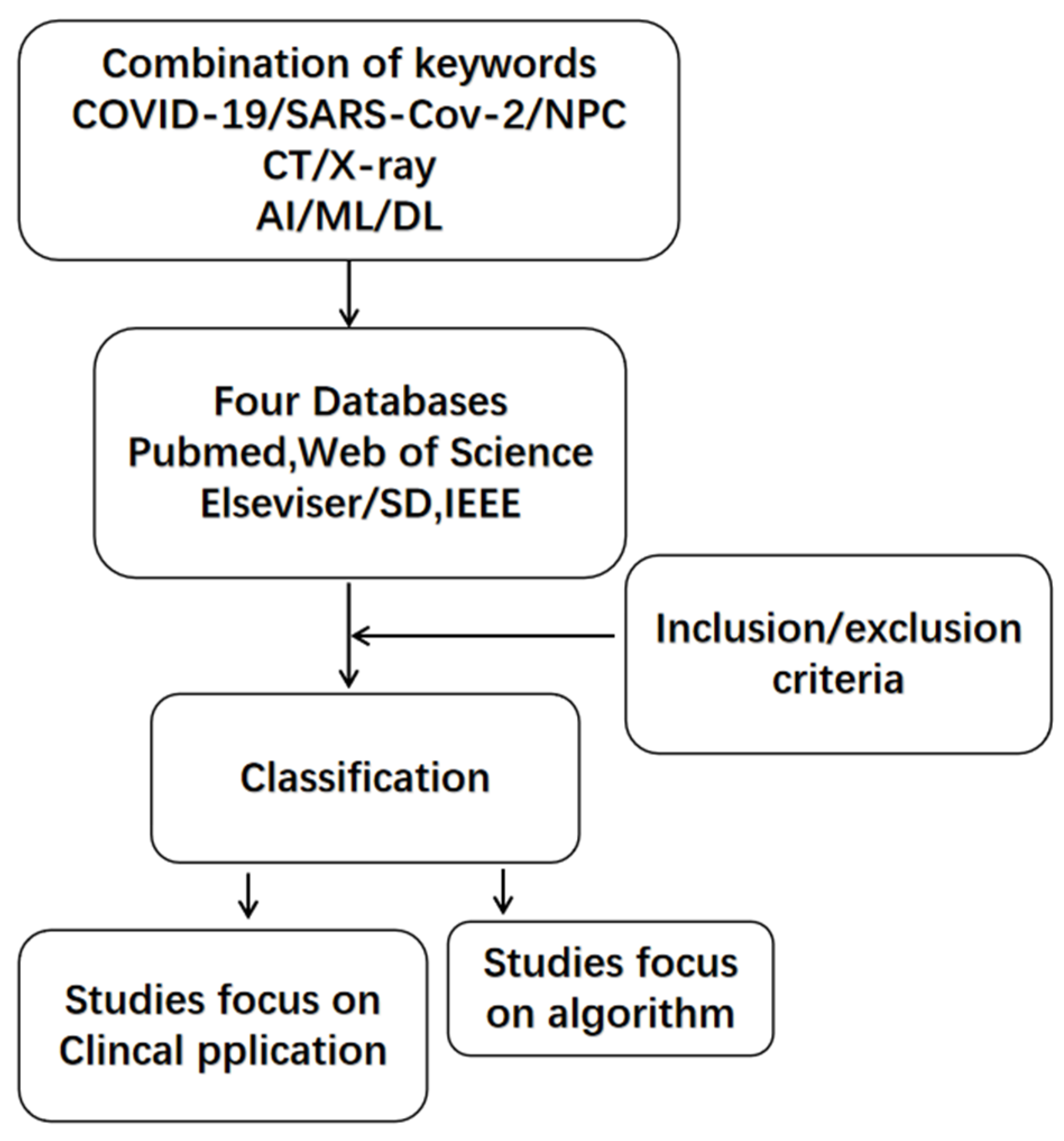
2. Methods
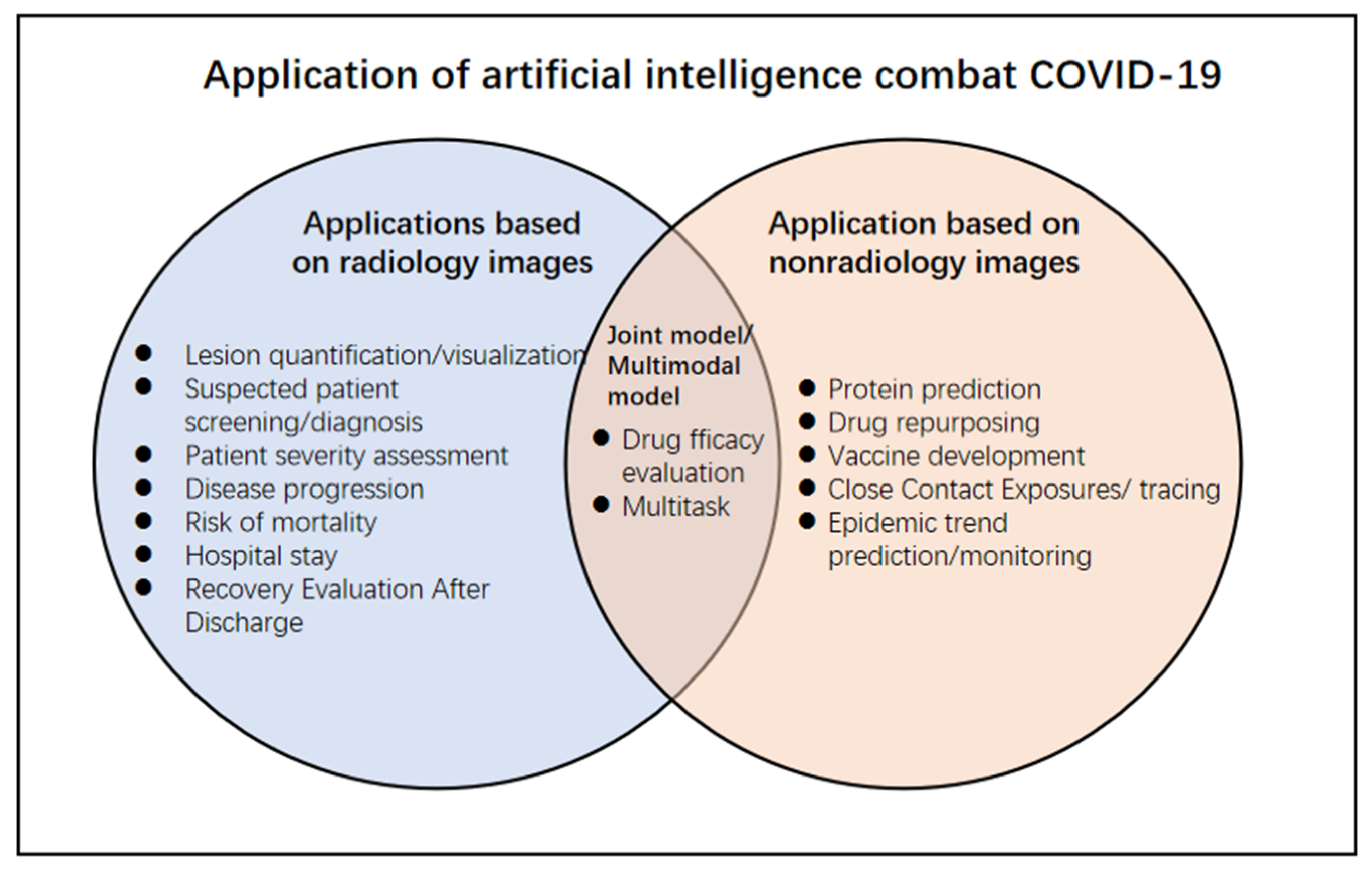
3. Application of Detection, Diagnosis, and Classification of COVID-19
4. Application of Early Intervention and Decision-Making
5. Comparison of Medical Images with Clinical Data
6. Comparison of the Diagnostic Performance of DL and AI Systems with Radiologists
7. Prediction of Curative Effect Evaluation and Prognosis in COVID-19
8. Follow-Up and Recovery Evaluation after Discharge
9. Constructing Image Multimodal Models to Evaluate COVID-19
10. Development of New DL and AI Algorithms
11. Other Nonimaging-Related Applications of DL and AI
12. Existing Limitations and Challenges
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
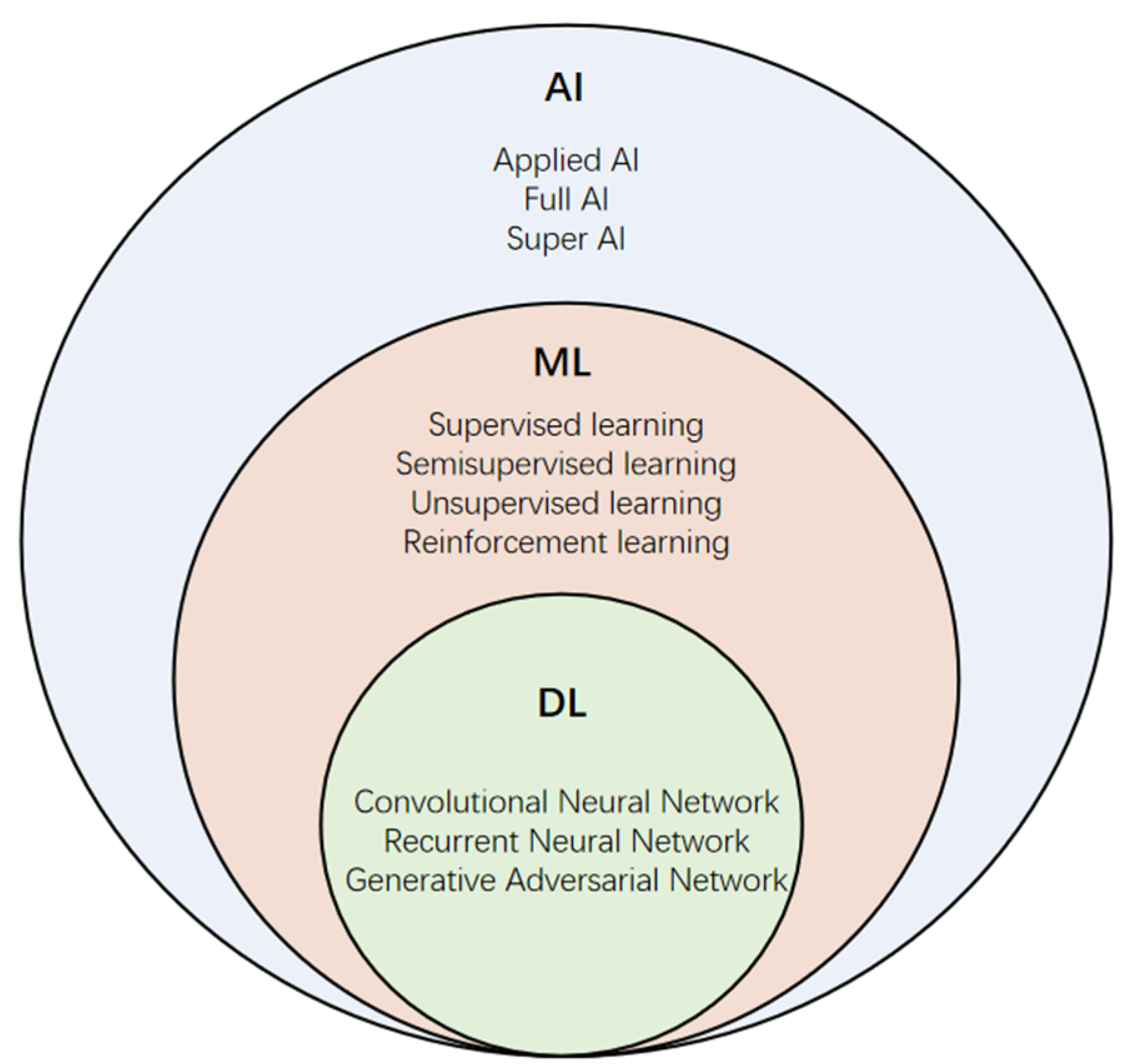
Abbreviations
| CXR | chest X-rays |
| CT | computed tomography |
| ML | machine learning |
| AI | artificial intelligence |
| DL | deep learning |
| RT-PCR | reverse transcription polymerase |
| HU | Hounsfield unit |
| AUC | the area under curve |
| ROC | the receiver operating character |
| CAP | community-acquired pneumonia |
| LR | logistic regression |
| RF | random forest |
| SVR | support vector regression |
| GGO | ground glass opacity |
Appendix A
References
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [Green Version]
- Nakaura, T.; Higaki, T.; Awai, K.; Ikeda, O.; Yamashita, Y. A primer for understanding radiology articles about machine learning and deep learning. Diagn. Interv. Imaging 2020, 101, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, M.; Merico, D.; Delong, A.; Frey, B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018, 36, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, K.; Zhang, K. Current status and future trends of clinical diagnoses via image-based deep learning. Theranostics 2019, 9, 7556–7565. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.O.; Alonso, E.; Andrés, M.; Buitrago, N.M.; Vigara, A.P.; Pajares, M.P.; López, E.C.; Moll, G.G.; Espin, I.M.; Barriocanal, M.B.; et al. Pediatric chest X-ray in COVID-19 infection. Eur. J. Radiol. 2020, 131, 109236. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Zhang, S. The indispensable role of chest CT in the detection of coronavirus disease 2019 (COVID-19). Eur. J. Nucl. Med. Mol. I 2020, 47, 1638–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, N.; Ebrahimzadeh, S.; Salameh, J.; Kazi, S.; Fabiano, N.; Treanor, L.; Absi, M.; Hallgrimson, Z.; Leeflang, M.M.; Hooft, L.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zhang, H.; Nan, Y.; Zhao, Y.; Fu, E.; Xie, Y.; Liu, W.; Li, W.; Zhang, H.; et al. Automated detection and quantification of COVID-19 pneumonia: CT imaging analysis by a deep learning-based software. Eur. J. Nucl. Med. Mol. I 2020, 47, 2525–2532. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Barbosa, E.J.M.; Georgescu, B.; Chaganti, S.; Aleman, G.B.; Cabrero, J.B.; Chabin, G.; Flohr, T.; Grenier, P.; Grbic, S.; Gupta, N.; et al. Machine learning automatically detects COVID-19 using chest CTs in a large multicenter cohort. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- Jin, C.; Chen, W.; Cao, Y.; Xu, Z.; Tan, Z.; Zhang, X.; Deng, L.; Zheng, C.; Zhou, J.; Shi, H.; et al. Development and evaluation of an artificial intelligence system for COVID-19 diagnosis. Nat. Commun. 2020, 11, 5088. [Google Scholar] [CrossRef]
- Harmon, S.A.; Sanford, T.H.; Xu, S.; Turkbey, E.B.; Roth, H.; Xu, Z.; Yang, D.; Myronenko, A.; Anderson, V.; Amalou, A.; et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020, 11, 4080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, X.; Shen, J.; Li, Z.; Sang, Y.; Wu, X.; Zha, Y.; Liang, W.; Wang, C.; Wang, K.; et al. Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography. Cell 2020, 181, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Society of Thoracic Radiology. Available online: https://thoracicrad.org/ (accessed on 6 October 2021).
- ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection American College of Radiology. Available online: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed on 6 October 2021).
- Wang, G.; Liu, X.; Shen, J.; Wang, C.; Li, Z.; Ye, L.; Wu, X.; Chen, T.; Wang, K.; Zhang, X.; et al. A deep-learning pipeline for the diagnosis and discrimination of viral, non-viral and COVID-19 pneumonia from chest X-ray images. Nat. Biomed. Eng. 2021, 5, 509–521. [Google Scholar] [CrossRef]
- Wehbe, R.M.; Sheng, J.; Dutta, S.; Chai, S.; Dravid, A.; Barutcu, S.; Wu, Y.; Cantrell, D.R.; Xiao, N.; Allen, B.D.; et al. Deep COVID-XR: An Artificial Intelligence Algorithm to Detect COVID-19 on Chest Radiographs Trained and Tested on a Large U.S. Clinical Data Set. Radiology 2021, 299, E167–E176. [Google Scholar] [CrossRef]
- Keidar, D.; Yaron, D.; Goldstein, E.; Shachar, Y.; Blass, A.; Charbinsky, L.; Aharony, I.; Lifshitz, L.; Lumelsky, D.; Neeman, Z.; et al. COVID-19 classification of X-ray images using deep neural networks. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- Cai, W.; Liu, T.; Xue, X.; Luo, G.; Wang, X.; Shen, Y.; Fang, Q.; Sheng, J.; Chen, F.; Liang, T. CT Quantification and Machine-learning Models for Assessment of Disease Severity and Prognosis of COVID-19 Patients. Acad. Radiol. 2020, 27, 1665–1678. [Google Scholar] [CrossRef]
- Wang, S.; Zha, Y.; Li, W.; Wu, Q.; Li, X.; Niu, M.; Wang, M.; Qiu, X.; Li, H.; Yu, H.; et al. A fully automatic deep learning system for COVID-19 diagnostic and prognostic analysis. Eur. Respir. J. 2020, 56, 2000775. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Yu, Q.; Liu, C.; Huang, Y.; Jiang, Z.; Shao, C.; Zhang, H.; Ma, B.; Wang, Y.; Xie, G.; et al. Machine learning-based CT radiomics method for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: A multicenter study. Ann. Transl. Med. 2020, 8, 859. [Google Scholar] [CrossRef]
- Wang, X.; Che, Q.; Ji, X.; Meng, X.; Zhang, L.; Jia, R.; Lyu, H.; Bai, W.; Tan, L.; Gao, Y. Correlation between lung infection severity and clinical laboratory indicators in patients with COVID-19: A cross-sectional study based on machine learning. BMC Infect. Dis. 2021, 21, 192. [Google Scholar] [CrossRef]
- Ma, H.; Ye, Q.; Ding, W.; Jiang, Y.; Wang, M.; Niu, Z.; Zhou, X.; Gao, Y.; Wang, C.; Menpes-Smith, W.; et al. Can Clinical Symptoms and Laboratory Results Predict CT Abnormality? Initial Findings Using Novel Machine Learning Techniques in Children With COVID-19 Infections. Front. Med. 2021, 8, 855. [Google Scholar] [CrossRef]
- Fung, D.L.X.; Liu, Q.; Zammit, J.; Leung, C.K.; Hu, P. Self-supervised deep learning model for COVID-19 lung CT image segmentation highlighting putative causal relationship among age, underlying disease and COVID-19. J. Transl. Med. 2021, 19, 358. [Google Scholar] [CrossRef]
- Murphy, K.; Smits, H.; Knoops, A.J.G.; Korst, M.B.J.M.; Samson, T.; Scholten, E.T.; Schalekamp, S.; Schaefer-Prokop, C.M.; Ginneken, B.V.; Rutten, M.J.C.M. COVID-19 on Chest Radiographs: A Multireader Evaluation of an Artificial Intelligence System. Radiology 2020, 296, E166–E172. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.J.; Kim, K.B.; Kim, J.Y.; Lim, J.; Nam, J.G.; Choi, H.; Kim, H.; Yoon, S.H.; Goo, J.M.; Park, C.M. COVID-19 pneumonia on chest X-rays: Performance of a deep learning-based computer-aided detection system. PLoS ONE 2021, 16, e252440. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.X.; Wang, R.; Xiong, Z.; Hsieh, B.; Chang, K.; Halsey, K.; Tran, T.M.L.; Choi, J.W.; Wang, D.; Shi, L.; et al. Artificial Intelligence Augmentation of Radiologist Performance in Distinguishing COVID-19 from Pneumonia of Other Origin at Chest CT. Radiology 2020, 296, E156–E165. [Google Scholar] [CrossRef]
- Shan, F.; Gao, Y.; Wang, J.; Shi, W.; Shi, N.; Han, M.; Xue, Z.; Shen, D.; Shi, Y. Abnormal lung quantification in chest CT images of COVID-19 patients with deep learning and its application to severity prediction. Med. Phys. 2021, 48, 1633–1645. [Google Scholar] [CrossRef]
- Li, M.D.; Arun, N.T.; Gidwani, M.; Chang, K.; Deng, F.; Little, B.P.; Mendoza, D.P.; Lang, M.; Lee, S.I.; O’Shea, A.; et al. Automated assessment of COVID-19 pulmonary disease severity on chest radiographs using convolutional Siamese neural networks. medRxiv 2020. [Google Scholar] [CrossRef]
- Lessmann, N.; Sánchez, C.I.; Beenen, L.; Boulogne, L.H.; Brink, M.; Calli, E.; Charbonnier, J.; Dofferhoff, T.; van Everdingen, W.M.; Gerke, P.K.; et al. Automated Assessment of COVID-19 Reporting and Data System and Chest CT Severity Scores in Patients Suspected of Having COVID-19 Using Artificial Intelligence. Radiology 2021, 298, E18–E28. [Google Scholar] [CrossRef]
- Fang, C.; Bai, S.; Chen, Q.; Zhou, Y.; Xia, L.; Qin, L.; Gong, S.; Xie, X.; Zhou, C.; Tu, D.; et al. Deep learning for predicting COVID-19 malignant progression. Med. Image Anal. 2021, 72, 102096. [Google Scholar] [CrossRef]
- Bartolucci, M.; Benelli, M.; Betti, M.; Bicchi, S.; Fedeli, L.; Giannelli, F.; Aquilini, D.; Baldini, A.; Consales, G.; Di Natale, M.E.; et al. The incremental value of computed tomography of COVID-19 pneumonia in predicting ICU admission. Sci. Rep. 2021, 11, 15619. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Huang, S.; Liu, S.; Zhou, Z.; Zhang, S.; Zhao, Z.; Yu, Y.; Yang, Y.; Ju, S. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics 2020, 10, 5641–5648. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yao, J.; Chen, A.; Lv, Q.; Zanin, M.; Liu, J.; Wong, S.; Li, Y.; Lu, J.; Liang, H.; et al. Early triage of critically ill COVID-19 patients using deep learning. Nat. Commun. 2020, 11, 3543. [Google Scholar] [CrossRef]
- Wei, J.; Yang, H.; Lei, P.; Fan, B.; Qiu, Y.; Zeng, B.; Yu, P.; Lv, J.; Jian, Y.; Wan, C. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J. X-ray Sci. Technol. 2020, 28, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, W.; Gao, P.; Zhang, J.; Sun, A.; Ding, J.; Liu, H.; Lei, Z. Novel Deep Learning Technique Used in Management and Discharge of Hospitalized Patients with COVID-19 in China. Ther. Clin. Risk Manag. 2020, 16, 1195–1201. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, J.; Yin, J.; Li, L.; Hou, W.; Zhang, L.; Zhou, Z.; Yu, Y.; Li, H.; Feng, Y.; et al. Lower Circulating Interferon-Gamma is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front. Immunol. 2020, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Gangloff, C.; Rafi, S.; Bouzillé, G.; Soulat, L.; Cuggia, M. Machine learning is the key to diagnose COVID-19: A proof-of-concept study. Sci. Rep. 2021, 11, 7166. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Dong, J.; Wang, N.; Huang, H.; Xu, H.; Xia, C. False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus 2: Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases. Korean J. Radiol. 2020, 21, 505. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.; Diao, K.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef]
- Purkayastha, S.; Xiao, Y.; Jiao, Z.; Thepumnoeysuk, R.; Halsey, K.; Wu, J.; Tran, T.M.L.; Hsieh, B.; Choi, J.W.; Wang, D.; et al. Machine Learning-Based Prediction of COVID-19 Severity and Progression to Critical Illness Using CT Imaging and Clinical Data. Korean J. Radiol. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Schiaffino, S.; Codari, M.; Cozzi, A.; Albano, D.; Alì, M.; Arioli, R.; Avola, E.; Bnà, C.; Cariati, M.; Carriero, S.; et al. Machine Learning to Predict In-Hospital Mortality in COVID-19 Patients Using Computed Tomography-Derived Pulmonary and Vascular Features. J. Pers. Med. 2021, 11, 501. [Google Scholar] [CrossRef]
- Jiao, Z.; Choi, J.W.; Halsey, K.; Tran, T.M.L.; Hsieh, B.; Wang, D.; Eweje, F.; Wang, R.; Chang, K.; Wu, J.; et al. Prognostication of patients with COVID-19 using artificial intelligence based on chest X-rays and clinical data: A retrospective study. Lancet Digit. Health 2021, 3, e286–e294. [Google Scholar] [CrossRef]
- Shiri, I.; Akhavanallaf, A.; Sanaat, A.; Salimi, Y.; Askari, D.; Mansouri, Z.; Shayesteh, S.P.; Hasanian, M.; Rezaei-Kalantari, K.; Salahshour, A.; et al. Ultra-low-dose chest CT imaging of COVID-19 patients using a deep residual neural network. Eur. Radiol. 2021, 31, 1420–1431. [Google Scholar] [CrossRef]
- Sengupta, K.; Srivastava, P.R. Quantum algorithm for quicker clinical prognostic analysis: An application and experimental study using CT scan images of COVID-19 patients. BMC Med. Inform. Decis. 2021, 21, 227. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.; Zhong, M.; Wang, J.; Shi, J. COVID-AL: The diagnosis of COVID-19 with deep active learning. Med. Image Anal. 2021, 68, 101913. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Lee, E.H.; Zheng, J.; Zhang, W.; Halabi, S.; Liu, C.; Deng, K.; Song, J.; Yeom, K.W. Decoding COVID-19 pneumonia: Comparison of deep learning and radiomics CT image signatures. Eur. J. Nucl. Med. Mol. I 2021, 48, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Polat, Ç.; Karaman, O.; Karaman, C.; Korkmaz, G.; Balcı, M.C.; Kelek, S.E. COVID-19 diagnosis from chest X-ray images using transfer learning: Enhanced performance by debiasing dataloader. J. X-Ray Sci. Technol. 2021, 29, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Goyal, M.; Gupta, D.; Khanna, A.; Al-Turjman, F.; Pinheiro, P.R. CovidGAN: Data Augmentation Using Auxiliary Classifier GAN for Improved Covid-19 Detection. IEEE Access 2020, 8, 91916–91923. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, S.; Ebadi, M.J.; Safaei, A.A.; Bajuri, M.Y.; Ahmadian, A. Efficient deep neural networks for classification of COVID-19 based on CT images: Virtualization via software defined radio. Comput. Commun. 2021, 176, 234–248. [Google Scholar] [CrossRef]
- Deng, H.; Li, X. AI-Empowered Computational Examination of Chest Imaging for COVID-19 Treatment: A Review. Front. Artif. Intell. 2021, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Posokhova, I.; Qureshi, H.N.; Masood, U.; Riaz, M.S.; Ali, K.; John, C.N.; Hussain, M.I.; Nabeel, M. AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an app. Inform. Med. Unlocked 2020, 20, 100378. [Google Scholar] [CrossRef]
- Ellison, G.T.H. COVID-19 and the epistemology of epidemiological models at the dawn of AI. Ann. Hum. Biol. 2020, 47, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Chimmula, V.K.R.; Zhang, L. Time series forecasting of COVID-19 transmission in Canada using LSTM networks. Chaos Solitons Fractals 2020, 135, 109864. [Google Scholar] [CrossRef]
- Ribeiro, M.H.D.M.; Da Silva, R.G.; Mariani, V.C.; Coelho, L.D.S. Short-term forecasting COVID-19 cumulative confirmed cases: Perspectives for Brazil. Chaos Solitons Fractals 2020, 135, 109853. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Ghosh, I. Real-time forecasts and risk assessment of novel coronavirus (COVID-19) cases: A data-driven analysis. Chaos Solitons Fractals 2020, 135, 109850. [Google Scholar] [CrossRef]
- Agbehadji, I.E.; Awuzie, B.O.; Ngowi, A.B.; Millham, R.C. Review of Big Data Analytics, Artificial Intelligence and Nature-Inspired Computing Models towards Accurate Detection of COVID-19 Pandemic Cases and Contact Tracing. Int. J. Environ. Res. Public Health 2020, 17, 5330. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Prashar, D.; Rashid, M.; Shafiq, M.; Khan, R.; Pruncu, C.I.; Siddiqui, S.T.; Kumar, M.S. Deep Learning Approach for Discovery of In Silico Drugs for Combating COVID-19. J. Healthc. Eng. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Wang, R.; Nguyen, D.D.; Wei, G. Review of COVID-19 Antibody Therapies. Annu. Rev. Biophys. 2021, 50, 1–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health 2020, 2, e667–e676. [Google Scholar] [CrossRef]
- Ong, E.; Wong, M.U.; Huffman, A.; He, Y. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front. Immunol. 2020, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Peng, T.; Yeh, T.; Huang, W.; Chang, S.; Wu, S.; Hung, H.; Hsu, T.; Lee, S.; Song, J.; et al. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed. J. 2020, 43, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Image Down-2019Novel Coronavirus Information Database(2019nCoVR). Available online: http://ncov-ai.big.ac.cn/download (accessed on 6 October 2021).
- Lieveld, A.W.E.; Azijli, K.; Teunissen, B.P.; van Haaften, R.M.; Kootte, R.S.; van den Berk, I.A.H.; van der Horst, S.F.B.; de Gans, C.; van de Ven, P.M.; Nanayakkara, P.W.B. Chest CT in COVID-19 at the ED: Validation of the COVID-19 Reporting and Data System (CO-RADS) and CT Severity Score. Chest 2021, 159, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, X.; Wang, X.; Fang, F.; Lv, X.; Zhang, D.; Sun, Y.; Hu, S.; Lin, Z.; Xiong, N. Radiology indispensable for tracking COVID-19. Diagn. Interv. Imaging 2021, 102, 69–75. [Google Scholar] [CrossRef] [PubMed]
| First Author | Modality | Technique | Subject | Numbers of Cases | Nation | Sensitivity (%) | Specificity (%) | Accuracy (%)/AUC |
|---|---|---|---|---|---|---|---|---|
| Zhang [9] | CT | uAI | Huoshenshan Hospital | 2460 patients | China | N/A | N/A | N/N |
| Li [10] | CT | COVNet | 6 medical centers | 4352 scans/3322 patients | China | 90 | 96 | N/0.96 |
| Bai [28] | CT | EfficientNet B4 DNN | 10 medical centers | 1186 patients/132,583 slices) | multinational | 95 | 96 | 96/0.95 |
| Harmoon [13] | CT | AH-Net/Densnet-121 | multinational datasets | 2724 scans/2617 patients | multinational | N/A | N/A | N/0.949 |
| Wang [17] | CXR | DeepLabv3 | CC-CXRI/CC-CXRI-P | SYSU 145202 CC-CXRI-P 16196 | China | 92.94 | 8704 | N/0.968 |
| Wehbe [18] | CXR | DeepCOVID-XR | multicenter | 2214 images/1192 COVID-19 | US | 75 | 93 | 83/0.90 |
| Jiao [44] | CXR | U-Net | 2 medical centers | 1834 patients | US | 73.8 | 85.3 | N/0.846 |
| Yu [34] | CT | CNN | multicenter | 421 patients | China | N/A | N/A | N/N |
| Mei [41] | CT | CNN | 18 medical centers | 905 patients | China | N/A | N/A | N/0.92 |
| Hwang [27] | CXR | Lunit INSIGHT CXR 2 | 4 medical centers | 172 CXRs/172 patients | Korea | 71.3 | 52.2 | 0.714 |
| Fung [25] | CT | 3D ResNet /LSTM | 2 medical centers | 1040 mild type/2543 patients | China | N/A | N/A | 0.92 |
| Wang [23] | CT | Full-uAI-Discover-NCP | single medical center | 31 patients | China | N/A | N/A | N/N |
| Shan [29] | CT | VB-Net | single medical center | 549 patients | China | N/A | N/A | N/N |
| Murphy [26] | CXR | CAD4COVID-XRay | 3 medical centers | 24,678 patients | the Netherlands | 85 | 61 | N/0.81 |
| Li [10] | CXR | Siamese neural network | CheXpert/1 medical center | 468 patients | US | N/A | N/A | N/0.80 |
| Lessman [31] | CT | CORADS-AI | 1 academic center/1 hospital | 843 patients | the Netherlands | 85.7 | 89.8 | N/0.95 |
| Wang [21] | CT | DenseNet121-FPN/COVID-19Net | multicenter | 5372 patients | China | 78.93 | 89.93 | N/0.90 |
| Schiaffino [43] | CT | SVM vs. MLP | 6 medical centers | 897 patients | Italy | N/A | N/A | N/0.747 vs. 0.844 |
| Bartolucci [33] | CT | 3DSlicer/RadAR | 2 medical centers | 115 patients | Italy | N/A | N/A | N/0.82 |
| Purkayastha [42] | CT | CNN | multicenter | 981 patients | multinational | N/A | N/A | N/0.868 |
| Ma [24] | CT | Advanced decision tree based machine | single medical center | 244 patients | China | 82 | 84 | N/0.84 |
| Cai [20] | CT | RF | single medical center | 99 patients | China | N/A | N/A | N/0.917 –0.940 |
| Yue [22] | CT | logistic regression /random forest | 31 patients/ 72scans | China | 100/75 | 100/89 | N/ 0.97/0.92 | |
| Shiri [45] | CT | ResNet | 9 medical centers | 1141 patients | Switzerland | N/A | N/A | N/N |
| Sengupta [46] | CT | QNN | 5 open-source datasets | 9500 + patients | India | 97.7 | N/A | 96.92/ N |
| Wu [47] | CT | COVID-AL | CC-CCII | 962 patients | China | N/A | N/A | 86.6/0.968 |
| Fouladi [51] | CT | ResNet-50/VGG-16/CNN/CAENN | COVID-19 dataset | 2482 patients | Iran | N/A | N/A | 94/N |
| No. | Source | Contents and Number of Images | Types of Images | Image Format | Links (10 September 2021) |
|---|---|---|---|---|---|
| 1 | COVID-19 Radiography Database | COVID-19 images: 3616, normal images: 10,192 viral pneumonia images: 1345 | CXR | PNG | https://www.kaggle.com/tawsifurrahman/covid19-radiographydatabase |
| 2 | COVID-19 Dataset | COVID-19 images: 3616 | CXR | DCM | |
| 3 | J. P. Cohen’s GitHub | N/A | CXR | JPG and PNG | https://github.com/ieee8023/covid-chestxray-dataset |
| 4 | CC-CCII | Total 617,775 COVID-19, CP and normal | CT | JPG | http://ncov-ai.big.ac.cn/download |
| 5 | UCSD-AI4H | COVID-19 images: 349, non-COVID-19 images: 397 | CT | JPG and PNG | https://github.com/UCSD-AI4H/COVID-CT |
| 6 | HUST-19-iCTCF | Total 19,685 | CT | DCM and JPEG | http://ictcf.biocuckoo.cn/ |
| 7 | European Society of Radiology | N/A | CXR and CT | https://www.eurorad.org/advanced-search?search=COVID | |
| 8 | COVID-19-AR | CXR 233 and CT23 31,935 images/105 patients | CXR and CT | DCM | https://wiki.cancerimagingarchive.net/pages/viewpage.action?pageId=70226443 |
| 9 | COVIDx Dataset | CXR 16,352 and CT 194,922 | CXR and CT | DCM | https://github.com/lindawangg/COVID-Net |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, Z.; Shang, Q.; Ma, C.; Chen, X.; Xiao, E. A Promising and Challenging Approach: Radiologists’ Perspective on Deep Learning and Artificial Intelligence for Fighting COVID-19. Diagnostics 2021, 11, 1924. https://doi.org/10.3390/diagnostics11101924
Wang T, Chen Z, Shang Q, Ma C, Chen X, Xiao E. A Promising and Challenging Approach: Radiologists’ Perspective on Deep Learning and Artificial Intelligence for Fighting COVID-19. Diagnostics. 2021; 11(10):1924. https://doi.org/10.3390/diagnostics11101924
Chicago/Turabian StyleWang, Tianming, Zhu Chen, Quanliang Shang, Cong Ma, Xiangyu Chen, and Enhua Xiao. 2021. "A Promising and Challenging Approach: Radiologists’ Perspective on Deep Learning and Artificial Intelligence for Fighting COVID-19" Diagnostics 11, no. 10: 1924. https://doi.org/10.3390/diagnostics11101924
APA StyleWang, T., Chen, Z., Shang, Q., Ma, C., Chen, X., & Xiao, E. (2021). A Promising and Challenging Approach: Radiologists’ Perspective on Deep Learning and Artificial Intelligence for Fighting COVID-19. Diagnostics, 11(10), 1924. https://doi.org/10.3390/diagnostics11101924






