1. Introduction
Numerous liver diseases can occur during pregnancy, and some of them can be accompanied by serious consequences for the mother and the baby. They often have very similar presentation, and their diagnosis does not seem to be obvious in all cases [
1,
2,
3]. Pregnancy-related liver disorders generally occur in the second or third trimester, with a mortality up to 25% [
4].
Differential diagnosis between hyperemesis gravidarum (HG), intrahepatic gestational cholestasis (ICP), preeclampsia (PE), acute fatty liver of pregnancy (AFLP), and/or HELLP syndrome is necessary to ensure the diagnostic outcome and the exact interdisciplinary management of the pregnancy [
5,
6,
7,
8].
A clinical and biochemical investigation does not fully discriminate between the various forms [
9,
10,
11]. In particular, AFLP and ICP1 share most clinical signs and occur in the same period of pregnancy, so that DNA analysis becomes imperative for a precise classification of the disease and the consequent therapeutic decisions. ICP1 causes severe itching that usually begins on palms and soles and then spreads to other parts of the body. Although the condition is often not strictly severe, it can cause problems for the fetus. In fact, it is associated with an increased risk of premature birth and stillbirth [
11]. Additionally, some babies born to ICP1 mothers present an increased risk of adverse perinatal outcomes [
12].
We describe the case of a woman with diamniotic dichorionic twin pregnancy (DCDA) who arrived at clinical observation at 32 + 3 weeks of pregnancy for generalized itching, evocative of intrahepatic cholestasis, with liver enlargement suggestive of severe steatosis. This result, together with leukocytosis and increased serum transaminases and uric acid led to the suspicion of AFLP, five Swansea criteria being met. Considering the importance of obtaining an accurate diagnosis to improve the clinical management of both the mother and the fetuses and to cope with subsequent pregnancies in the best possible way, we decided to elucidate the basis of the disease through DNA investigation also by a noninvasive procedure.
In fact, since variants of some genes associated with AFLP can result in severe disease of the newborn if at homozygous or compound heterozygous state, we decided to analyze at the same time both fetal and parents’ DNA.
2. Materials and Methods
2.1. Patient
The study conformed to the guidelines outlined by the 1975 Declaration of Helsinki, and written consent was obtained from the patient. A 35-year-old female primigravida at 32 + 3 weeks of a dichorionic diamniotic (DCDA) twin pregnancy from homologous in vitro fertilization (IVF) was admitted to the division of prenatal medicine of Sant’Orsola-Malpighi Hospital. In line with the IVF protocol, the patient received combined estrogen and progesterone supplementation until gestation week 12. The woman had a BMI of 33 kg/m
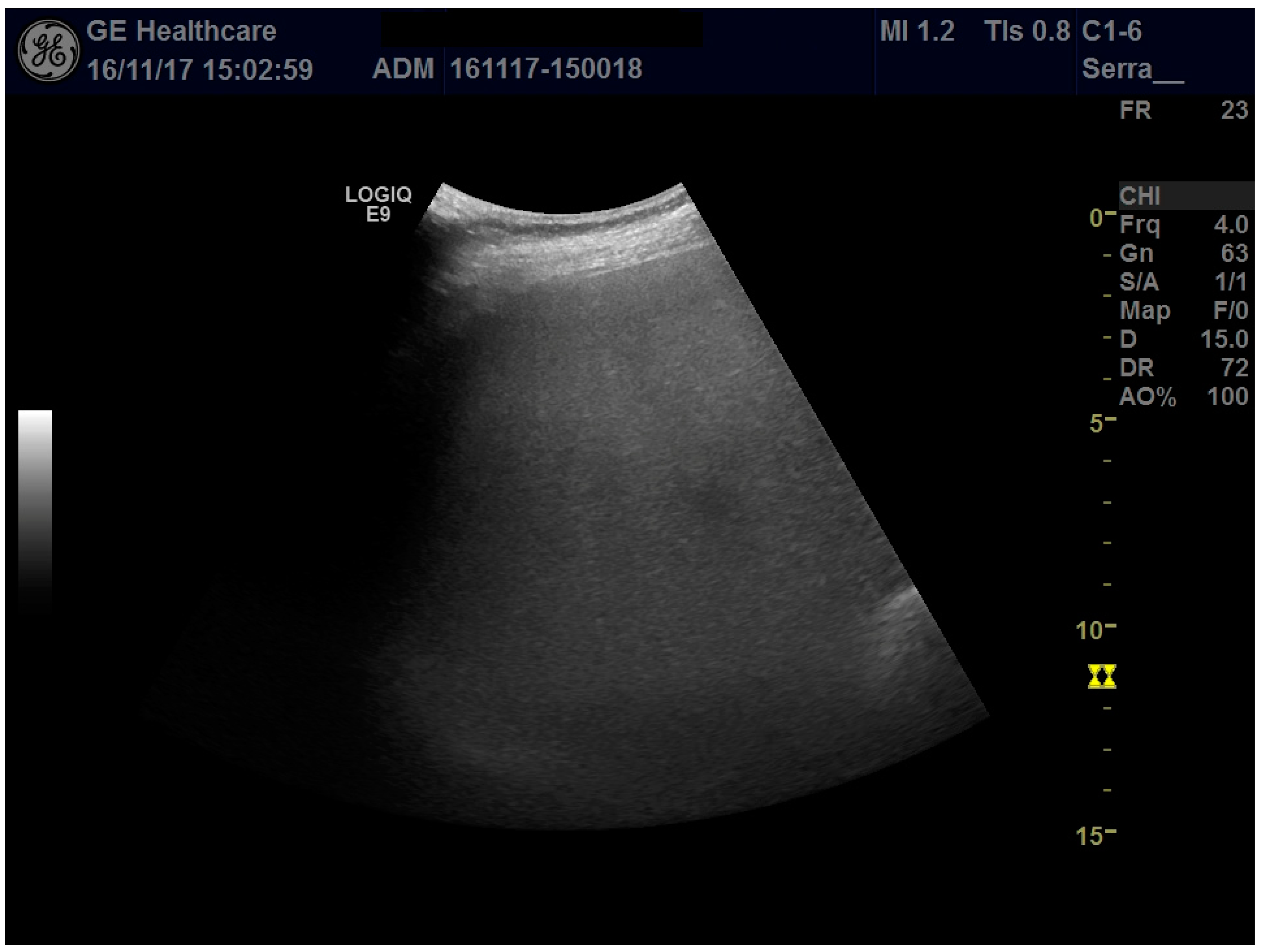
2 at the appearance of the symptoms (having gained 12 kg in weight). At the time of admission, due to the sudden appearance of abdominal pain as well as generalized itching, laboratory tests showed a mild increase in transaminases above the upper limit of normal and elevated serum bile acid concentrations (23 μmol/L), a picture consistent with ICP; therefore, treatment with ursodeoxycholic acid 1500 mg/day was started. Abdominal ultrasonography revealed an enlarged liver (longitudinal right lobe diameter, 20 cm) with regular margins and marked hyperechogenicity determining attenuation of the ultrasound beam in the deeper segments of the liver, suggestive of severe steatosis (
Figure 1); due to the ultrasound beam attenuation, the suprahepatic veins were not visible. Liver elastography with 2Dlogic E9 gave a liver stiffness value of 1.48 ± 0.13 m/s.
During her stay in the ward, neutrophil leukocytosis (>15,000 cells/μL), increased serum transaminases (up to a 3-fold upper limit of normal), and increased serum uric acid (399 μmol/L) were observed. In addition to worsening transaminases, hyperuricemia, leukocytosis, nausea, abdominal pain, and a marked bright liver on ultrasound, the patient fulfilled five Swansea criteria for AFLP [
13].
Given the possible fetal involvement in AFLP, we opted for WES analysis of the blood DNA of the woman and her partner and of the cell-free fetal DNA (cffDNA) of the ongoing pregnancy to search for fetal variants. The parents signed informed written consent prior to sample collection.
WES analysis of the blood DNA of the woman and her partner and of the cell-free fetal DNA (cffDNA) of the ongoing pregnancy led to a diagnosis of ICP1.
During hospitalization, the patient exhibited worsening serum bilirubin (from 0.7 to 17 μmol/L) and therefore underwent a cesarean delivery at 35 weeks after receiving antenatal betamethasone to accelerate fetal lung maturation. After delivery and then every six months, the patient was followed up. The last known follow-up, carried out 20 months after pregnancy, showed normal liver function, a residual hyperechoic area of 16 × 10 mm at liver segment VI (also investigated by contrast US imaging and suggestive of an area of residual steatosis), and normal liver dimensions.
After birth, twins auxological parameters and Apgar scores were appropriate. Clinical examination at 2 and 4 months of age confirmed normal growth and psychomotor development.
2.2. DNA Extraction, Library Construction, and Sequencing
Cell-free fetal DNA (cffDNA) and genomic DNA (gDNA) extraction and sequencing were performed as previously described [
14]
2.3. Estimation of the cffDNA Fraction
To determine the cffDNA fraction, the proportion of reads on the Y chromosome, in particular, the presence of the variants in the SRY gene, was estimated. Taking into consideration that in twin pregnancies the cffDNA fraction ranges from 5.4% to 23.5% [
15], the distribution of the cffDNA fraction in twins has an average of 12.1% as compared to 9.6% in individual pregnancies in our in-house samples. The presence of the Y chromosome and 32 non polymorphic Y-linked loci indicated that the twins were dizygotic males. After normalization of the frequency of the X and the Y chromosomes combined with the cffDNA fraction and the analysis of autosomal single-nucleotide polymorphisms (SNPs) having a frequency greater than 2%, we confirmed the dizygosity of the male twins, as expected by IVF, and estimated the total cffDNA as approximately 10%.
2.4. Data Analysis
WES analysis was performed by examining the filtered FastQ data of all genes hitherto known to be associated with pregnancy and liver disease and following manual IGV screening of BAM files.
Variant calling was carried out using Varscan software (version 2.4.0, Washington University, Greater St. Louis, MO, USA) to call variants which met the desired thresholds for read depth, base quality, variant allele frequency, and statistical significance. This software allows the analysis of the variants present in regions with low coverage, somatic mutations and multisamples, as well as germline variants. This strategy was applied to both cffDNA and genomic WES.
3. Results
WES was performed in 2 weeks, while the confirmation of the DNA analysis was carried out a few days after the birth of the twins.
Trio-WES (cffDNA plus maternal and paternal gDNA) identified the c.913T > A variant (Phe305Ile) in the ATP8B1 gene in maternal cffDNA and lymphocytes DNA. No variants were detected along other liver disease-associated genes. Phe305Ile (rs150860808) is reported in the gnomAD database (v.2.1.1) with a frequency of 0.18% in the non-Finnish European population (NFE) (0.19% in males and 0.16% in females), and no homozygotes are reported, while in general populations, the frequency is 0.12% and 0.1% in males and females, respectively. In the ClinVar database, the variant is reported with conflicting interpretations of pathogenicity.
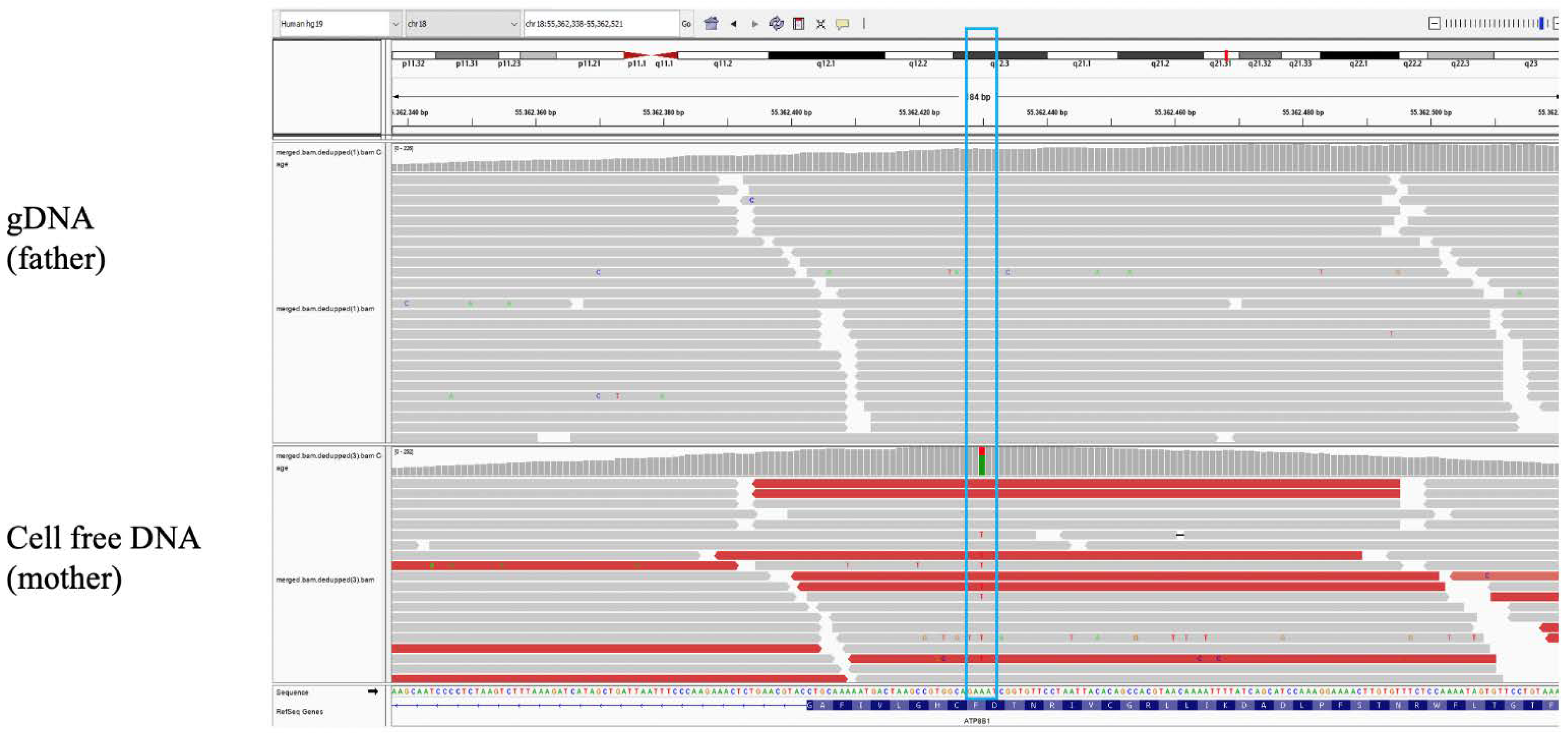
The cffDNA was analyzed at a greater reading depth than the maternal and paternal DNA (
Figure 2), and the lymphocytes’ DNA of the twins was also analyzed after birth. Since in cffDNA the fetal fraction was ~10% and the variant reading rate was ~31%, a clear imbalance towards the wild-type allele emerged (
Figure 2), suggesting that the variant was exclusively maternal. All lymphocytes DNA data confirmed the interpretation.
The repeated bile acid analysis in the mother 10 after delivery of the twins was just above the normal values.
4. Discussion
Disease framing through DNA analysis can significantly increase the diagnostic rate and lead to better management, regardless of the specificity of the clinical signs. This takes on effective significance in the prenatal setting, where an exact diagnosis can greatly influence the management of both pregnancy and the newborn.
AFLP is a rare disease of the third trimester of pregnancy (1 in 7000–10,000 pregnancies), associated with variants of the LCHAD gene alone, while ICP, a disorder of the second or third trimester of pregnancy, is found in 3–5% of pregnancies, with variants associated with 13 genes of the ABC membrane protein complex, and ATP8B1, TJB2, NR1H4, and ANO8 involved in the transport and metabolism of bile salts. On a clinical basis, these pregnancy disorders are more severe in AFLP, with the need for immediate termination of the pregnancy to avoid maternal disseminated intravascular coagulation and death. If the newborn is a carrier of the mother’s genetic defect, the risk of death from dilated cardiomyopathy or progressive neuropathy is high. Instead, ICP can be treated with ursodeoxycholic acid (UDCA) to reduce the risk of perinatal morbidity and mortality and relieve maternal symptoms. The timing of delivery reflects the balance between fetal death, risk of severe prematurity, and sudden maternal death with high concentrations of bile acids. In the absence of genetic tests, the differential diagnosis between AFLP and ICP can be challenging, and the analysis of fetal and maternal DNA may be appropriate to identify any risks to the fetus, if it is a carrier of the maternal genetic variant.
In our case, WES identified a maternal heterozygous variant (c.913T > A) located in exon 10 of the
ATP8B1 gene (NM_005603.6), leading to diagnosis of ICP1, anticipation of the delivery, and setting up of a follow-up program for liver function checking. ATP8B1 is a flippase involved in phospholipid translocation from the outer to the inner leaflet of the cell membrane, expressed in the canalicular membrane of liver cells as well as in the apical membranes of intestinal and pancreatic cells [
16,
17]. The variant we detected has previously been reported in individuals with ICP1, all showing post-partum resolution [
18]. Thus, the variant, although predicted as possibly deleterious, does not seem to have effects other than impaired liver function during pregnancy, at least at the heterozygous state. Indeed, combined heterozygosity for this variant as well as for other variants of
ATP8B1 is associated with progressive familial intrahepatic cholestasis (#243300), characterized by episodic cholestatic disease with intervals during which there is no clinical or biochemical evidence of cholestasis. This indicates that intrahepatic cholestasis linked to this gene is triggered not only by variants that alter the stability of the protein, its folding, or the ability to interact with other proteins involved in the transport of bile salts, but also by the environment. During pregnancy, the higher estrogen levels can impair biliary salts transport to the liver, determining cholestasis in predisposed individuals [
19]. It is possible that even an excessive increase in body weight, as was the case for our proband, can increment the predisposition to cholestasis. As to intermittent cholestasis associated to
ATP8B1, bacterial endotoxins such as lipopolysaccharides released from sites of infection [
20] might induce the failure of bile transport, thus explaining intermittent cholestasis. Therefore, drugs that act on the transport of bile salts may prevent the occurrence of these conditions.
Our study demonstrates that (i) clinical examination alone is not sufficient for a correct diagnosis, even in the presence of specific clinical/biochemical signs; (ii) diagnosis by noninvasive procedures can be highly efficient even for twin pregnancies; (iii) WES on cffDNA and parental genomic DNA can provide reliable results in few weeks and at a cost comparable to that of SN or CGH-array, even if deeper sequencing is required for cffDNA; (iv) genetic data strongly help in the management of the patient and, in prenatal diagnosis, even of the unborn child.
Finally, this study confirms that the effect on health and disease of frequent variants depends on the burden of environmental factors [
21].
5. Take Home Message
WES may help the diagnosis of conditions where the phenotypes are not immediately interpretable. This assumes an effective significance in prenatal diagnosis, since a correct diagnosis can notably influence the management of the patient in terms of drug therapy and the management of the newborn.
Liver disease can occur during pregnancy where signs and symptoms can sometimes overlap, and usually its features become evident in the third trimester of pregnancy. Since these are complex conditions that often share clinical manifestations, it is essential to find a method for a rapid and precise diagnosis in order to distinguish between the different liver diseases.
Author Contributions
Conceptualization, A.P., A.F. and S.R.G.; methodology, A.P., A.S., F.A. and C.S. software, A.P.; validation, A.P. and A.D.G.; formal analysis, A.P. and A.F.; investigation, A.P. and S.R.G.; resources, S.R.G.; data curation, A.P., A.F., O.Z. and S.R.G.; writing—original draft preparation, A.P. and A.F.; writing—review and editing, O.Z. and S.R.G.; supervision, S.R.G. and O.Z.; funding acquisition, S.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received found from Fondazione di Sardegna.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code 102 and approved on date 9 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval
The study was conducted according to the Declaration of Helsinki statement. Ethics Committee. The patient enrolled in the study provided signed informed consent on genetics investigation and publication. Compliance with Ethical Standards.
References
- Sasamori, Y.; Tanaka, A.; Ayabe, T. Liver disease in pregnancy. Hepatol. Res. 2020, 24, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Filipec-Kanizaj, T.; Jakopcic, I.; Majurec, I.; Brncic-Fischer, A.; Sobocan, N.; Hrstic, I.; Stimac, T.; Stimac, D.; Milic, S. Liver disease during pregnancy: A challenging clinical issue. Med. Sci. Monit. 2018, 24, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Rachel, H. Westbrook, geoffrey dusheiko, catherine williamson. Pregnancy and liver disease. J. Hepatol. 2016, 64, 933–945. [Google Scholar]
- Murali, A.R.; Devarbhavi, H.; Venkatachala, P.R.; Singh, R.; Sheth, K.A. Factors that predict 1-month mortality in patients with pregnancy-specific liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.W. Liver disease in pregnancy: What’s new. Hepatol. Commun. 2020, 4, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, K.; Abe, H.; Kawai, H.; Kamimura, H.; Kobayashi, Y.; Nomoto, M.; Aoyagi, Y.; Terai, S. Advances in understanding and treating liver diseases during pregnancy: A review. World J. Gastroenterol. 2015, 21, 5183–5190. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Tonismae, T. HELLP syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Azzaroli, F.; Mazzella, G.; Marchesini, G.; Brodosi, L.; Petroni, M.L. Fatty liver in pregnancy: A narrative review of two distinct conditions. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Morton, A. Acute fatty liver of pregnancy-differential diagnosis. Am. J. Gastroenterol. 2017, 112, 1342. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.K.; Ibdah, J.A. Role of 3-hydroxy fatty acid-induced hepatic lipotoxicity in acute fatty liver of pregnancy. Int. J. Mol. Sci. 2018, 19, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, C.; Geenes, V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2014, 124, 120–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-Furrer, R.; et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. Lancet 2019, 393, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Morton, A.; Laurie, J. Physiological changes of pregnancy and the Swansea criteria in diagnosing acute fatty liver of pregnancy. Obstet. Med. 2018, 11, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, A.; Palazzo, V.; Reho, P.; Pagliazzi, A.; Marozza, A.; Farina, A.; Zuffardi, O.; Giglio, S. Noninvasive prenatal diagnosis in a family at risk for Fraser syndrome. Prenat. Diagn. 2020, 40, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Villela, D.; Che, H.; Van Ghelue, M.; Dehaspe, L.; Brison, N.; Van Den Bogaert, K.; Devriendt, K.; Lewi, L.; Bayindir, B.; Vermeesch, J.R. Fetal sex determination in twin pregnancies using non-invasive prenatal testing. NPJ Genom. Med. 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsu, H.; Tanaka, G.; Segawa, K.; Suzuki, J.; Nagata, S.; Nakayama, K.; Shin, H.W. Phospholipid flippase activities and substrate specificities of human type IV P-type ATPases localized to the plasma membrane. J. Biol. Chem. 2014, 289, 33543–33556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulusma, C.C.; Groen, A.; Kunne, C.; Ho-Mok, K.S.; Spijkerboer, A.L.; Rudi de Waart, D.; Hoek, F.J.; Vreeling, H.; Hoeben, K.A.; van Marle, J.; et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology 2006, 44, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Müllenbach, R.; Bennett, A.; Tetlow, N.; Patel, N.; Hamilton, G.; Cheng, F.; Chambers, J.; Howard, R.; Taylor-Robinson, S.D.; Williamson, C. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex. Differ. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Marrone, J.; Danielli, M.; Gaspari, C.I.; Marinelli, R.A. Adenovirus-mediated human aquaporin-1 expression in hepatocytes improves lipopolysaccharide-induced cholestasis. IUBMB Life 2017, 69, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari, S.; Luo, Y.; Akbari, A.; Belbin, G.M.; Li, X.; Harris, D.N.; Selig, M.; Bartell, E.; Roger Calderon, R.; Slowikowski, K.; et al. A positively selected FBN1 missense variant reduces height in Peruvian individuals. Nature 2020, 582, 234–239. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).