Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Sample Size
2.3. Assessments and Procedures
2.4. Myotonometric Measurements
2.5. Clinical Variables
2.6. Statistical Analysis
3. Results
3.1. Differences in MMPs, Sociodemographic, and Clinical Variables between Groups
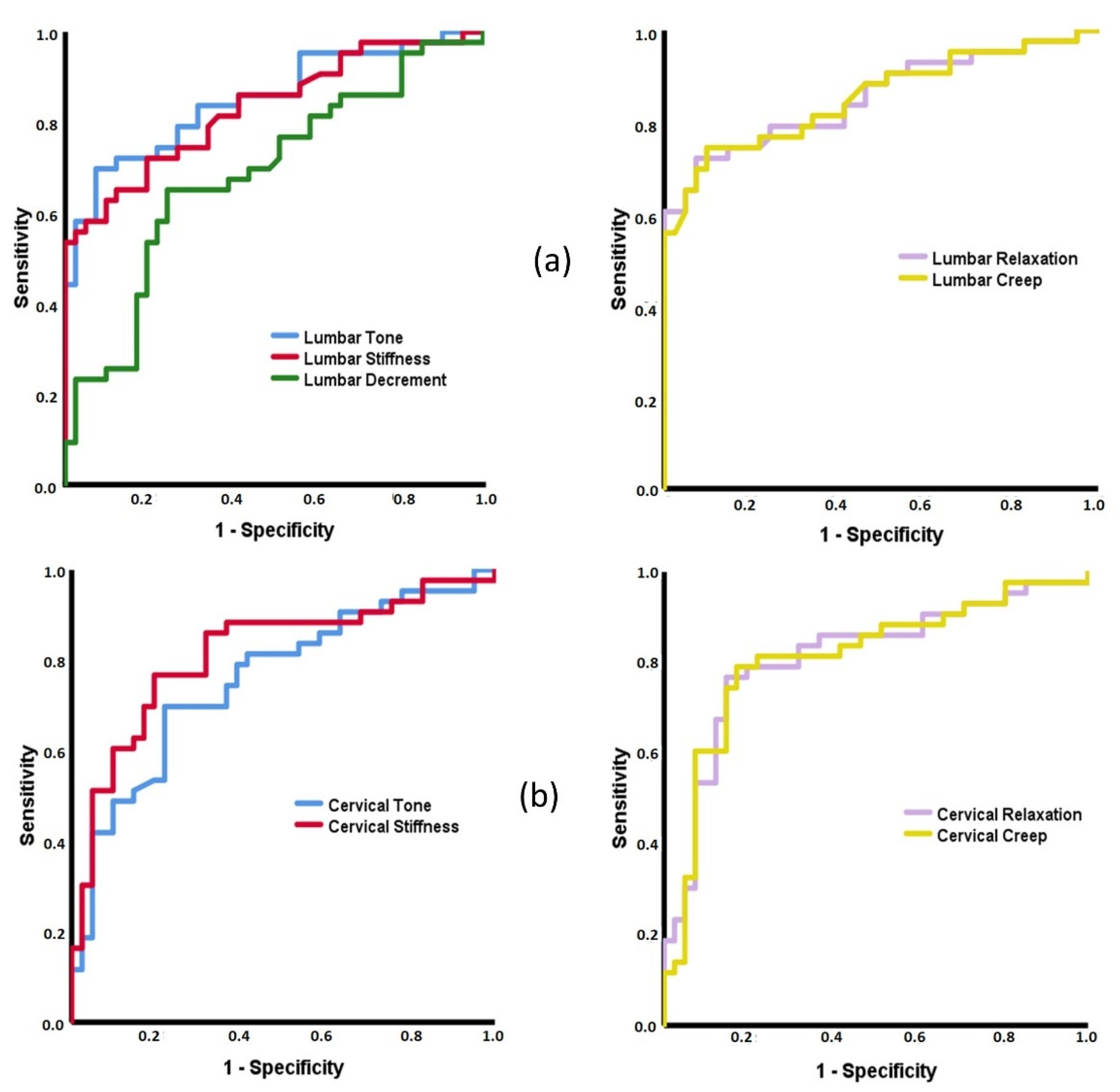
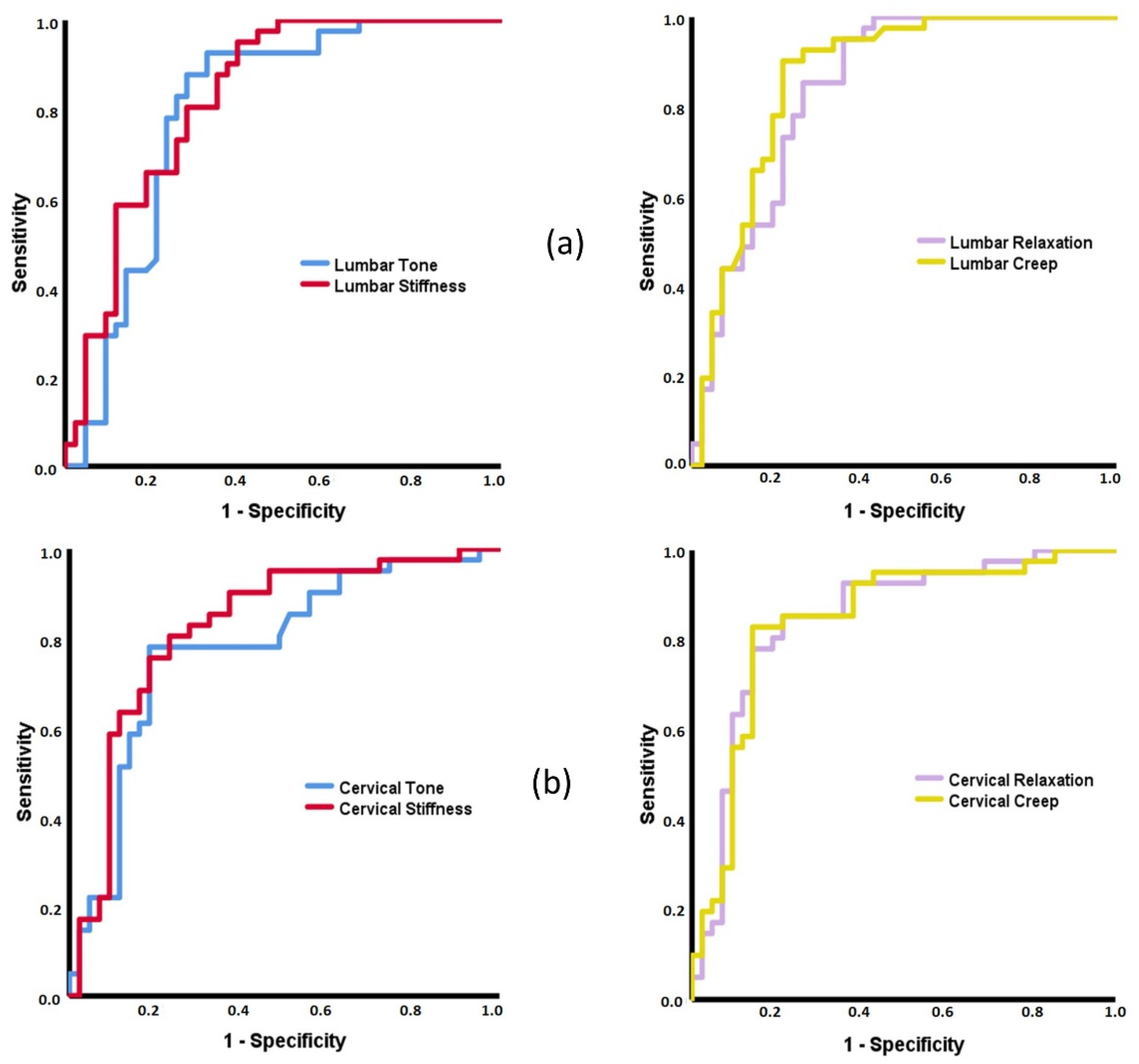
3.2. ROC Curves Based on MMPs
3.3. Intra-Group Associations among MMPs, Sociodemographic, and Clinical Variables
4. Discussion
4.1. Differences in MMPs, Spinal Mobility, Pain, and Quality of Life between Groups
4.2. Capacity of MMPs to Discriminate between Patients with Inflammatory and Mechanical Low Back Pain and Healthy Subjects
4.3. Associations between MMPs with Sociodemographic and Clinical Data
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ASAS | SpondyloArthritis International Society |
| axSpA | Axial spondyloarthritis |
| AUC | Area Under the Curve |
| BASDAI | Bath Ankylosing Spondylitis Disease Activity Index |
| BASFI | Bath Ankylosing Spondylitis Function Index |
| BASMI | Bath Ankylosing Spondylitis Metrology Index |
| BMI | Body Mass Index |
| cLBP | Chronic low back pain |
| ICC | Intraclass Correlation Coefficient |
| LBP | Low Back Pain |
| MCS-12 | Mental Component Summary of 12-item Short-Form Health Survey |
| MMP | Muscle Mechanical Property |
| mSASSS | Modified Stoke Ankylosing Spondylitis Spinal Score |
| NPRS | Numerical Pain Rating Scale |
| NS | Not Significant |
| NWC | Number of Words Chosen |
| PCS-12 | Physical Component Summary of 12-item Short-Form Health Survey |
| QoL | Quality of life |
| ROC | Receiver Operating Characteristic |
| ROM | Range Of Motion |
| SF-12 | 12-item Short-Form Health Survey |
| sLBP | Subacute LBP |
| 95%CI | 95% Confidence Interval |
References
- Raciborski, F.; Gasik, R.; Kłak, A. Disorders of the Spine. A Major Health and Social Problem. Reumatologia 2016, 54, 196–200. [Google Scholar] [CrossRef]
- Palacios-Ceña, D.; Alonso-Blanco, C.; Hernández-Barrera, V.; Carrasco-Garrido, P.; Jiménez-García, R.; Fernández-de-las-Peñas, C. Prevalence of Neck and Low Back Pain in Community-Dwelling Adults in Spain: An Updated Population-Based National Study (2009/10-2011/12). Eur. Spine J. 2015, 24, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Coenen, P.; Smith, A.; Paananen, M.; O’Sullivan, P.; Beales, D.; Straker, L. Trajectories of Low Back Pain From Adolescence to Young Adulthood. Arthritis Care Res. 2017, 69, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Wei, C.; Lin, C.; François, J.; Maurits, C.; Bart, V.T. Clinical Practice Guidelines for the Management of Non-Specific Low Back Pain in Primary Care: An Updated Overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, R.A.; Keating, J.L.; Ussing, K.; Li, P.; Kent, P. Does Movement Matter in People with Back Pain? Investigating “atypical” Lumbo-Pelvic Kinematics in People with and without Back Pain Using Wireless Movement Sensors. BMC Musculoskelet. Disord. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaraz-Clariana, S.; García-Luque, L.; Garrido-Castro, J.L.; Fernández-de-las-Peñas, C.; Carmona-Pérez, C.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F. Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study. Diagnostics 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Kent, P.; Laird, R.; Haines, T. The Effect of Changing Movement and Posture Using Motion-Sensor Biofeedback, versus Guidelines-Based Care, on the Clinical Outcomes of People with Sub-Acute or Chronic Low Back Pain-a Multicentre, Cluster-Randomised, Placebo-Controlled, Pilot Trial. BMC Musculoskelet. Disord. 2015, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hodges, P.; van den Hoorn, W.; Dawson, A.; Cholewicki, J. Changes in the Mechanical Properties of the Trunk in Low Back Pain May Be Associated with Recurrence. J. Biomech. 2009, 42, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Watanabe, Y.; Kutsuna, T.; Futohashi, T.; Kusumoto, Y.; Chiba, H.; Kubo, M.; Takasaki, H. Spinal Movement Variability Associated with Low Back Pain: A Scoping Review. PLoS ONE 2021, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; Chhabra, A.; Colbert, R.A. Ankylosing Spondylitis and Axial Spondyloarthritis. N. Engl. J. Med. 2016, 374, 2563–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urizar, E.; Antepara, C.; Urtaran-laresgoiti, M. Informe Sobre La Atención de La Espondiloartritis En España. Tech. Rep. 2019. [Google Scholar] [CrossRef]
- Navarro-Compán, V. An Update on Diagnosis and Classification of Axial Spondyloarthritis. Curr. Rheumatol. Rep. 2019, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; van der Heijde, D.; Landewé, R.; Brandt, J.; Burgos-Vagas, R.; Collantes-Estevez, E.; Dijkmans, B.; Dougados, M.; Khan, M.A.; Leirisalo-Repo, M.; et al. New Criteria for Inflammatory Back Pain in Patients with Chronic Back Pain: A Real Patient Exercise by Experts from the Assessment of SpondyloArthritis International Society (ASAS). Ann. Rheum. Dis. 2009, 68, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Akgul, O.; Gulkesen, A.; Akgol, G.; Ozgocmen, S. MR-Defined Fat Infiltration of the Lumbar Paravertebral Muscles Differs between Non-Radiographic Axial Spondyloarthritis and Established Ankylosing Spondylitis. Mod. Rheumatol. 2013, 23, 811–816. [Google Scholar] [CrossRef]
- Braun, J.; Van Den Berg, R.; Baraliakos, X.; Boehm, H.; Burgos-Vargas, R.; Collantes-Estevez, E.; Dagfinrud, H.; Dijkmans, B.; Dougados, M.; Emery, P.; et al. 2010 Update of the ASAS/EULAR Recommendations for the Management of Ankylosing Spondylitis. Ann. Rheum. Dis. 2011, 70, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, G.O.; Mcdougall, J.; Mills, K.R.; Isenberg, D.A.; Ebringer, A. Muscle Changes in Ankylosing Spondylitis. Rheumatology 1983, 22, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Andonian, B.J.; Masi, A.T.; Aldag, J.C.; Barry, A.J.; Coates, B.A.; Emrich, K.; Henderson, J.; Kelly, J.; Nair, K. Greater Resting Lumbar Extensor Myofascial Stiffness in Younger Ankylosing Spondylitis Patients Than Age-Comparable Healthy Volunteers Quantified by Myotonometry. Arch. Phys. Med. Rehabil. 2015, 96, 2041–2047. [Google Scholar] [CrossRef]
- Nair, K.; Masi, A.T.; Andonian, B.J.; Barry, A.J.; Coates, B.A.; Dougherty, J.; Schaefer, E.; Henderson, J.; Kelly, J. Stiffness of Resting Lumbar Myofascia in Healthy Young Subjects Quantified Using a Handheld Myotonometer and Concurrently with Surface Electromyography Monitoring. J. Bodyw. Mov. Ther. 2016, 20, 388–396. [Google Scholar] [CrossRef]
- Masi, A.T. Might Axial Myofascial Properties and Biomechanical Mechanisms Be Relevant to Ankylosing Spondylitis and Axial Spondyloarthritis? Arthritis Res. Ther. 2014, 16, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, E.C.; Yagci, I. The Structural, Functional and Electrophysiological Assessment of Paraspinal Musculature of Patients with Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthropathy. Rheumatol. Int. 2021, 41, 595–603. [Google Scholar] [CrossRef]
- Cedraschi, C.; Luthy, C.; Allaz, A.F.; Herrmann, F.R.; Ludwig, C. Low Back Pain and Health-Related Quality of Life in Community-Dwelling Older Adults. Eur. Spine J. 2016, 25, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low Back Pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global Low Back Pain Prevalence and Years Lived with Disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.J.; Bajwa, Z.H. What Is Mechanical Back Pain and How Best to Treat It? Curr. Pain Headache Rep. 2008, 12, 406–411. [Google Scholar] [CrossRef]
- Bardin, L.D.; King, P.; Maher, C.G. Diagnostic Triage for Low Back Pain: A Practical Approach for Primary Care. Med. J. Aust. 2017, 206, 268–273. [Google Scholar] [CrossRef]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are MRI-Defined Fat Infiltrations in the Multifidus Muscles Associated with Low Back Pain? BMC Med. 2007, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered Muscle Recruitment during Extension from Trunk Flexion in Low Back Pain Developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef]
- Hildebrandt, M.; Fankhauser, G.; Meichtry, A.; Luomajoki, H. Correlation between Lumbar Dysfunction and Fat Infiltration in Lumbar Multifidus Muscles in Patients with Low Back Pain. BMC Musculoskelet. Disord. 2017, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ranger, T.A.; Cicuttini, F.M.; Jensen, T.S.; Peiris, W.L.; Hussain, S.M.; Fairley, J.; Urquhart, D.M. Are the Size and Composition of the Paraspinal Muscles Associated with Low Back Pain? A Systematic Review. Spine J. 2017, 17, P1729–P1748. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Kocur, P.; Wilski, M.; Lewandowski, J.; Łochyński, D. Female Office Workers With Moderate Neck Pain Have Increased Anterior Positioning of the Cervical Spine and Stiffness of Upper Trapezius Myofascial Tissue in Sitting Posture. Pm&r 2018, 11, 476–482. [Google Scholar]
- Liu, Y.; Pan, A.; Hai, Y.; Li, W.; Yin, L.; Guo, R. Asymmetric Biomechanical Characteristics of the Paravertebral Muscle in Adolescent Idiopathic Scoliosis. Clin. Biomech. 2019, 65, 81–86. [Google Scholar] [CrossRef]
- Gatchel, R.; Bevers, K.; Licciardone, J.; Su, J.; Du, Y.; Brotto, M. Transitioning from Acute to Chronic Pain: An Examination of Different Trajectories of Low-Back Pain. Healthcare 2018, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Gatchel, R.J.; Reuben, D.B.; Dagenais, S.; Turk, D.C.; Chou, R.; Hershey, A.D.; Hicks, G.E.; Licciardone, J.C.; Horn, S.D. Research Agenda for the Prevention of Pain and Its Impact: Report of the Work Group on the Prevention of Acute and Chronic Pain of the Federal Pain Research Strategy. J. Pain 2018, 19, 837–851. [Google Scholar] [CrossRef] [Green Version]
- Falla, D.; Farina, D. Neuromuscular Adaptation in Experimental and Clinical Neck Pain. J. Electromyogr. Kinesiol. 2008, 18, 255–261. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Alexandre, A.M.; Pepa, G.M.D.; Altavilla, R.; Zobel, B.B. Modic Changes: Anatomy, Pathophysiology and Clinical Correlation. Acta Neurochir. Suppl. 2011, 108, 49–53. [Google Scholar]
- Colosimo, C.; Gaudino, S.; Alexandre, A.M. Imaging in Degenerative Spine Pathology. Acta Neurochir. Suppl. 2011, 108, 9–15. [Google Scholar] [PubMed]
- Ilahi, S.; Masi, A.T.; White, A.; Devos, A.; Henderson, J.; Nair, K. Quantified Biomechanical Properties of Lower Lumbar Myofascia in Younger Adults with Chronic Idiopathic Low Back Pain and Matched Healthy Controls. Clin. Biomech. 2020, 73, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mustalampi, S.; Ylinen, J.; Korniloff, K.; Weir, A.; Häkkinen, A. Reduced Neck Muscle Strength and Altered Muscle Mechanical Properties in Cervical Dystonia Following Botulinum Neurotoxin Injections: A Prospective Study. J. Mov. Disord. 2016, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.A.; Yu, Q.; Mao, Y.; Li, W.; Hu, C.; Li, L. Lumbar Muscles Biomechanical Characteristics in Young People with Chronic Spinal Pain. BMC Musculoskelet. Disord. 2019, 20, 559. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, Y.F.; Ding, W.L.; Yu, J.Y.; Song, L.; Feng, Y.N.; Zhang, Z.J. Quantification of the Masseter Muscle Hardness of Stroke Patients Using the Myotonpro Apparatus: Intra- And Inter-Rater Reliability and Its Correlation with Masticatory Performance. Med. Sci. Monit. 2020, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Santos, F.M.; Manica, S.R.; Alfonse, T.M.; Lagoas-Gomes, J.; Santos, M.B.; Ramiro, S.; Sepriano, A.; Nair, K.; Costa, J.; Gomes-Alves, P.; et al. Lumbar Myofascial Physical Properties in Healthy Adults: Myotonometry vs. Shear Wave Elastography Measurements. Acta Reumatol. Port. 2021, 46, 110–119. [Google Scholar] [PubMed]
- White, A.; Abbott, H.; Masi, A.T.; Henderson, J.; Nair, K. Biomechanical Properties of Low Back Myofascial Tissue in Younger Adult Ankylosing Spondylitis Patients and Matched Healthy Control Subjects. Clin. Biomech. 2018, 57, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Xu, W.; Liang, J.; Guan, Y.; Xu, X. Analysis of Biomechanical Properties of the Lumbar Extensor Myofascia in Elderly Patients with Chronic Low Back Pain and That in Healthy People. Biomed. Res. Int. 2020, 2020, 7649157. [Google Scholar] [CrossRef]
- Garrido-Castro, J.L.; Aranda-Valera, I.C.; Peña-Amaro, J.; Martínez-Galisteo, A.; González-Navas, C.; Rodrigues-de-Souza, D.P.; Alcaraz-Clariana, S.; García-Luque, L.; Martínez-Sánchez, I.R.; López-Medina, C.; et al. Mechanical Properties of Lumbar and Cervical Paravertebral Muscles in Patients with Axial Spondyloarthritis: A Case—Control Study. Diagnostics 2021, 11, 1662. [Google Scholar] [CrossRef]
- Aranda-Valera, I.; Alcaraz-Clariana, S.; Garcia-Luque, L.; Garrido-Castro, J.; Martinez-Sanchez, I.; Gonzalez, C.; Gardiner, P.; Machado, P.; Collantes, E. Lumbar Muscles Stiffness in Patients with Axial Spondyloarthritis Is Altered in Comparison with Healthy Subjects. Ann. Rheum. Dis. 2018, 77, 1561–1562. [Google Scholar]
- Rudwaleit, M.; Van Der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The Development of Assessment of SpondyloArthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef] [Green Version]
- Abbott, J.H.; Schmitt, J. Minimum Important Differences for the Patient-Specific Functional Scale, 4 Region-Specific Outcome Measures, and the Numeric Pain Rating Scale. J. Orthop. Sports Phys. Ther. 2014, 44, 560–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armijo-Olivo, S.; Warren, S.; Fuentes, J.; Magee, D.J. Clinical Relevance vs. Statistical Significance: Using Neck Outcomes in Patients with Temporomandibular Disorders as an Example. Man Ther. 2011, 16, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between Age, BMI, Head Posture and Superficial Neck Muscle Stiffness and Elasticity in Adult Women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Yang, D.J.; Kim, J.H.; Heo, J.W.; Uhm, Y.H.; Yoon, J.H. Analysis of Mechanical Properties of Cervical Muscles in Patients with Cervicogenic Headache. J. Phys. Ther. Sci. 2017, 29, 332–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavronski, G.; Veraksitš, A.; Vasar, E.; Maaroos, J. Evaluation of Viscoelastic Parameters of the Skeletal Muscles in Junior Triathletes. Physiol. Meas. 2007, 28, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Agyapong-Badu, S.; Warner, M.B.; Samuel, D.; Koutra, V.; Stokes, M. Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender. J. Clin. Med. 2021, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- Selva-Sevilla, C.; Ferrara, P.; Geronimo-Pardo, M. Psychometric Properties Study of the Oswestry Disability Index in a Spanish Population with Previous Lumbar Disc Surgery: Homogeneity and Validity. Spine 2019, 1–44, 430–437. [Google Scholar] [CrossRef]
- Ware, J.E.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Vaishnav, A.S.; Gang, C.H.; Iyer, S.; McAnany, S.; Albert, T.; Qureshi, S.A. Correlation between NDI, PROMIS and SF-12 in Cervical Spine Surgery. Spine J. 2020, 20, 409–416. [Google Scholar] [CrossRef]
- Resnick, B.; Parker, B. Simplified Scoring and Psychometrics of the Revised 12-Item Short-Form Health Survey. Nursing 2001, 5, 161–166. [Google Scholar]
- Chapman, J.R.; Norvell, D.C.; Hermsmeyer, J.T.; Bransford, R.J.; Devine, J.; McGirt, M.J.; Lee, M.J. Evaluating Common Outcomes for Measuring Treatment Success for Chronic Low Back Pain. Spine 2011, 36, S54–S68. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Assessing the Fit of the Model. In Applied Logistic Regression; Hosmer, D.W., Lemeshow, S., Sturdivant, R.X., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 153–226. [Google Scholar]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Tirrell, T.F.; Cook, M.S.; Carr, J.A.; Lin, E.; Ward, S.R.; Lieber, R.L. Human Skeletal Muscle Biochemical Diversity. J. Exp. Biol. 2012, 215, 2551–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Lei, D.; Li, L.; Leng, Y.; Yu, Q.; Wei, X.; Lo, W.L.A. Quantifying Paraspinal Muscle Tone and Stiffness in Young Adults with Chronic Low Back Pain: A Reliability Study. Sci. Rep. 2018, 8, 14343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of Tensiomyography and Myotonometry in Detecting Mechanical and Contractile Characteristics of the Lumbar Erector Spinae in Healthy Volunteers. Eur J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef]
- Aranda-Valera, I.C.; Cuesta-Vargas, A.; Garrido-Castro, J.L.; Gardiner, P.V.; López-Medina, C.; Machado, P.M.; Condell, J.; Connolly, J.; Williams, J.M.; Muñoz-Esquivel, K.; et al. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Validation Study in Axial Spondyloarthritis. Diagnostics 2020, 10, 426. [Google Scholar] [CrossRef]
- Díaz-Arribas, M.J.; Fernández-Serrano, M.; Royuela, A.; Kovacs, F.M.; Gallego-Izquierdo, T.; Ramos-Sánchez, M.; Llorca-Palomera, R.; Pardo-Hervás, P.; Martín-Pariente, O.S. Minimal Clinically Important Difference in Quality of Life for Patients with Low Back Pain. Spine 2017, 42, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Megari, K. Quality of Life in Chronic Disease Patients. Health Psychol. Res. 2013, 1, e27. [Google Scholar] [CrossRef]
- Packham, J. Optimizing Outcomes for Ankylosing Spondylitis and Axial Spondyloarthritis Patients: A Holistic Approach to Care. Rheumatology 2018, 57, vi29–vi34. [Google Scholar] [CrossRef] [Green Version]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of Ageing Effects on Muscle Tone and Mechanical Properties of Rectus Femoris and Biceps Brachii in Healthy Males and Females Using a Novel Hand-Held Myometric Device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A Regional Interdependence Model of Musculoskeletal Dysfunction: Research, Mechanisms, and Clinical Implications. J. Man. Manip. Ther. 2013, 21, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Jacques, P.; McGonagle, D. The Role of Mechanical Stress in the Pathogenesis of Spondyloarthritis and How to Combat It. Best Pract. Res. Clin. Rheumatol. 2014, 28, 703–710. [Google Scholar] [CrossRef]
- Masaki, M.; Aoyama, T.; Murakami, T.; Yanase, K.; Ji, X.; Tateuchi, H.; Ichihashi, N. Association of Low Back Pain with Muscle Stiffness and Muscle Mass of the Lumbar Back Muscles, and Sagittal Spinal Alignment in Young and Middle-Aged Medical Workers. Clin. Biomech. 2017, 49, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felicio, D.C.; Pereira, D.S.; Diz, J.B.M.; De Queiroz, B.Z.; Da Silva, J.P.; Leopoldino, A.A.O.; Pereira, L.S.M. Anterior Trunk Mobility Does Not Predict Disability in Elderly Women with Acute Low Back Pain: Brazilian Back Complaints in the Elders (BACE-Brazil) Study Results. Spine 2017, 42, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, A.; Corò, L.; Paradiso, R.; Dall’Aglio, R.; Alexandre, A.M.; Fraschini, F.; Spaggiari, P.G. Treatment of Symptomatic Lumbar Spinal Degenerative Pathologies by Means of Combined Conservative Biochemical Treatments. Acta Neurochir. Suppl. 2011, 108, 127–135. [Google Scholar] [PubMed]
- Agrawal, P.; Machado, P.M. Recent Advances in Managing Axial Spondyloarthritis. F1000Research 2020, 9, F1000 Faculty Rev-697. [Google Scholar] [CrossRef]
- Akkoc, N.; Can, G.; D’Angelo, S.; Padula, A.; Olivieri, I. Therapies of Early, Advanced, and Late Onset Forms of Axial Spondyloarthritis, and the Need for Treat to Target Strategies. Curr. Rheumatol. Rep. 2017, 19, 8. [Google Scholar] [CrossRef]
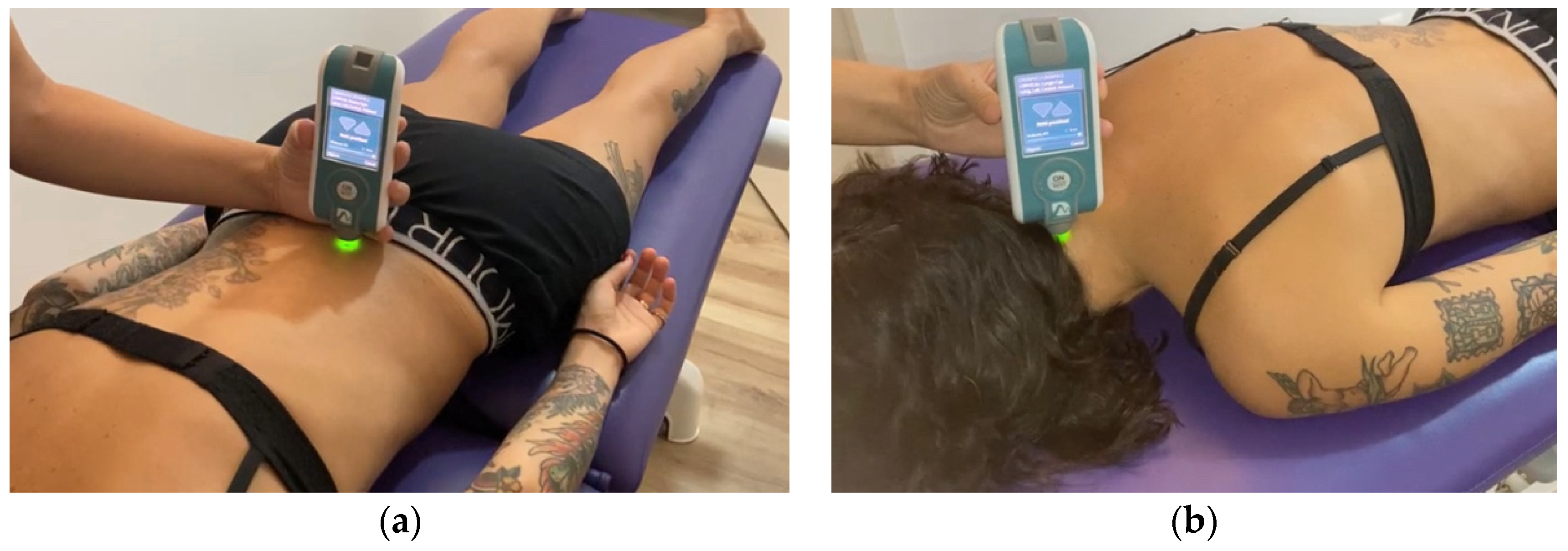
| axSpA Group (n = 43) | sLBP Group (n = 43) | Control Group (n = 43) | p-Value | |
|---|---|---|---|---|
| Age (years) | 41.9 ± 9.5 | 40.2 ± 12.3 | 39.2 ± 11.3 | 0.581 |
| Sex (female/male) | 15/28 | 15/28 | 15/28 | 1.000 |
| BMI (Kg/m2) | 24.6 ± 3.4 | 24.7 ± 3.0 | 23.9 ± 3.4 | 0.440 |
| PCS-12 | 42.5 ± 9.8 | 41.0 ± 8.3 | 53.9 ± 4.3 | <0.001 ‡ |
| MCS-12 | 50.4 ± 9.1 | 51.0 ± 9.0 | 53.2 ± 6.6 | 0.483 |
| NPRS | 4.4 ± 2.6 | 4.9 ± 1.8 | 0.341 | |
| Lateral spinal flexion (cm) | 13.8 ± 8.4 | 18.5 ± 5.2 | 21.7 ± 11.5 | <0.001 † |
| Tragus to wall distance (cm) | 12.5 ± 4.0 | 11.9 ± 1.6 | 11.5 ± 1.4 | 0.197 |
| Modified Schöber test (cm) | 5.1 ± 1.5 | 4.8 ± 1.4 | 5.1 ± 1.2 | 0.562 |
| Intermalleolar distance (cm) | 98.0 ± 16.6 | 114.3 ± 20.0 | 116.3 ± 14.1 | <0.001 † |
| Cervical rotation (°) | 61.2 ± 17.3 | 70.3 ± 13.3 | 79.3 ± 7.5 | <0.001 § |
| Evolution time (years) | 17.6 ± 12.0 | |||
| BASMI | 3.0 ± 1.6 | 1.9 ± 0.6 | 1.5 ± 0.6 | <0.001 † |
| BASFI | 2.8 ± 2.6 | |||
| BASDAI | 3.8 ± 2.5 | |||
| mSASSS | 15.3 ± 14.7 | |||
| ODI | 18.0 ± 12.6 | |||
| Muscle Mechanical Properties (MMPs) | ||||
| Lumbar tone (Hz) | 18.23 ± 1.67 | 16.01 ± 2.34 | 15.28 ± 2.21 | <0.001 † |
| Lumbar stiffness (N/m) | 383.13 ± 53.22 | 303.81 ± 64.79 | 284.23 ± 82.61 | <0.001 † |
| Lumbar decrement | 1.45 ± 0.29 | 1.38 ± 0.28 | 1.25 ± 0.31 | 0.009 * |
| Lumbar relaxation (ms) | 14.03 ± 1.64 | 17.88 ± 3.70 | 18.99 ± 4.54 | <0.001 † |
| Lumbar creep (Deborah number) | 0.88 ± 0.09 | 1.09 ± 0.18 | 1.13 ± 0.25 | <0.001 † |
| Cervical tone (Hz) | 16.56 ± 1.70 | 14.76 ± 1.85 | 14.71 ± 1.99 | <0.001 † |
| Cervical stiffness (N/m) | 314.71 ± 43.87 | 250.60 ± 54.76 | 247.40 ± 61.21 | <0.001 † |
| Cervical decrement | 1.25 ± 0.20 | 1.35 ± 0.36 | 1.25 ± 0.25 | 0.134 |
| Cervical relaxation (ms) | 16.53 ± 20.13 | 20.69 ± 3.49 | 16.53 ± 2.22 | <0.001 † |
| Cervical creep (Deborah number) | 1.01 ± 0.12 | 1.23 ± 0.18 | 1.19 ± 0.20 | <0.001 † |
| Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.520 ** | 0.326 * | 0.696 ** | NS | NS | 0.323 * | NS | 0.573 ** | NS | NS |
| Height | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Weight | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| BMI | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Evolution time | 0.622 ** | 0.513 ** | 0.743 ** | −0.473 ** | −0.405 ** | 0.627 ** | 0.505 ** | NS. | −0.407 * | NS |
| Total pain | 0.370 * | 0.412 ** | 0.504 ** | −0.336 * | NS. | 0.478 ** | NS | NS | −0.316 * | −0.315 * |
| PCS-12 | −0.617 ** | −0.551 ** | −0.369 * | 0.494 ** | 0.476 ** | −0.610 ** | −0.462 * | NS. | 0.417 * | NS |
| MCS-12 | −0.546 ** | −0.497 ** | NS | 0.538 ** | 0.540 ** | −0.592 ** | −0.467 ** | NS | 0.481 ** | 0.444 * |
| BASMI | 0.449 ** | 0.419 ** | 0.385 * | −0.330 * | NS | NS | NS | NS | NS | NS |
| BASDAI | 0.416 ** | 0.437 ** | 0.445 ** | −0.352 * | NS | 0.389 * | NS | NS | NS | NS |
| BASFI | 0.500 ** | 0.513 ** | 0.533 ** | −0.423 ** | −0.362 * | 0.356 * | NS | NS | NS | NS |
| mSASSS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Lateral spinal flexion | −0.407 ** | −0.388 * | NS | 0.370 * | 0.342 * | −0.456 ** | −0.456 ** | NS. | 0.384 * | NS. |
| Tragus to wall distance | 0.491 ** | 0.560 ** | NS. | −0.469 ** | −0.441 ** | NS | NS | NS | NS | NS |
| Modified Schöber test | −0.402 * | −0.469 ** | NS. | 0.455 ** | 0.453 ** | NS | NS | NS | NS | NS |
| Intermalleolar distance | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Cervical rotation | −0.346 * | −0.373 * | NS | NS | NS | NS | NS | NS | NS | NS |
| Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.470 ** | 0.579 ** | 0.605 ** | −0.394 ** | NS | 0.464 ** | 0.523 ** | 0.767 ** | −0.360 * | NS |
| Height | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.339 * |
| Weight | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.379 * |
| BMI | NS | NS | NS | NS | NS | NS | NS | −0.342 * | 0.381 * | 0.327 * |
| Total pain | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| PCS-12 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| MCS-12 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| ODI | 0.366 * | 0.322 * | NS | NS | NS | NS | NS | NS | NS | NS |
| Lateral spinal flexion | −0.348 * | −0.333 * | NS. | −0.311 * | NS | NS | NS | NS | NS | NS |
| Tragus to wall distance | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Modified Schöber test | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Intermalleolar distance | NS. | −0.349 * | −0.309 * | NS | NS | NS | −0.342 * | −0.475 ** | NS | NS |
| Cervical rotation | −0.384 * | −0.332 * | NS. | 0.398 ** | 0.393 ** | NS | NS | NS | NS | NS |
| Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.685 ** | 0.670 ** | 0.570 ** | −0.604 ** | −0.513 ** | 0.312 * | NS. | 0.475 ** | NS | NS |
| Height | NS | NS | NS | NS | NS | NS | NS | −0.463 ** | 0.420 ** | NS. |
| Weight | NS | NS | NS | NS | NS | −0.401 ** | −0.413 ** | −0.442** | 0.665 ** | 0.603 ** |
| BMI | NS | NS | NS | NS | NS | −0.408 ** | −0.445 ** | −0.306* | 0.642 ** | 0.702 ** |
| PCS-12 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| MCS-12 | NS | NS | NS | NS | NS | NS | NS | −0.448 * | NS | NS |
| Lateral spinal flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tragus to wall distance | NS | NS | −0.315 * | NS | NS | NS | NS | −0.428 ** | 0.372 * | NS |
| Modified Schöber test | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Intermalleolar distance | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Cervical rotation | −0.335 * | −0.340 * | −0.521 ** | NS | NS | NS | −0.311 * | −0.382 * | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz-Clariana, S.; García-Luque, L.; Garrido-Castro, J.L.; Aranda-Valera, I.C.; Ladehesa-Pineda, L.; Puche-Larrubia, M.Á.; Carmona-Pérez, C.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F. Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study. Diagnostics 2021, 11, 1898. https://doi.org/10.3390/diagnostics11101898
Alcaraz-Clariana S, García-Luque L, Garrido-Castro JL, Aranda-Valera IC, Ladehesa-Pineda L, Puche-Larrubia MÁ, Carmona-Pérez C, Rodrigues-de-Souza DP, Alburquerque-Sendín F. Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study. Diagnostics. 2021; 11(10):1898. https://doi.org/10.3390/diagnostics11101898
Chicago/Turabian StyleAlcaraz-Clariana, Sandra, Lourdes García-Luque, Juan Luis Garrido-Castro, I. Concepción Aranda-Valera, Lourdes Ladehesa-Pineda, María Ángeles Puche-Larrubia, Cristina Carmona-Pérez, Daiana Priscila Rodrigues-de-Souza, and Francisco Alburquerque-Sendín. 2021. "Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study" Diagnostics 11, no. 10: 1898. https://doi.org/10.3390/diagnostics11101898
APA StyleAlcaraz-Clariana, S., García-Luque, L., Garrido-Castro, J. L., Aranda-Valera, I. C., Ladehesa-Pineda, L., Puche-Larrubia, M. Á., Carmona-Pérez, C., Rodrigues-de-Souza, D. P., & Alburquerque-Sendín, F. (2021). Paravertebral Muscle Mechanical Properties in Patients with Axial Spondyloarthritis or Low Back Pain: A Case-Control Study. Diagnostics, 11(10), 1898. https://doi.org/10.3390/diagnostics11101898








