Usefulness of a Fork-Tip Needle in Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Gastric Subepithelial Lesions
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Patients
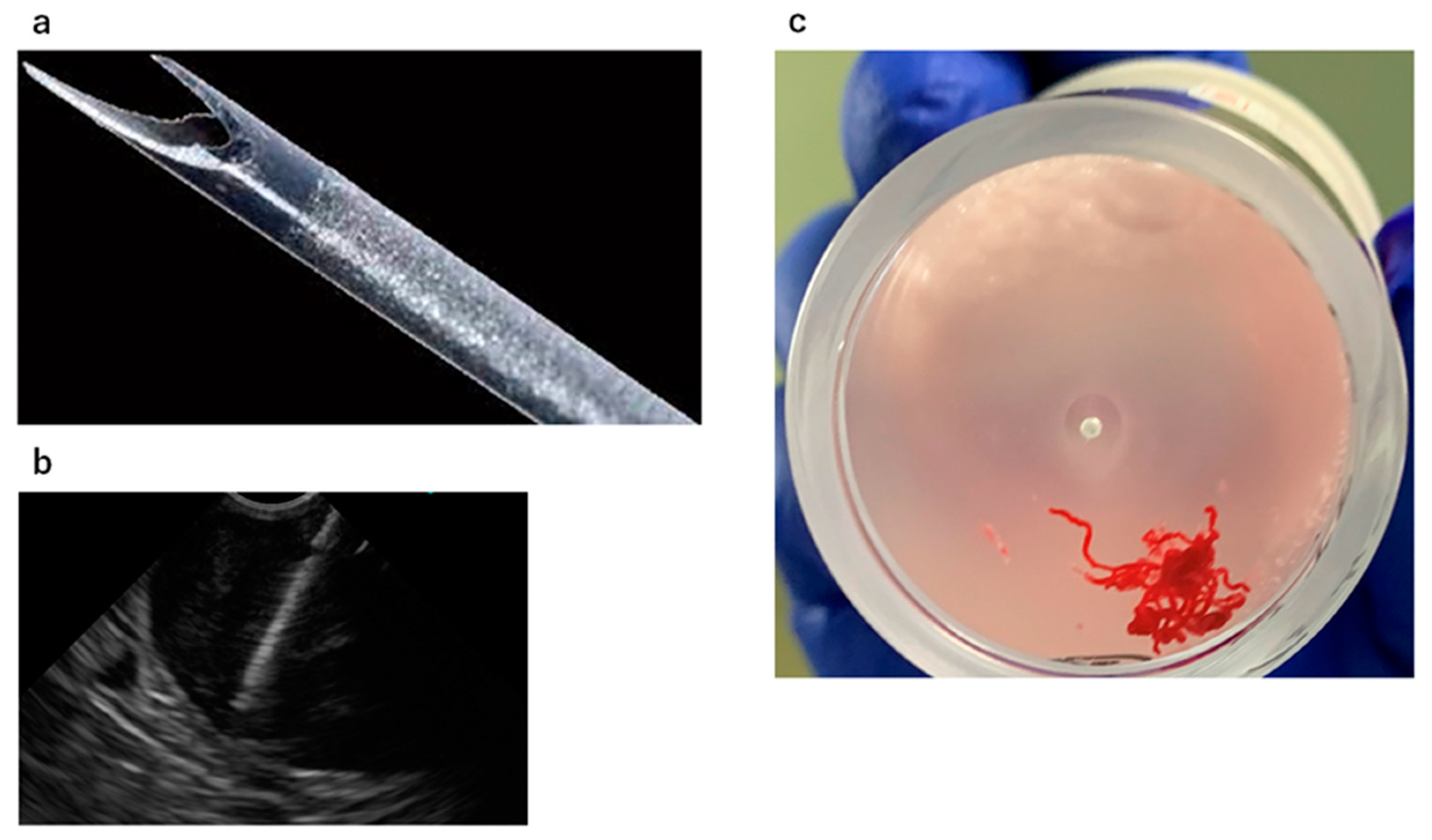
2.2. EUS-FNA/B Indication and Procedure
2.3. Endpoints of This Study
2.4. Statistical Analyses
3. Results
3.1. Patient and Lesion Characteristics
3.2. Diagnostic Ability and AEs of EUS-FNA/Bs
3.3. Factors Influencing Diagnostic Accuracy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Mekky, M.A.; Yamao, K.; Sawaki, A.; Mizuno, N.; Hara, K.; Nafeh, M.A.; Osman, A.M.; Koshikawa, T.; Yatabe, Y.; Bhatia, V. Diagnostic utility of EUS-guided FNA in patients with gastric subepithelial tumors. Gastrointest. Endosc. 2010, 71, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Oya, M.; Koga, T.; Koga, H.; Motomura, Y.; Kubokawa, M.; Gibo, J.; Nakamura, K. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J. Gastrointest Liver Dis. 2014, 23, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larghi, A.; Fuccio, L.; Chiarello, G.; Attili, F.; Vanella, G.; Paliani, G.B.; Napoleone, M.; Rindi, G.; Larocca, L.M.; Costamagna, G.; et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy 2014, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Goto, H.; Niwa, Y.; Arisawa, T.; Hirooka, Y.; Hayakawa, T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal subepithelial tumors. Endoscopy 1998, 30, 750–755. [Google Scholar] [CrossRef]
- Wiersema, M.J.; Vilmann, P.; Giovannini, M.; Chang, K.J.; Wiersema, L.M. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology 1997, 112, 1087–1095. [Google Scholar] [CrossRef]
- Tian, L.; Tang, A.L.; Zhang, L.; Liu, X.W.; Li, J.B.; Wang, F.; Shen, S.R.; Wang, X.Y. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: A prospective comparison study. Surg. Endosc. 2018, 32, 3533–3539. [Google Scholar] [CrossRef] [Green Version]
- Ang, T.L.; Li, J.W.; Kwek, A.B.E.; Thurairajah, P.H.; Wang, L.M. The difference in histological yield between 19G EUS-FNA and EUS-fine-needle biopsy needles. Endosc. Ultrasound 2019, 8, 255–260. [Google Scholar] [CrossRef]
- Ka, C.; Loh, T.; Kah, L.L.; Gek, S.T.; Ying, H.; Tan, D.; Khor, C.; Lim, T.; Soetikno, R. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study R.; Yung. Endosc. Int. Open 2019, 7, e955–e963. [Google Scholar]
- El Hajj, I.I.; Wu, H.; Reuss, S.; Randolph, M.; Harris, A.; Gromski, M.A.; Al-Haddad, M. Prospective assessment of the performance of a new fine needle biopsy device for EUS-guided sampling of solid lesions. Clin. Endosc. 2018, 51, 576–583. [Google Scholar] [CrossRef]
- de Moura, D.T.H.; McCarty, T.R.; Jirapinyo, P.; Ribeiro, I.B.; Flumignan, V.K.; Najdawai, F.; Ryou, M.; Lee, L.S.; Thompson, C.C. EUS-guided fine-needle biopsy sampling versus FNA in the diagnosis of subepithelial lesions: A large multicenter study. Gastrointest. Endosc. 2020, 92, 108–119.e3. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Irie, H.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Nakamura, J.; Takasumi, M.; Hashimoto, M.; et al. Efficacy of EUS-guided FNB using a Franseen needle for tissue acquisition and microsatellite instability evaluation in unresectable pancreatic lesions. BMC Cancer 2020, 20, 1094. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Okumura, F.; Sano, H.; Mizushima, T.; Tsukamoto, H.; Fujita, Y.; Ibusuki, M.; Kitano, R.; Kobayashi, Y.; Ishii, N.; et al. Impact of endoscopic ultrasound-guided fine-needle biopsy on the diagnosis of subepithelial tumors: A propensity score-matching analysis. Dig. Endosc. 2019, 31, 156–163. [Google Scholar] [CrossRef]
- Kandel, P.; Tranesh, G.; Nassar, A.; Bingham, R.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Wallace, M.B. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest. Endosc. 2016, 84, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Asokkumar, R.; Jayaraj, M.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Comparison of Franseen and fork-tip needles for EUS-guided fine-needle biopsy of solid mass lesions: A systematic review and meta-analysis. Endosc. Ultrasound 2019, 8, 382–391. [Google Scholar] [PubMed]
- Ashat, M.; Klair, J.S.; Rooney, S.L.; Vishal, S.J.; Jensen, C.; Sahar, N.; Murali, A.R.; El-Abiad, R.; Gerke, H. Randomized controlled trial comparing the Franseen needle with the Fork-tip needle for EUS-guided fine-needle biopsy. Gastrointest. Endosc. 2021, 93, 140–150.e2. [Google Scholar] [CrossRef]
- Song, Z.; Trujillo, C.N.; Song, H.; Tongson-Ignacio, J.E.; Chan, M.Y. Endoscopic ultrasound-guided tissue acquisition using fork-tip needle improves histological yield, reduces needle passes, without on-site cytopathological evaluation. J. Pancreat. Cancer 2018, 4, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Crinò, S.F.; Le Grazie, M.; Manfrin, E.; Conti Bellocchi, M.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658.e2. [Google Scholar] [CrossRef]
- Oppong, K.W.; Bekkali, N.L.H.; Leeds, J.S.; Johnson, S.J.; Nayar, M.K.; Darné, A.; Egan, M.; Bassett, P.; Haugk, B. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized crossover study. Endoscopy 2020, 52, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Hikichi, T.; Irisawa, A.; Bhutani, M.S.; Takagi, T.; Shibukawa, G.; Yamamoto, G.; Wakatsuki, T.; Imamura, H.; Takahashi, Y.; Sato, A.; et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J. Gastroenterol. 2009, 44, 322–328. [Google Scholar] [CrossRef]
- Attam, R.; Arain, M.A.; Bloechl, S.J.; Trikudanathan, G.; Munigala, S.; Bakman, Y.; Singh, M.; Wallace, T.; Henderson, J.B.; Catalano, M.F.; et al. ‘Wet suction technique (WEST)’: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gage needle for EUS-FNA of solid lesions. Gastrointest. Endosc. 2015, 81, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; et al. Can the wet suction technique change the efficacy of endoscopic ultrasound-guided fine-needle aspiration for diagnosing autoimmune pancreatitis type 1? A prospective single-arm study. World J. Clin. Cases 2020, 8, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Villa, N.A.; Berzosa, M.; Wallace, M.B.; Raijman, I. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc. Ultrasound 2016, 5, 17–20. [Google Scholar] [PubMed] [Green Version]
- Takasumi, M.; Hikichi, T.; Hashimoto, M.; Nakamura, J.; Kato, T.; Kikuchi, H.; Waragai, Y.; Watanabe, K.; Takagi, T.; Suzuki, R.; et al. A pilot randomized crossover trial of wet suction and conventional techniques of endoscopic ultrasound-guided fine-needle aspiration for upper gastrointestinal subepithelial lesions. Gastroenterol. Res. Pract. 2021, 2021, 4913107. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, B.; Klausen, P.; Vilmann, P. Franseen versus fork-tip: Crowning the king of crown-cut needles? Gastrointest. Endosc. 2021, 93, 151–153. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Buccino, V.R.; Purohit, P.; Setia, P.; Muscatiello, N. Diagnostic yield of Franseen and Fork-Tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: A meta-analysis. Endosc. Int. Open 2019, 7, E1221–E1230. [Google Scholar] [CrossRef] [Green Version]
- Fujita, A.; Ryozawa, S.; Kobayashi, M.; Araki, R.; Nagata, K.; Minami, K.; Tanisaka, Y.; Kobatake, T.; Mizuide, M. Diagnostic ability of a 22G Franseen needle in endoscopic ultrasound-guided fine needle aspiration of subepithelial lesions. Mol. Clin. Oncol. 2018, 9, 527–531. [Google Scholar] [CrossRef]
- El Chafic, A.H.; Loren, D.; Siddiqui, A.; Mounzer, R.; Cosgrove, N.; Kowalski, T. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest. Endosc. 2017, 86, 510–515. [Google Scholar] [CrossRef]
- Facciorusso, A.; Sunny, S.P.; Del Prete, V.; Antonino, M.; Muscatiello, N. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest. Endosc. 2020, 91, 14–22.e2. [Google Scholar] [CrossRef]
- Suzuki, T.; Arai, M.; Matsumura, T.; Arai, E.; Hata, S.; Maruoka, D.; Tanaka, T.; Nakamoto, S.; Imazeki, F.; Yokosuka, O. Factors associated with inadequate tissue yield in EUS-FNA for Gastric SMT. ISRN Gastroenterol. 2011, 2011, 619128. [Google Scholar] [CrossRef] [Green Version]
| Fork-Tip Group (n = 13) | FNA Group (n = 66) | p Value | |
|---|---|---|---|
| Age (y) * | 68 (41–80) | 65 (19–91) | 0.449 |
| Sex (male/female) | 9/4 | 29/37 | 0.131 |
| Tumor size (mm) * | 20 (15–95) | 20 (8–200) | 0.881 |
| Tumor location (U/M/L) | 9/4/0 | 38/18/10 | 0.322 |
| Needle gage (22G/25G) | 13/0 | 64/2 | 1.000 |
| Treatment, n Resection Chemotherapy Follow-up | 0.019 ** | ||
| Resection | 12 | 33 | |
| Chemotherapy | 0 | 4 | |
| Follow-up | 1 | 29 |
| Fork-Tip Group (n = 13) | FNA Group (n = 66) | |
|---|---|---|
| GIST | 10 | 41 |
| Leiomyoma | 1 | 8 |
| Schwannoma | 0 | 1 |
| Ectopic pancreas | 0 | 5 |
| Carcinoma | 1 | 1 |
| Malignant lymphoma | 1 | 0 |
| Unknown | 0 | 10 |
| Fork-Tip Group (n = 13) | FNA Group (n = 66) | p Value | |
|---|---|---|---|
| Rate of adequate sampling, % (n) | 100 (13) | 90.9 (60) | 0.582 |
| Diagnostic accuracy, % (n) | 92.3 (12) | 81.8 (54) | 0.682 |
| Number of punctures * | 5 (3–9) | 5 (1–9) | 0.886 |
| Adverse events, % (n) | 7.7 (1) | 0 (0) | 0.153 |
| Parameters | Univariate Analysis † | Multivariate Analyses ‡ | ||
|---|---|---|---|---|
| p Value | Adjusted OR (95% CI) | p Value | ||
| Age (1-y increments) | 0.095 | 1.035 (0.971–1.102) | 0.291 | |
| Sex | Female (35) | 0.642 | 1.0 [Reference] | |
| Male (44) | 0.674 (0.119–3.834) | 0.657 | ||
| Tumor size (1-cm increments) | 0.004 * | 1.477 (1.114–1.958) | 0.007 * | |
| Tumor location | Lower stomach (10) Middle stomach (22) Upper stomach (47) | 0.009 * | 1.0 [Reference] 14.186 (1.069–188.201) 33.150 (2.321–473.487) | 0.044 * 0.010 * |
| Number of punctures (1-time increments) | 0.886 | 0.729 (0.397–1.338) | 0.308 | |
| Puncture needle | FNA needle (66) | 0.682 | 1.0 [Reference] | |
| Fork-tip needle (13) | 3.543 (0.194–64.854) | 0.394 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takasumi, M.; Hikichi, T.; Hashimoto, M.; Nakamura, J.; Kato, T.; Kobashi, R.; Yanagita, T.; Suzuki, R.; Sugimoto, M.; Sato, Y.; et al. Usefulness of a Fork-Tip Needle in Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Gastric Subepithelial Lesions. Diagnostics 2021, 11, 1883. https://doi.org/10.3390/diagnostics11101883
Takasumi M, Hikichi T, Hashimoto M, Nakamura J, Kato T, Kobashi R, Yanagita T, Suzuki R, Sugimoto M, Sato Y, et al. Usefulness of a Fork-Tip Needle in Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Gastric Subepithelial Lesions. Diagnostics. 2021; 11(10):1883. https://doi.org/10.3390/diagnostics11101883
Chicago/Turabian StyleTakasumi, Mika, Takuto Hikichi, Minami Hashimoto, Jun Nakamura, Tsunetaka Kato, Ryoichiro Kobashi, Takumi Yanagita, Rei Suzuki, Mitsuru Sugimoto, Yuki Sato, and et al. 2021. "Usefulness of a Fork-Tip Needle in Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Gastric Subepithelial Lesions" Diagnostics 11, no. 10: 1883. https://doi.org/10.3390/diagnostics11101883
APA StyleTakasumi, M., Hikichi, T., Hashimoto, M., Nakamura, J., Kato, T., Kobashi, R., Yanagita, T., Suzuki, R., Sugimoto, M., Sato, Y., Irie, H., Takagi, T., Kobayakawa, M., Hashimoto, Y., & Ohira, H. (2021). Usefulness of a Fork-Tip Needle in Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Gastric Subepithelial Lesions. Diagnostics, 11(10), 1883. https://doi.org/10.3390/diagnostics11101883







