Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Hemodynamic Analysis
2.3. Physical Performance
2.4. Blood Sampling
2.5. Atherogenic Markers
2.6. Inflammatory Markers and Progenitor Cells
2.7. Statistical Analysis
3. Results
3.1. Subjects
3.2. Physical Performance
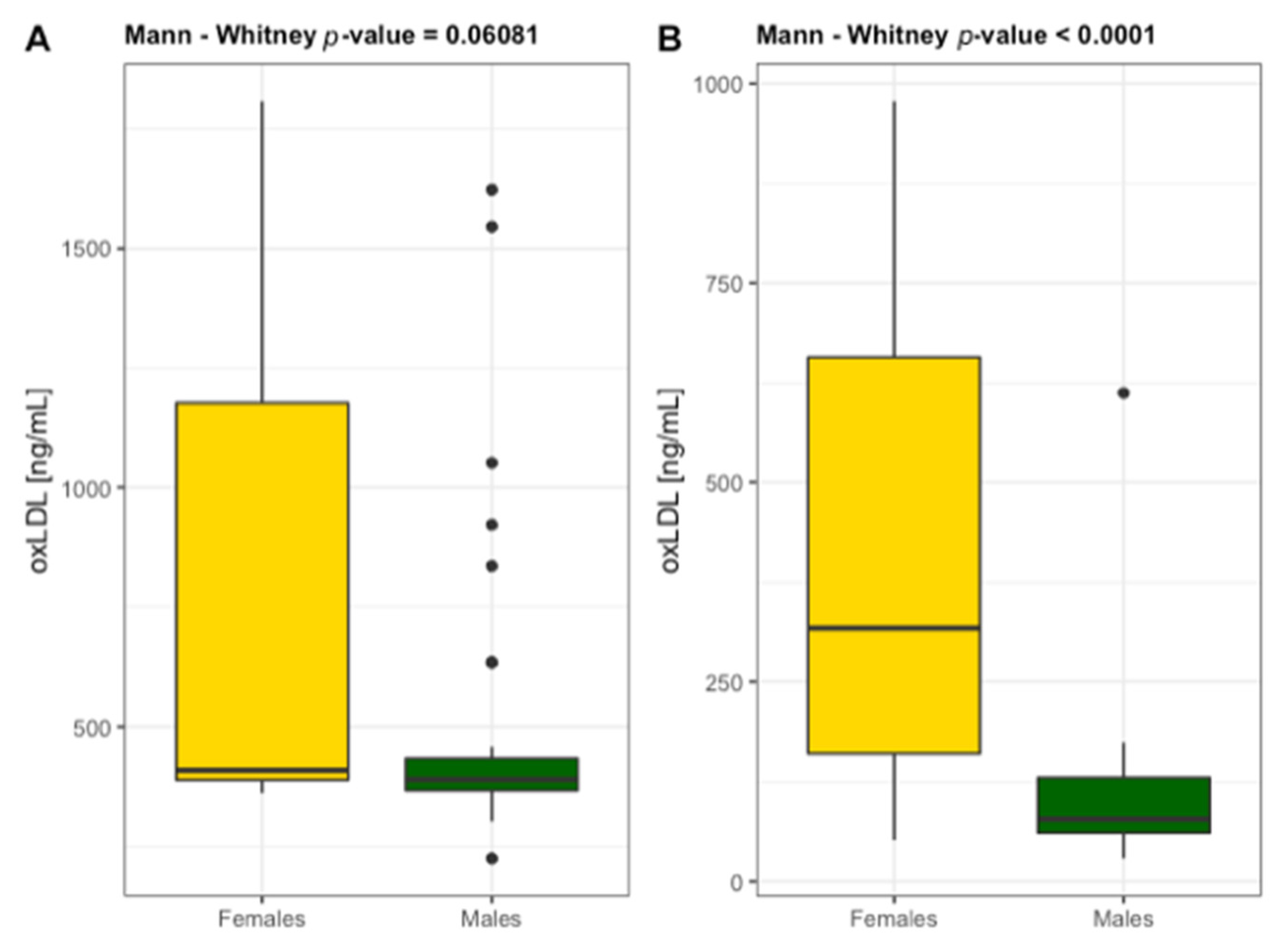
3.3. Atherogenic Markers
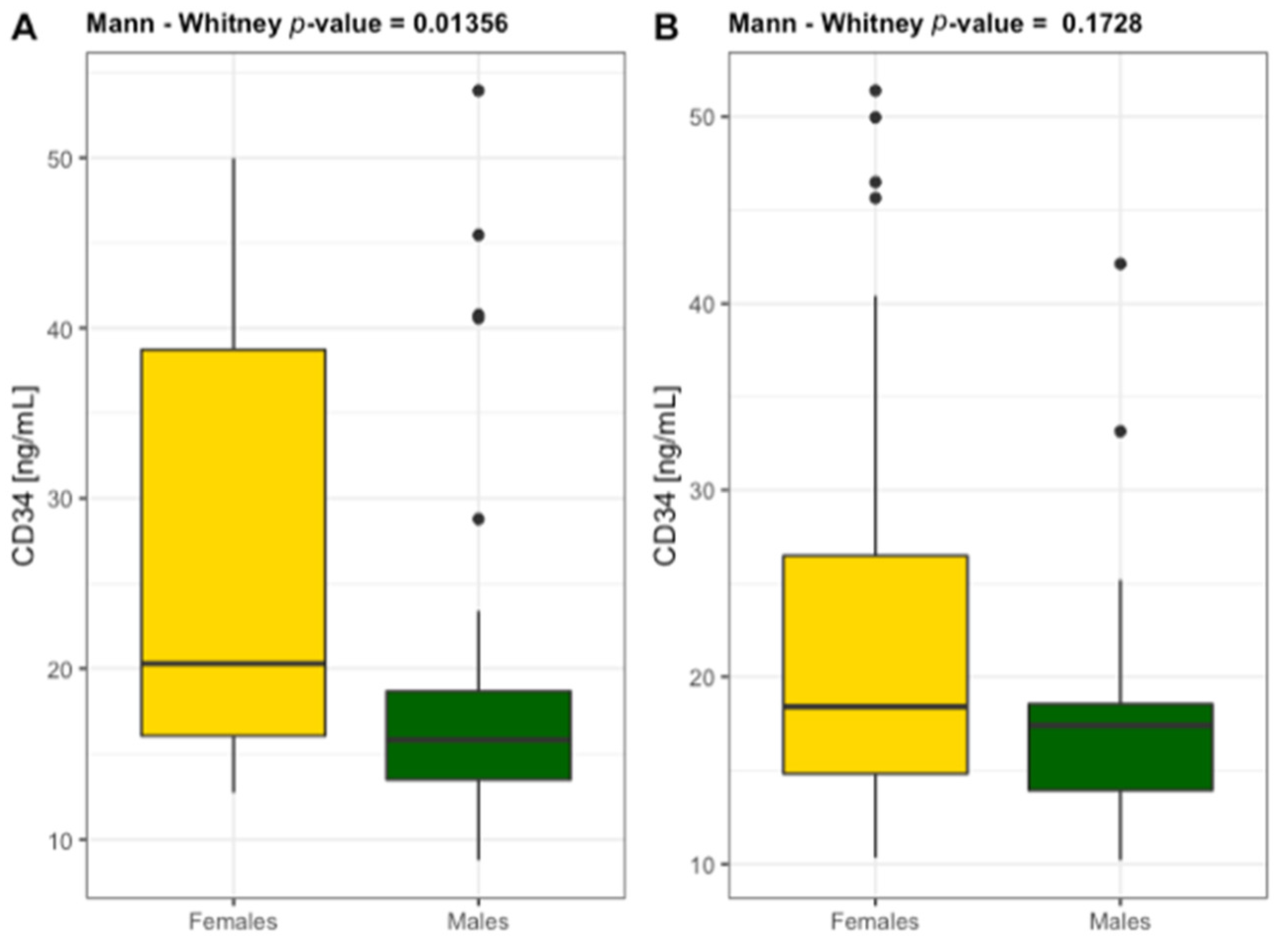
3.4. Inflammatory Markers and Progenitor Cells
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saenz-Pipaon, G.; Martinez-Aguilar, E.; Orbe, J.; González Miqueo, A.; Fernandez-Alonso, L.; Paramo, J.A.; Roncal, C. The Role of Circulating Biomarkers in Peripheral Arterial Disease. Int. J. Mol. Sci. 2021, 22, 3601. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Vanella, L.; Abraham, N.G.; Scuto, S.; Marino, E.; Rocic, P. Pathophysiology of Chronic Peripheral Ischemia: New Perspectives. Ther. Adv. Chronic Dis. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.A.; Yacoub, M. Inflammatory Biomarkers in Peripheral Arterial Disease. Semin. Vasc. Surg. 2014, 27, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The Immune Response in Atherosclerosis: A Double-Edged Sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Shirai, R.; Sato, K.; Yamashita, T.; Yamaguchi, M.; Okano, T.; Watanabe-Kominato, K.; Watanabe, R.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S.; et al. Neopterin Counters Vascular Inflammation and Atherosclerosis. J. Am. Heart Assoc. 2018, 7, e007359. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Fu, Y.-C.; Zhang, D.-W.; Yin, K.; Tang, C.-K. Foam Cells in Atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef]
- Obikane, H.; Abiko, Y.; Ueno, H.; Kusumi, Y.; Esumi, M.; Mitsumata, M. Effect of Endothelial Cell Proliferation on Atherogenesis: A Role of p21Sdi/Cip/Waf1 in Monocyte Adhesion to Endothelial Cells. Atherosclerosis 2010, 212, 116–122. [Google Scholar] [CrossRef]
- Adachi, T.; Naruko, T.; Itoh, A.; Komatsu, R.; Abe, Y.; Shirai, N.; Yamashita, H.; Ehara, S.; Nakagawa, M.; Kitabayashi, C.; et al. Neopterin Is Associated with Plaque Inflammation and Destabilisation in Human Coronary Atherosclerotic Lesions. Heart 2007, 93, 1537–1541. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Anzaldi, M.; Fiore, V.; Candido, S.; Di Marco, R.; Mangano, K.; Quattrocchi, C.; Neri, S. Neopterin: A Potential Marker in Chronic Peripheral Arterial Disease. Mol. Med. Rep. 2013, 7, 1855–1858. [Google Scholar] [CrossRef]
- Melichar, B.; Gregor, J.; Slochova, D.; Lukes, J.; Tichy, M.; Pidrman, V. Increased urinary neopterin in acute myocardial infarction. Clin. Chem. 1994, 40, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Willeit, J.; Kiechl, S.; Fuchs, D.; Jarosch, E.; Oberhollenzer, F.; Reibnegger, G.; Tilz, G.P.; Gerstenbrand, F.; Wachter, H. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis 1994, 106, 263–271. [Google Scholar] [CrossRef]
- Lanser, L.; Pölzl, G.; Fuchs, D.; Weiss, G.; Kurz, K. Neopterin Is Associated with Disease Severity and Outcome in Patients with Non-Ischaemic Heart Failure. J. Clin. Med. 2019, 8, 2230. [Google Scholar] [CrossRef]
- Avanzas, P.; Arroyo-Espliguero, R.; Quiles, J.; Roy, D.; Kaski, J.C. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur. Heart J. 2005, 26, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ozkaramanli Gur, D.; Gur, O.; Guzel, S.; Akyuz, A.; Gurkan, S.; Alpsoy, S.; Gulec, N.S.; Koc, F. Inflammatory Mediators Across the Spectrum of Ankle-Brachial Index. J. Atheroscler. Thromb. 2019, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Grammer, T.B.; Fuchs, D.; Boehm, B.O.; Winkelmann, B.R.; Maerz, W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin. Chem. 2009, 55, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar] [PubMed]
- Arain, F.A.; Cooper, L.T. Peripheral Arterial Disease: Diagnosis and Management. Mayo Clin. Proc. 2008, 83, 944–950. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking Speed: The Functional Vital Sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 17 June 2021).
- Zuo, W.; Liu, N.; Zeng, Y.; Liu, Y.; Li, B.; Wu, K.; Xiao, Y.; Liu, Q. CD38: A Potential Therapeutic Target in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2020, 35, 815–828. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, C. Oxidized LDL Causes Endothelial Apoptosis by Inhibiting Mitochondrial Fusion and Mitochondria Autophagy. Front. Cell Dev. Biol. 2020, 8, 600950. [Google Scholar] [CrossRef] [PubMed]
- Avanzas, P. Markers of Inflammation and Multiple Complex Stenoses (Pancoronary Plaque Vulnerability) in Patients with Non-ST Segment Elevation Acute Coronary Syndromes. Heart 2004, 90, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Pankow, J.S.; Tracy, R.P.; Arnett, D.K.; Peacock, J.M.; Hong, Y.; Djoussé, L.; Eckfeldt, J.H. Association of C-Reactive Protein with Markers of Prevalent Atherosclerotic Disease. Am. J. Cardiol. 2001, 88, 112–117. [Google Scholar] [CrossRef]
- Chen, X.; Stoner, J.A.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Chen, S.; Janitz, A.E.; Gardner, A.W. Prediction of 6-Minute Walk Performance in Patients with Peripheral Artery Disease. J. Vasc. Surg. 2017, 66, 1202–1209. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.; Yancey, P.; Davies, S.; Jerome, W.; Linton, E.; Song, W.; Doran, A.; Vickers, K. The Role of Lipids and Lipoproteins in Atherosclerosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343489/ (accessed on 17 June 2021).
- Aday, A.W.; Everett, B.M. Dyslipidemia Profiles in Patients with Peripheral Artery Disease. Curr. Cardiol. Rep. 2019, 21, 42. [Google Scholar] [CrossRef]
- Smith, F.B.; Lowe, G.D.; Fowkes, F.G.; Rumley, A.; Rumley, A.G.; Donnan, P.T.; Housley, E. Smoking, Haemostatic Factors and Lipid Peroxides in a Population Case Control Study of Peripheral Arterial Disease. Atherosclerosis 1993, 102, 155–162. [Google Scholar] [CrossRef]
- Bergmark, C.; Wu, R.; de Faire, U.; Lefvert, A.K.; Swedenborg, J. Patients with Early-Onset Peripheral Vascular Disease Have Increased Levels of Autoantibodies against Oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 441–445. [Google Scholar] [CrossRef]
- Tsimikas, S.; Kiechl, S.; Willeit, J.; Mayr, M.; Miller, E.R.; Kronenberg, F.; Xu, Q.; Bergmark, C.; Weger, S.; Oberhollenzer, F.; et al. Oxidized Phospholipids Predict the Presence and Progression of Carotid and Femoral Atherosclerosis and Symptomatic Cardiovascular Disease: Five-Year Prospective Results from the Bruneck Study. J. Am. Coll. Cardiol. 2006, 47, 2219–2228. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Tylutka, A.; Wacka, E.; Wawrzyniak-Gramacka, E.; Hiczkiewicz, D.; Kasperska, A.; Czuba, M. Intermittent Hypoxic Exposure Reduces Endothelial Dysfunction. Biomed. Res. Int. 2020, 2020, 6479630. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Uzui, H.; Nakano, A.; Mitsuke, Y.; Geshi, T.; Ueda, T.; Lee, J.-D. Number of Endothelial Progenitor Cells in Peripheral Artery Disease as a Marker of Severity and Association with Pentraxin-3, Malondialdehyde-Modified Low-Density Lipoprotein and Membrane Type-1 Matrix Metalloproteinase. J. Atheroscler. Thromb. 2012, 19, 149–158. [Google Scholar] [CrossRef]
- Hu, T.; She, Q.; Jiang, Y.; Su, L.; Yin, Y. Level of CD14+-Endothelial Progenitor Cells Is Not Associated with Coronary Artery Disease or Cardiovascular Risk Factors. Age 2008, 30, 319–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Maruyama, S.; Ozaki, T.; Taguchi, A.; Meigs, J.; Dimmeler, S.; Zeiher, A.M.; de Kreutzenberg, S.; Avogaro, A.; Nickenig, G.; et al. Circulating Progenitor Cell Count for Cardiovascular Risk Stratification: A Pooled Analysis. PLoS ONE 2010, 5, e11488. [Google Scholar] [CrossRef]
- Hayek, S.S.; MacNamara, J.; Tahhan, A.S.; Awad, M.; Yadalam, A.; Ko, Y.-A.; Healy, S.; Hesaroieh, I.; Ahmed, H.; Gray, B.; et al. Circulating Progenitor Cells Identify Peripheral Arterial Disease in Patients With Coronary Artery Disease. Circ. Res. 2016, 119, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Asahara, T. Role of Progenitor Endothelial Cells in Cardiovascular Disease and Upcoming Therapies. Catheter. Cardiovasc. Interv. 2007, 70, 477–484. [Google Scholar] [CrossRef]
- Boslett, J.; Hemann, C.; Christofi, F.L.; Zweier, J.L. Characterization of CD38 in the Major Cell Types of the Heart: Endothelial Cells Highly Express CD38 with Activation by Hypoxia-Reoxygenation Triggering NAD(P)H Depletion. Am. J. Physiol. Cell Physiol. 2018, 314, C297–C309. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M. Rationale and Tutorial for Analysing and Reporting Sex Differences in Cardiovascular Associations. Heart 2019, 105, 1701–1708. [Google Scholar] [CrossRef]
- Dogan, S.; Deshpande, D.A.; White, T.A.; Walseth, T.F.; Kannan, M.S. Regulation of CD38 Expression and Function by Steroid Hormones in Myometrium. Mol. Cell. Endocrinol. 2006, 246, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; Huang, W.; Deng, K.-Y.; Qian, Y.; Xin, H.-B. 17β-Estradiol Promotes Apoptosis in Airway Smooth Muscle Cells Through CD38/SIRT1/P53 Pathway. Front. Endocrinol. 2018, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
| Patients | Controls | Patients vs. Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Females n = 17 | Males n = 42 | Females vs. Males | Females n = 42 | Males n = 18 | Females vs. Males | Females | Males |
| Age [y] | 68.5 ± 7.4 | 66.4 ± 8.5 | 0.387 | 68.9 ± 4.7 | 73.9 ± 5.5 | <0.01 | 0.349 | <0.01 |
| Height [cm] | 158.7 ± 5.4 | 171.6 ± 7.1 | <0.001 | 160.6 ± 4.7 | 168.5 ± 4.7 | <0.0001 | 0.197 | 0.077 |
| Weight [kg] | 66.4 ± 11.0 | 82.3 ± 13.0 | <0.001 | 66.1 ± 6.7 | 80.0 ± 8.4 | <0.001 | 0.902 | 0.051 |
| BMI [kg/m2] | 26.3 ± 4.0 | 27.9 ± 3.5 | 0.159 | 25.8 ± 2.7 | 27.9 ± 2.6 | <0.5 | 0.644 | 0.921 |
| Diabetes, n (%) | 7 (41.2) | 13 (30.9) | - | 3 (7.1) | 4 (22.2) | - | - | - |
| SBP [mmHg] | 134 ± 15 | 138 ± 15 | 0.414 | 129 ± 15 | 133 ± 13 | 0.421 | 0.371 | 0.220 |
| DBP [mmHg] | 78 ± 7 | 80 ± 8 | 0.436 | 78 ± 10 | 81 ± 9 | 0.175 | 0.628 | 0.543 |
| 6MWT [m] | 328 ± 62 | 371 ± 63 | <0.05 | 502 ± 66 | 504 ± 57 | 0.824 | <0.0001 | <0.0001 |
| Gait speed [m/s] | 0.91 ± 0.17 | 1.03 ± 0.18 | <0.05 | 1.39 ± 0.18 | 1.40 ± 0.16 | 0.824 | <0.0001 | <0.0001 |
| Medication | Females n = 17 | Males n = 42 | Total n = 59 |
|---|---|---|---|
| Beta-blockers | 7 (41.2%) | 17 (40.5%) | 24 (40.7%) |
| ASA | 13 (76.5%) | 33 (78.6%) | 46 (78.0%) |
| Anticoagulants | 7 (41.2%) | 16 (38.1%) | 23 (39.0%) |
| Statin | 14 (82.4%) | 27 (64.3%) | 41 (69.5%) |
| Fenofibrate | 0 | 3 (7.1%) | 3 (5.1%) |
| ACEi | 7 (41.2%) | 17 (40.5%) | 24 (40.7%) |
| ARB | 2 (11.8%) | 8 (19.0%) | 10 (16.9%) |
| CCB | 6 (35.3%) | 14 (33.3%) | 20 (33.9%) |
| Clonidine | 2 (11.8%) | 2 (4.8%) | 4 (6.8%) |
| Doxazosin | 3 (17.6%) | 0 | 3 (5.1%) |
| Diuretics | 7 (41.2%) | 15 (35.7%) | 22 (37.3%) |
| Insulin | 2 (11.8%) | 5 (11.9%) | 7 (11.9%) |
| Metformin | 6 (35.3%) | 9 (21.4%) | 15 (25.4%) |
| OAD | 7 (41.2%) | 7 (16.7%) | 14 (23.7%) |
| Bencyclane | 2 (11.8%) | 8 (19.0%) | 10 (16.9%) |
| Pentoxifylline | 4 (23.5%) | 8 (19.0%) | 12 (20.3%) |
| Biomarker | Patients | ABI ≤ 0.9 vs. ABI ≤ 0.5 | η2 | Controls | Controls vs. ABI ≤ 0.9 | η2 | Controls vs. ABI ≤ 0.5 | η2 | |
|---|---|---|---|---|---|---|---|---|---|
| ABI ≤ 0.9 n = 43 | ABI ≤ 0.5 n = 16 | ABI ≥ 0.9 n = 60 | |||||||
| TG [mg/dL] | 147 ± 94 | 129 ± 57 | 0.878 | 0.017 | 140 ± 29 | <0.05 | 0.038 | <0.05 | 0.043 |
| TC [mg/dL] | 178 ± 37 | 185 ± 43 | 0.838 | 0.017 | 224 ± 35 | <0.0001 | 0.287 | <0.01 | 0.128 |
| LDL [mg/dL] | 100 ± 33 | 107 ± 29 | 0.627 | 0.013 | 84 ± 28 | <0.05 | 0.056 | <0.05 | 0.076 |
| HDL [mg/dL] | 47 ± 8 | 45 ± 7 | 0.393 | 0.005 | 72 ± 13 | <0.0001 | 0.605 | <0.0001 | 0.431 |
| non-HDL [mg/dL] | 131 ± 38 | 141 ± 44 | 0.627 | 0.013 | 151 ± 38 | <0.01 | 0.058 | 0.209 | 0.008 |
| oxLDL [ng/mL] | 467 ± 159 | 467 ± 137 | 0.946 | 0.017 | 319 ± 284 | <0.001 | 0.131 | <0.01 | 0.080 |
| 3NT [nmol/mL] | 3.89 ± 2.38 | 5.33 ± 2.95 | 0.054 | 0.048 | 1.29 ± 0.81 | <0.0001 | 0.532 | <0.0001 | 0.413 |
| TG [mg/dL] | TC [mg/dL] | LDL [mg/dL] | HDL [mg/dL] | Non-HDL [mg/dL] | EPC [ng/mL] | CD34 [ng/mL] | CD38 [ng/mL] | |
|---|---|---|---|---|---|---|---|---|
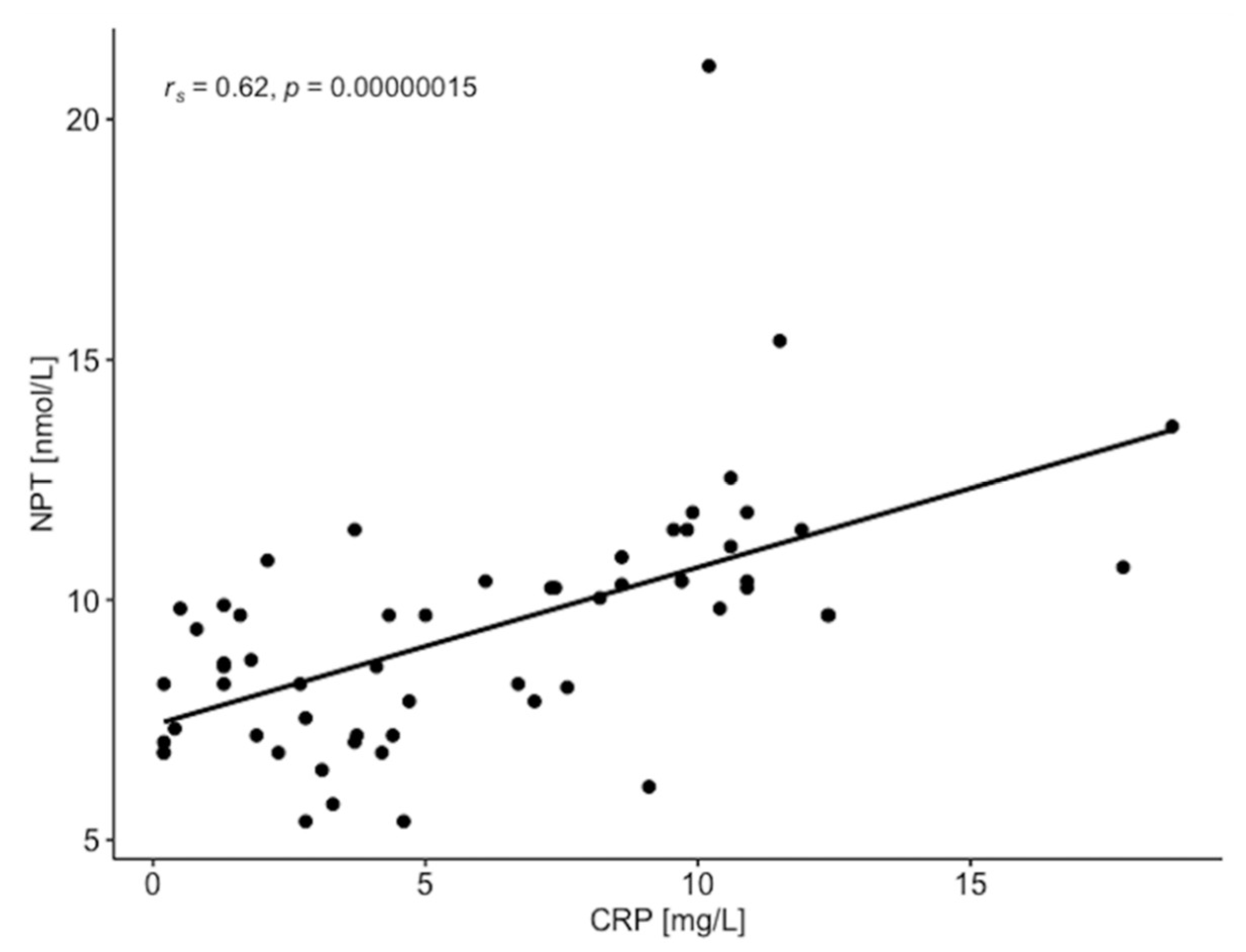
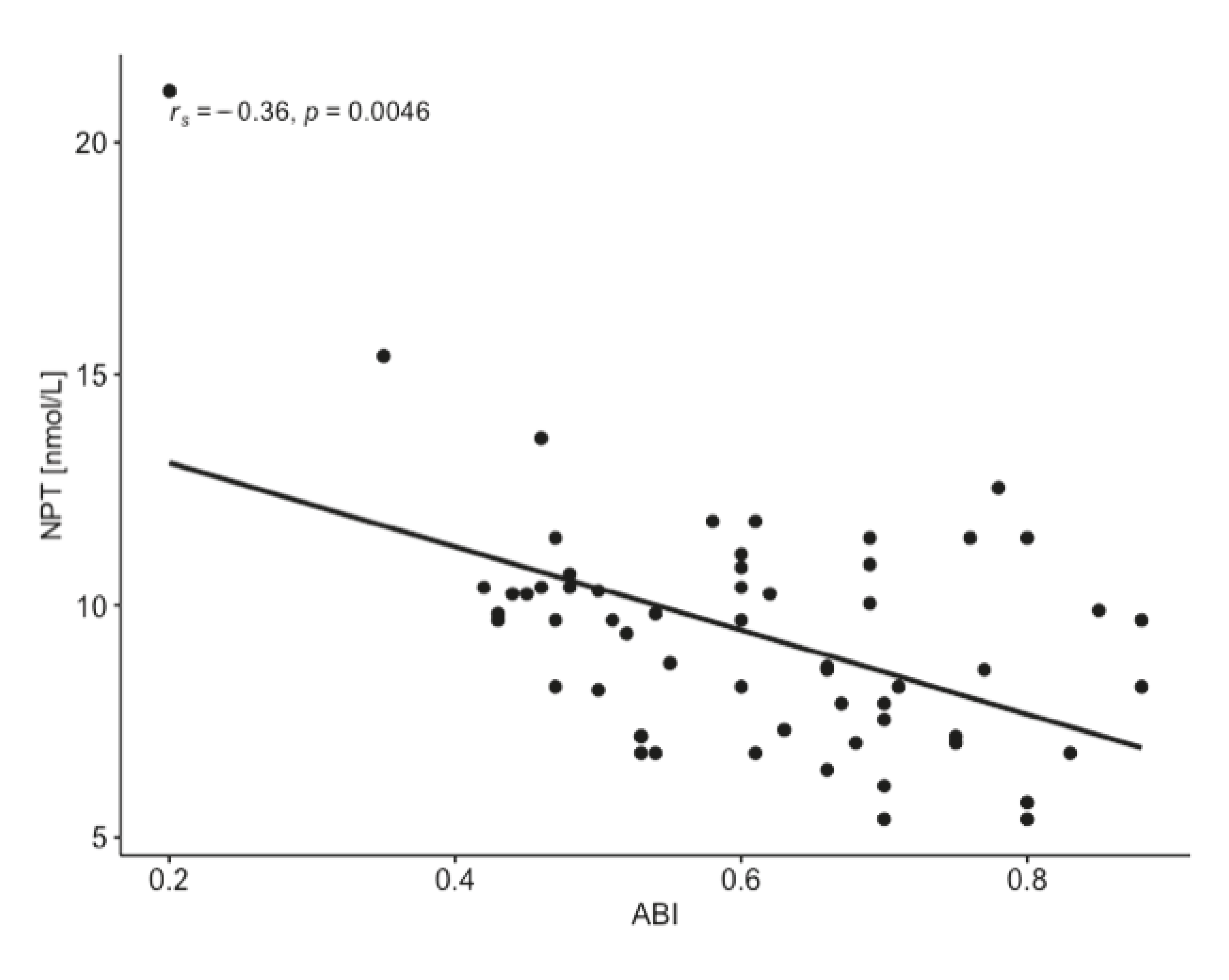
| NPT [nmol/L] | rs = −0.103 p = 0.436 | rs = 0.514 p < 0.001 | rs = 0.394 p < 0.01 | rs = 0.043 p = 0.747 | rs = 0.501 p < 0.001 | rs = −0.286 p < 0.05 | rs = −0.109 p = 0.408 | rs = −0.105 p = 0.428 |
| CRP [mg/L] | rs = −0.132 p = 0.318 | rs = 0.394 p < 0.01 | rs = 0.363 p < 0.01 | rs = 0.023 p = 0.861 | rs = 0.394 p < 0.01 | rs = −0.324 p < 0.05 | rs = −0.162 p = 0.218 | rs = −0.120 p = 0.365 |
| Biomarker | Patients | ABI ≤ 0.9 vs. ABI ≤ 0.5 | η2 | Controls | Controls vs. ABI ≤ 0.9 | η2 | Controls vs. ABI ≤ 0.5 | η2 | |
|---|---|---|---|---|---|---|---|---|---|
| ABI ≤ 0.9 n = 43 | ABI ≤ 0.5 n = 16 | ABI ≥ 0.9 n = 60 | |||||||
| NPT [nmol/L] | 8.70 ± 1.95 | 11.24 ± 3.17 | <0.01 | 0.114 | 8.15 ± 1.33 | 0.261 | 0.003 | <0.0001 | 0.349 |
| CRP [mg/L] | 4.37 ± 3.54 | 10.69 ± 3.52 | <0.0001 | 0.349 | 2.98 ± 1.94 | 0.129 | 0.013 | <0.0001 | 0.464 |
| EPCs [ng/mL] | 15.64 ± 6.82 | 8.90 ± 2.22 | <0.0001 | 0.256 | 18.90 ± 8.53 | <0.05 | 0.039 | <0.0001 | 0.361 |
| CD34 [ng/mL] | 21.30 ± 11.24 | 14.92 ± 3.69 | <0.05 | 0.078 | 21.21 ± 10.43 | 0.730 | 0.009 | <0.05 | 0.055 |
| CD38 [ng/mL] | 0.935 ± 0.582 | 0.781 ± 0.442 | 0.344 | 0.000 | 1.115 ± 0.793 | 0.126 | 0.013 | <0.05 | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zembron-Lacny, A.; Dziubek, W.; Tylutka, A.; Wacka, E.; Morawin, B.; Bulinska, K.; Stefanska, M.; Wozniewski, M.; Szuba, A. Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease. Diagnostics 2021, 11, 1911. https://doi.org/10.3390/diagnostics11101911
Zembron-Lacny A, Dziubek W, Tylutka A, Wacka E, Morawin B, Bulinska K, Stefanska M, Wozniewski M, Szuba A. Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease. Diagnostics. 2021; 11(10):1911. https://doi.org/10.3390/diagnostics11101911
Chicago/Turabian StyleZembron-Lacny, Agnieszka, Wioletta Dziubek, Anna Tylutka, Eryk Wacka, Barbara Morawin, Katarzyna Bulinska, Malgorzata Stefanska, Marek Wozniewski, and Andrzej Szuba. 2021. "Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease" Diagnostics 11, no. 10: 1911. https://doi.org/10.3390/diagnostics11101911
APA StyleZembron-Lacny, A., Dziubek, W., Tylutka, A., Wacka, E., Morawin, B., Bulinska, K., Stefanska, M., Wozniewski, M., & Szuba, A. (2021). Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease. Diagnostics, 11(10), 1911. https://doi.org/10.3390/diagnostics11101911






