Abstract
Introduction: Urbanization has caused dramatic changes in lifestyle, and these rapid transitions have led to an increased risk of noncommunicable diseases, such as type 2 diabetes. In terms of cost-effectiveness, screening for diabetic retinopathy is a critical aspect in diabetes management. The aim of this study was to review the imaging modalities employed for retinal examination in diabetic retinopathy screening. Methods: The PubMed and Web of Science databases were the main sources used to investigate the medical literature. An extensive search was performed to identify relevant articles concerning “imaging”, “diabetic retinopathy” and “screening” up to 1 June 2021. Imaging techniques were divided into the following: (i) mydriatic fundus photography, (ii) non-mydriatic fundus photography, (iii) smartphone-based imaging, and (iv) ultrawide-field imaging. A meta-analysis was performed to analyze the performance and technical failure rate of each method. Results: The technical failure rates for mydriatic and non-mydriatic digital fundus photography, smartphone-based and ultrawide-field imaging were 3.4% (95% CI: 2.3–4.6%), 12.1% (95% CI: 5.4–18.7%), 5.3% (95% CI: 1.5–9.0%) and 2.2% (95% CI: 0.3–4.0%), respectively. The rate was significantly different between all analyzed techniques (p < 0.001), and the overall failure rate was 6.6% (4.9–8.3%; I2 = 97.2%). The publication bias factor for smartphone-based imaging was significantly higher than for mydriatic digital fundus photography and non-mydriatic digital fundus photography (b = −8.61, b = −2.59 and b = −7.03, respectively; p < 0.001). Ultrawide-field imaging studies were excluded from the final sensitivity/specificity analysis, as the total number of patients included was too small. Conclusions: Regardless of the type of the device used, retinal photographs should be taken on eyes with dilated pupils, unless contraindicated, as this setting decreases the rate of ungradable images. Smartphone-based and ultrawide-field imaging may become potential alternative methods for optimized DR screening; however, there is not yet enough evidence for these techniques to displace mydriatic fundus photography.
1. Introduction
Dramatic changes in lifestyle have led to an increased risk of noncommunicable diseases, such as type 2 diabetes [1]. The prevalence of diabetes mellitus (DM) has been steadily increasing over the past three decades from an estimated 108 million in 1990 to over 415 million people worldwide, or 1 in every 11 adults [2,3,4]. The most prominent increase is noted in low- and middle-income countries.
Diabetic retinopathy (DR) is the leading cause of vision loss both of working-age adults and of preventable blindness globally. In a meta-analysis, Yau et al. estimated that the prevalence of any DR among diabetic subjects might reach 34.6% (95% confidence interval [CI]: 34.5–34.8), while the prevalence of vision-threatening diabetic retinopathy (VTDR) is 10.2% (95% CI: 10.1–10.3) [5]. The risk of DR is higher in individuals with type 1 diabetes, compared to those with type 2 diabetes. Hyperglycemia remains the most important modifiable risk factor for DR [6].
The development of a screening program for DR in Europe was encouraged by the St. Vincent Declaration in 1989 [7], which has set a target to reduce new cases of blindness by one third within a 5-year period. In terms of cost-effectiveness, screening for DR is a critical aspect of DM management [8,9]. Screening for DR is predominantly warranted by the fact that the major complications—macular edema and proliferative DR—respond to treatment [10,11]. According to the International Council of Ophthalmology Guidelines for Diabetic Eye Care 2017, examinations performed for DR screening should involve visual acuity assessment with current spectacles and retinal evaluation (ophthalmoscopy or fundus photography) [12]. In recent years, an important development was noted, particularly in retinal imaging techniques. The aim of this study was to review the imaging modalities employed for retinal examination in diabetic retinopathy screening. The article did not evaluate methods for DR grading, nor deep-learning algorithms for automated DR detection, which may, however, play a significant role in the future [13].
2. Materials and Methods
2.1. Literature Search
The PubMed and Web of Science databases were the main sources used to investigate medical literature. An extensive search was performed to identify relevant articles concerning “imaging”, “diabetic retinopathy” and “screening” up to 1 June 2021 (Appendix A). The following keywords were used in various combinations: diabetes, diabetic, retinopathy, macular edema, screening, imaging, fundus, photography, and scanning laser ophthalmoscopy. Of the studies retrieved, we reviewed all publications in English and abstracts of non-English publications. The reference lists of the articles analyzed were also considered as a potential source of information. We attempted to present all publications, analyzing the accuracy of various retinal imaging methods employed for DR screening. Emphasis was placed on studies published after the meta-analysis by Bragge et al. [14] and Hu et al. [15]; however, in contrast to those studies, we did not evaluate the performance of different manual methods of the eye examination and aimed to analyze the performance of different technologies. Our study did not aim to compare the accuracy of automated vs. manual analysis, but solely to evaluate the utility of technical methods for obtaining images. Studies were critically reviewed to create an overview and guidance for further research. No attempts were made to discover unpublished data. In addition to the PubMed and Web of Science searches, selected chapters from relevant textbooks were included.
2.2. Statistical Analysis
Articles were included in our statistical analysis if they met the following criteria: (i) the study evaluated an imaging modality, and the outcome of interest was the detection of diabetic retinopathy; (ii) the study defined a reference standard for DR detection to which the imaging method was compared; (iii) a threshold for DR detection was defined; and (iv) the sensitivity and specificity for DR detection was specified, or data were given to calculate them. If any investigation presented more than one threshold level for DR detection (e.g., the detection of any DR or alternatively VTDR) the performance for all thresholds was analyzed. In studies comparing the performance of conventional photography and digital photography with the reference standard, we analyzed only outcomes for the digital method. If more than two imaging techniques were applied within the study, all methods were included in the analysis. Meta-analyses were performed by using Stata 14.2 (StataCorp, College Station, TX, USA) environment, i.e., by employing two Stata routines, namely, METAAN (random-effects meta-analysis command) for failure rate calculations and MIDAS (Meta-analytical Integration of Diagnostic Accuracy Studies) for appraisal of sensitivity and specificity of the investigated diagnostic tests. Due to the observed heterogeneity between the studies, random-effects models were applied. When assessing subgroup differences in the meta-analyses, a Chi-squared test was used. The level of p < 0.05 was deemed statistically significant.
3. Results
The search identified 2137 unique articles. After removing duplicates and irrelevant studies, 148 articles were included in the review. Table 1 presents the testing accuracy in studies on imaging modalities used for detecting diabetic retinopathy.

Table 1.
Testing accuracy in studies on imaging modalities used for detecting diabetic retinopathy.
3.1. Technical Failure Rate
The technical failure rates for mydriatic digital fundus photography, non-mydriatic digital fundus photography, smartphone-based imaging and ultrawide-field imaging were 3.4% (95% CI: 2.3–4.6%), 12.1% (95% CI: 5.4–18.7%), 5.3% (95% CI: 1.5–9.0%) and 2.2% (95% CI: 0.3–4.0%), respectively (Figure 1). The failure rate was significantly different between all pairs of the analyzed techniques (p < 0.001). The overall failure rate for all techniques was 6.6% (4.9–8.3%; heterogeneity [I2] = 97.2%).
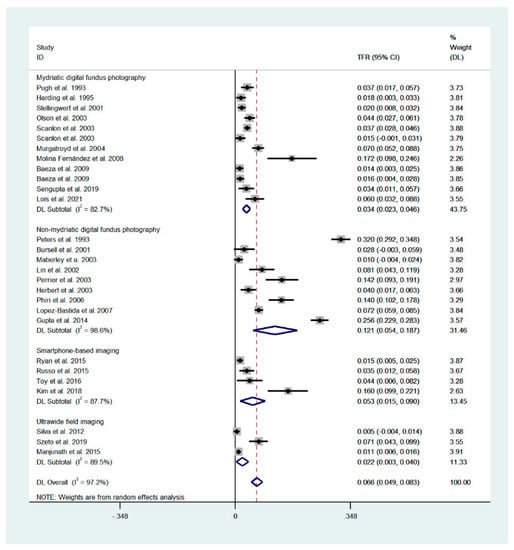
Figure 1.
Forest plot presenting the technical failure of the analyzed techniques. Overall values for mydriatic fundus photography: 3.4% (95% CI: 2.3–4.6%, I2 = 82.7%), for non-mydriatic fundus imaging: 12.1% (95% CI: 5.4–18.7%, I2 = 98.6%), smartphone-based imaging: 5.3% (1.5–9.0%; I2 = 87.7%), ultrawide-field imaging: 2.2% (0.3–4.0%; I2 = 89.5%). Overall, for all techniques: 6.6% (4.9–8.3%; I2 = 97.2%). Weights are calculated from random effects analysis.
3.2. Sensitivity and Specificity in Cases without Technical Failure
The articles included in the study are presented in Table 1; ultrawide-field imaging studies were excluded from the final analysis, as the total number of patients included was too small.
The pooled sensitivity for all the methods was 0.84 (95% CI: 0.8–0.88) (Figure 2). In terms of sensitivity, mydriatic fundus photography (0.85 [95% CI: 0.77–0.91], I2 = 0.0; Figure 3) and non-mydriatic fundus photography (0.85 [95% CI: 0.77–0.9], I2 = 38.85; Figure 4) had lower sensitivity than smartphone-based imaging (0.91 [95% CI: 0.85–0.94], I2 = 98.47; Figure 5). There was a statistically significant difference between all three groups (p < 0.001). Due to the high heterogeneity of smartphone-based imaging studies, the results should be taken with caution.
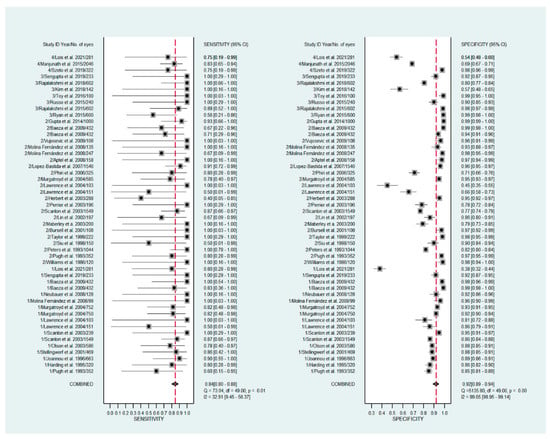
Figure 2.
Forest plots for the sensitivity and specificity of mydriatic, non-mydriatic and smartphone-based imaging fundus imaging.
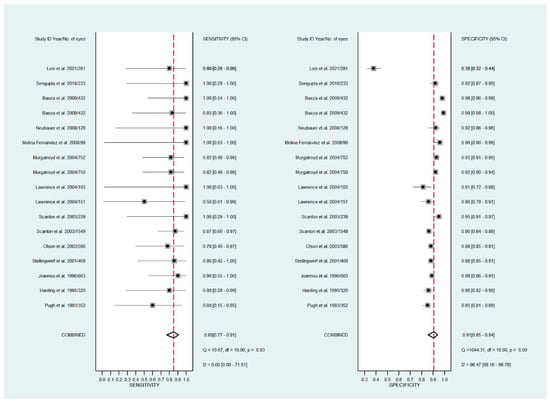
Figure 3.
Forest plots for the sensitivity and specificity of mydriatic fundus photography.
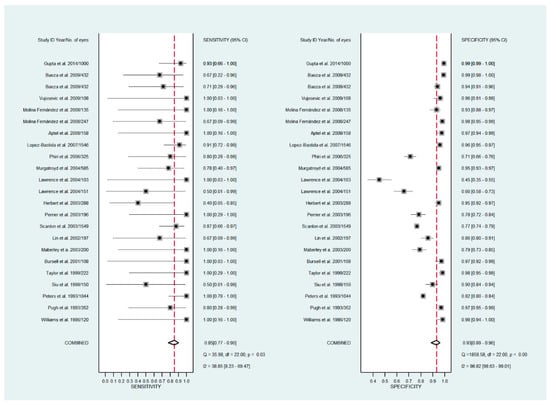
Figure 4.
Forest plots for the sensitivity and specificity of non-mydriatic fundus photography.
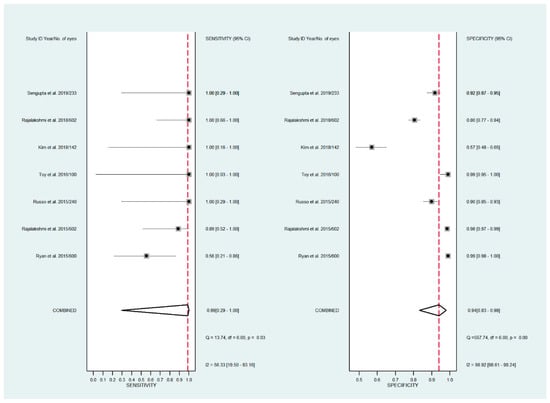
Figure 5.
Forest plots for the sensitivity and specificity of smartphone-based fundus imaging.
The pooled specificity for all methods was 0.92 (95% CI: 0.89–0.94) (Figure 2). The specificity of mydriatic fundus photography (0.91 [95% CI: 0.84–0.94], I2 = 98.47) was not different from that of non-mydriatic fundus photography (0.93 [95% CI: 0.89–0.96], I2 = 98.82). The pooled specificity of smartphone-based imaging studies (0.94 [95% CI: 0.83–0.98], I2 = 98.92) was significantly better than that of mydriatic (p < 0.001) and non-mydriatic fundus photography (p < 0.001). There was no difference observed in the specificity for mydriatic and non-mydriatic photography (p > 0.05). The receiver operating characteristic (ROC) curves of the analyzed methods are shown in Figure 6, Figure 7 and Figure 8.
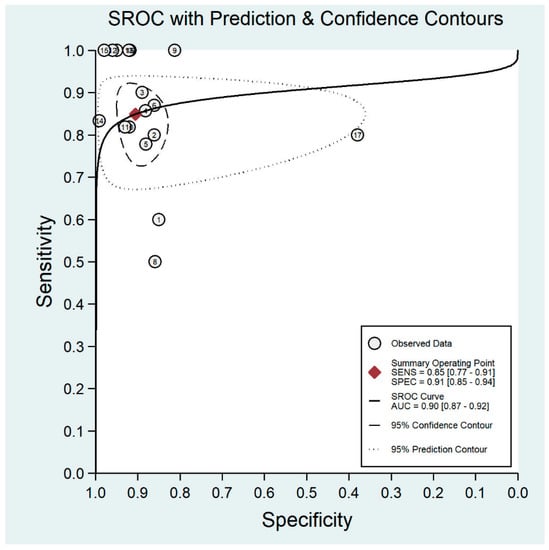
Figure 6.
Receiver operating characteristic curve for mydriatic fundus imaging.
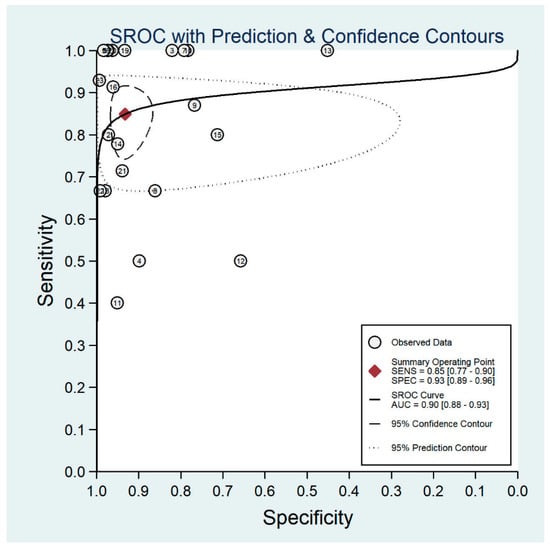
Figure 7.
Receiver operating characteristic curve for non-mydriatic fundus imaging.
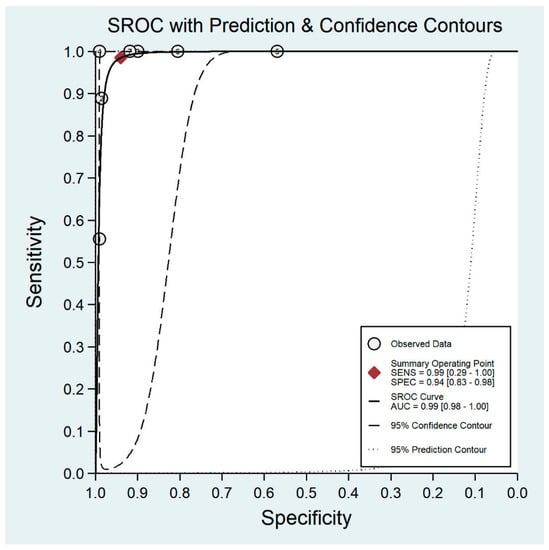
Figure 8.
Receiver operating characteristic curve for smartphone-based imaging.
The total sample size was the lowest in smartphone-based imaging studies. Moreover, the publication bias factor for smartphone-based imaging was significantly higher than for mydriatic digital fundus photography and non-mydriatic digital fundus photography (b = −8.61, b = −2.59 and b = −7.03, respectively). The pooled sensitivity and specificity for mydriatic methods, i.e., mydriatic fundus photography and smartphone imaging, was 0.85 (95% CI: 0.78–0.90) and 0.92 (95% CI: 0.87–0.95), respectively; it was not different to the sensitivity and specificity of non-mydriatic fundus photography (p = 0.827 and p = 0.921, respectively).
4. Discussion
4.1. Fundus Examination vs. Retinal Photography
A DR screening examination could hypothetically include a complete ophthalmic check-up with best-corrected visual acuity after refraction, pupil dilation and state-of-art retinal imaging including wide-field retinal photography with optical coherence tomography [53,54]. This is not performed even in high-resource settings; ideally, a DR screening program should have as few components as possible, be affordable and available, but should ensure appropriate referral [55].
With the increasing prevalence of diabetes, one could consider ophthalmology as under-resourced in some parts of the world. However, even with a sufficient number of ophthalmologists available, employing them to screen every individual with DM is not feasible and likely to be inefficient use of resources [14,56]. As a consequence, in some studies fundoscopy for DR screening was successfully performed by ophthalmological optometrists [20,57,58,59,60], general practitioners [61,62], trained technicians [63] or nurses [64]. Although in a single study consultants performed better than non-consultant staff in grading DR, the variability of opinions was significant even for consultants [65]. In another study, the sensitivity and specificity of slit-lamp examination for DR detection performed by optometrists was 73% and 90%, respectively, compared to the reference slit-lamp biomicroscopy by ophthalmologists with interest in medical retina [20]. In a Norwegian investigation the sensitivity and specificity of optometrists for DR evaluation of 7-field fundus images was 67% (62–72%) and 84% (95% CI: 80–89%), respectively, when compared to reading by ophthalmologists [66]. Only 5% of optometrists met the required standard of at least 80% sensitivity and 95% specificity which was postulated as the ultimate requirement for DR screening programs [66]. Still, these differences might rather be a matter of briefing for specific tasks, than reflect the competence based on the actual educational background.
Additional criteria should be considered for a screening test — the test should be inexpensive and non-invasive. Screening techniques cannot be expected to perform as well as detailed investigative techniques but should be comparable with the original method [67]. In clinical studies the agreement between ophthalmoscopy and color fundus photography grading by various methods ranges from 34.0% to 86.3% [37]. Interestingly, regarding the grading of DR, there is evidence indicating that color photography is superior to fundoscopy alone [10,20,37,63,65,68,69], and particularly to direct ophthalmoscopy [17,33]. Schachat et al. reported that clinical examination underestimates the prevalence of DR when compared to photography gradings (7.7% vs. 8.7%, respectively) [10]. In another study, the sensitivity and specificity for ophthalmoscopy compared to grading of 7-field fundus photographs for the detection of any DR was 51% and 91%, respectively [70]. Even worse rates of performance were reported in an investigation by Lin et al. where the sensitivity of ophthalmoscopy for DR screening compared with 7-field photography was 34%, with a specificity of 100% [37]. Pugh et al. found that the sensitivity of an ophthalmologist in detecting DR was 33% and it was even worse (sensitivity 14%) for a physician’s assistant when compared to the reference standard, the 7-field photography [16]. Another study reported that ophthalmoscopy missed approximately 50% of eyes with microaneurysms only when compared to fundus photography [71].
It was hypothesized that macular edema with a few hard exudates could be easier to detect in fundoscopy than in non-stereoscopic photography [68]. Nevertheless, such a finding was not confirmed in clinical studies [31,65]. In an investigation by Taylor et al. maculopathy was reported in 147/4312 eyes with camera screening and only in 95/4312 eyes by ophthalmoscopy alone (p < 0.001); moreover, ophthalmoscopy underestimated the presence of hard exudates (p < 0.001) [65]. A disadvantage of the fundus camera is its cost; however, without such a camera, documenting minimal changes over time might be difficult [63]. However, fundus photography offers the benefit of providing a record of retinopathy which can be used at a later date to document the progression of retinopathy or response to treatment. Currently, it might be difficult to consider eye fundus examinations as a method for DR screening using the resources efficiently.
4.2. Monoscopic vs. Stereoscopic Fundus Photography
Both the original Airlie House DR classification used in the Diabetic Retinopathy Study [72,73,74], and the modified DR classification used in the Early Treatment Diabetic Retinopathy Study, employed 7-field stereographic photography [75] to determine the grade of DR. In stereographic retinal photography a stereo image is obtained by taking photographs from two slightly different positions and merging these images enables a perception of depth [76].
The perception of depth in assessing DR severity should help us to determine the presence of macular edema, to differentiate neovascularization from intraretinal microvascular abnormalities, and to detect pre- and intraretinal hemorrhages [77]. Despite the potential benefits, acquisition and grading of stereoscopic images is time-consuming and doubles the number of light flashes that the patient must endure [76]. Moreover, the technique depends on the experience of photographers, as left and right images must be equally sharp and illuminated in each pair [78,79]. For the graders, special equipment such as optical viewers or goggles is needed to achieve the stereo depth and to review them [76]. The perception of stereoscopy is dependent on the observer’s capability to fuse stereoscopically [76]. There is evidence indicating that obtaining stereoscopy is not critical for the assessment of DR severity, and monoscopic photography can equal the reliability of stereo photography for full ETDRS DR severity scale grading [76]. Moreover, it might be questionable whether the cost and logistical concerns involved in obtaining 7-field images either conventionally, or digitally, would make the method practical and cost-effective for widespread screening [63,80].
4.3. DR Grading
Within the analyzed studies, two thresholds for DR detection were most commonly used: VTDR or any symptoms DR. VTDR is usually defined as severe non-proliferative, proliferative retinopathy and/or macular oedema in at least one eye [81]. Treatment for VTDR is agreed upon universally [82]: laser treatment is effective [83,84], and vascular endothelial growth factor inhibitors (anti-VEGFs) can improve the results of treatment in diabetic maculopathy [85,86] and in some cases of proliferative DR [87,88]. Patients with mild nonproliferative DR (which is indicated by the presence of at least 1 microaneurysm) do not require any ophthalmic treatment. Thus, positive screening of patients with any symptoms of DR could not be considered appropriate. On the other hand, the rate of DR deterioration is reduced by improved control of blood glucose [89,90,91] and blood pressure [92,93], and this could be some benefit of screening patients with any DR.
In terms of methodological correctness and the principles of meta-analysis, future DR screening research should focus solely on the epidemiology of VTDR. One should consider that the lower the prevalence of a specific disease, the greater the meticulousness and usefulness of the meta-analysis performed as regards the investigated diagnostic tests, which are employed in clinical practice. As mentioned previously, the estimated prevalence of any DR among diabetic is significantly higher than the prevalence of VTDR (34.6% vs. 10.2%, respectively) [5]. Also VTDR could be considered as the main outcome of interest of DR screening programmes.
4.4. Mydriatic Versus Non-Mydriatic Fundus Photography
Seven-field mydriatic photography is considered as the gold standard for fundus imaging, however, the inconvenience and risks associated with mydriasis must be considered. Even when using a short acting mydriatic (tropicamide), dilating the pupils can cause discomfort, especially for those who plan to return to work after being screened or need to drive a car to reach the screening facility [65]. Moreover, pupil dilation is time-consuming, both for the patient and also for the examiner, thus negatively influencing efficiency. Finally, as the use of such agents is not popular with patients, it might lead to poorer compliance [25,94]. For example, in a study by Natarajan et al. 9.4% of patients did not agree to participate in the screening due to waiting time and potential discomfort associated with pupil dilation [95]. Non-mydriatic imaging is a faster and less expensive option than mydriatic photography [68].
Importantly, diabetes is concerned as a risk factor for presenting with a small pupil [96,97]. The pupillary dysfunction demonstrated in diabetes is related to autonomic neuropathy and primarily involves the sympathetic innervation of the iris dilator [98]. Applying a mydriatic agent could potentially lead to improving the quality of imaging in these cases. However, the loss of sympathetic tonus in individuals with diabetes restricts the utility of commonly used topical anticholinergic agents resulting in inadequate pupil dilation [99]. Sympathetic denervation is correlated with the duration of the disease and the development of systemic autonomic neuropathy [100]. Diabetic patients might respond relatively poorly to mydriasis with topical tropicamide 1%; pupil dilation might be achieved in these patients by additional application of topical phenylephrine [23,96,101].
In a clinical DR screening study by Murgatroyd et al. mydriasis reduced the proportion of ungradable photographs from 26% to 5% (p < 0.001) [24]. In another study up to 29.2% of non-mydriatic images were poorly focused, and as a consequence, partly ungradable [68]. In an investigation by Pugh et al. 14% of undilated and 3.7% of dilated images were found ungradable; importantly, after mydriasis most of the ungradable photographs (42/50) became gradable [16]. Similar results were noted by Baeza et al. who reported that 15.3–17.6% of non-mydriatic images but only 1.4–2.1% of the mydriatic images were ungradable [28]. In a study by Peters et al. the rate of ungradable non-mydriatic images was 32%; patients with ungradable images were older (56.0 vs. 46.6 years) and had a pupil size <4 mm (27% vs. 7%) [32]. Pharmacologic dilation might not only enhance the gradability of fundus photographs but also their accuracy [16,43]. After pupil dilation, some retinal findings such as venous beading or nerve fiber layer hemorrhages, are more probable to be detected [25]. Moreover, in a dark iris population e.g. in Indian eyes, non-mydriatic digital imaging might result in an even higher (30.6–31%) rate of poor quality photographs, resulting in low sensitivity and restricting the use of this technique [43]. The diminished sensitivity of non-mydriatic photographs could be acceptable if a greater percentage of patients would agree to complete the screening process [102]; however, such a finding was not confirmed in clinical trials. Pupil dilation might be used when the quality of the obtained images is poor, e.g. in older patients with advanced cataract or senile miosis [25]. Scotland introduced the concept of staged mydriasis into their screening programme, only dilating those patients having poor-quality images without mydriasis [82]. The image quality is assessed by the technician taking the images. Recently, the numbers needing dilation have currently risen to 34% [82]. In a single study by Molina Fernández et al. selective mydriasis, based upon the decision taken by the family doctor who performed the imaging, did not improve the screening performance [26]. Regardless of the type of the device used, the photographs should be taken on the dilated eye, as this significantly improves the sensitivity and decreases the rate of ungradable images. Selective mydriasis did not improve performance of DR screening.
Several of the analyzed studies are more than 10 years old, and one must consider that in recent years there has been a technical development in fundus cameras. First, advancements in the field of optical sources and detectors have led to miniaturization of optical assemblies at a lower cost. In line with these developments, miniature table-top fundus camera system designs have emerged that provide retinal images comparable to those of traditional fundus cameras [103].Camera systems have evolved to boast sharper images, having a higher resolution, pupil tracking, and, most recently, portability. Potentially, an improvement in camera optics could result in decreasing the TFR rate. On the other hand, this has not been proved in clinical trials.
4.5. Single vs. Multiple-Field Imaging
One major concern in single-field imaging is that a smaller area of the retina is imaged; particularly the nasal retina is of importance for a valid evaluation of the DR stage [78]. From a mathematical point of view, a 30° angle field-of-view is equal to visualizing the retinal area of 56.4 mm2, while a 45° angle equals to the visualization of a 124.8 mm2 area [104]. In these terms, a retinal area visualized with a single 45° image cannot be considered equivalent to seven 30° shots; with two- or three-field 45° images the area could be comparable.
Different protocols were applied with regional DR screening programs, e.g., a single-field 45° photography in Singapore [105], two-field 45° photography in England [106], or five-field 45° photography in France [107]. In the study of Aptel et al. there was a major difference seen in the sensitivity of detecting DR between single-field and three-field 45° non-mydriatic photographs (76.92% vs. 92.31%, respectively; p < 0.001) [25]. The study by Perrier et al. presented no significant difference in sensitivity between two, three and four-field non-mydriatic photography (95.7%, 97.6% and 97.6%, respectively) [38]. Moreover, additional images reduced the specificity (which was 78.1%, 71.9% and 65.6% for two-, three- and four-field imaging, respectively) and led to a higher rate of ungradable images (14.2%, 18.3% and 18.3%, respectively; p values not stated) [38]. The poor quality of adding extra-field to two-field imaging translated into an increase of 6.2% in the rate of referral to an ophthalmologist [38]. Baeza et al. noted that by increasing the number of fields from one to three the sensitivity slightly increased (from 68% to 79%) [28]. Importantly, applying mydriasis led to a decrease in the rate of ungradable images (from 15.3–18.3% to 1.4–2.1%) [28]. In another study the performance of two-field evaluation was similar to single-field photography; including nasal images did not bring added value to macular images [20]. Moreover, despite mydriasis, the nasal images had poorer quality than macular images (3.5–8.1% of nasal images were ungradable) [20]. These findings are analogous to the meta-analysis by Hu et al. who reported that single-field non-mydriatic photography might be inadequate to detect DR [15].
4.6. Handheld and Smartphone-Based Devices
To expand screening programs into rural areas it would be beneficial to have access to low-cost portable, easy to operate, and high image quality fundus cameras [108]. Tran et al. have shown that it is possible to construct a hand-held mydriatic fundus camera prototype at a cost of less than 1000 USD [109]. Their front-end module was retrofitted to go with several consumer cameras; however, those with smaller CMOS (Complementary Metal Oxide Semiconductor) sensors showed loss of image detail or increased image noise compared to larger CMOS devices [109]. In the following years, several portable eye fundus cameras were developed which have a digital camera incorporated. These include the Smartscope Pro (Optomed, Oulu, Finland) commercialized as Pictor (Volk Optical, Mentor, OH, USA), Horus DEC 200 (MiiS, Nsinchu, Taiwan), Genesis-D (Kowa, Nagoya, Japan), Signal (Topcon Corporation, Tokyo, Japan), Dragonfly (Eyefficient; Aurora, OH, USA), VersaCamTM DS-10 (Nidek, Gamagori, Japan) or Visuscout 100 (Carl Zeiss Meditec AG, Jena, Germany).
Another option for retinal imaging is the use of a smartphone’s in-built camera. A smartphone can be used to capture pictures of the posterior segment of the eye during slit-lamp indirect ophthalmoscopy with a 78 D lens [110,111]. Haddock et al. [112] and Bastawrous [113] suggested using the coaxial light source of the phone rather than that of the slit-lamp; in their technique the phone is being kept in one of the examiners hand, while the other hand is holding a 20 D or 28 D lens. For examinations performed in general anesthesia, additionally a Koeppe contact lens was applied, which was useful in receiving a wider field of view, keeping the lids open and the cornea wet [112]. Images obtained with a 20 D lens have a smaller imaging area of <45° when compared to a combination of a 60 D with a 90 mm focal length lens (area of 92°) [114,115]. A special attachment which is designed to hold a specific lens at a prescribed, but adjustable distance from the camera lens, might improve the ease-of-use of such imaging methods [116,117]; and such an attachment can be 3D-printed [116].
Currently, several adapters for cell phones have become commercially available: D-Eye (D-Eye, Padova, Italy), PanOptic + iExaminer (Welch Allyn, Skaneateles Falls, NY, USA), MII RetCam (MII RetCam Inc., Coimbatore, India), iNview/Vistaview (Volk Optical, Mentor, OH, USA) or the Peek Vision (Nesta, London, UK) [29,118]. The PanOptic and D-Eye have limited imaging fields (25° and 20°, respectively). Interestingly, the Fundus-On-Phone System (Remidio, Bengaluru, India) is smartphone based, but not handheld. The technical details and a review of the currently developed systems was published elsewhere [103] and does not fall within the scope of this paper.
A significant limitation of several smartphone-based systems is the requirement of mydriasis. Moreover, it might be difficult to consider the resolution of a smartphone’s in-built camera (particularly in older phones, which have been used in several studies) to that of a professional desktop camera. Another problem is glare, improper exposure or difficulties in capturing ideally sharp images [103,119]. For example, iPhone’s built-in flash has a fairly high intensity, and efforts are made to design imaging systems with an external light source with varying intensity levels [103]. Finally, sophisticated skill is required to perform the imaging as the beam alignment is problematic, and stability of the camera is required [103]. Thus, unless the examiner is already adept at indirect ophthalmoscopy, it can be challenging to obtain high-quality images that are useful for evaluation [120]. Some might prefer to use portable cameras that have slit lamp attachments. On the other hand, a single study has shown that medical students who were previously unfamiliar with indirect ophthalmoscopy were able to successfully acquire images after 15 minutes of training [121], and some of them preferred smartphone ophthalmoscopy compared to conventional direct ophthalmoscopy [122].
4.7. Ultrawide-Field Imaging
Ultrawide-field scanning laser ophthalmoscopy (UWF-SLO) employs confocal laser scanning microscopy combined with a concave elliptical mirror, having the capability of capturing up to 200° of the retina in a single image, without pupil dilation in less than one second [123]. With the steering function it is possible to obtain a greater field under mydriasis with a light inside the camera guiding the patients’ eye [79]. During the examination a low-powered green (532 nm) and red light (633 nm) simultaneously scan the retina and choroidal tissue; a composite picture is created by digital combination of the two wavelengths [50]. By scanning a smaller area (100° instead of 200°) it is possible to obtain images having higher resolution up to 11 µm [50]. Although ultrawide images can be obtained with or without mydriasis, a study by Rasmussen et al. showed that the quality of mydriatic and non-mydriatic images obtained with Optos 200Tx (Optos, Dunfermline, United Kingdom) did not differ significantly [79]. One should mention that currently there are a variety of Optos devices enabling UWF-SLO imaging; it is also possible to obtain 102-degree UWF-SLO images with Spectralis (Heidelberg Engineering, Heidelberg, Germany) [124].
An advantage of UWF-SLO is assessment of peripheral pathologies which could be overlooked if a smaller angle is imaged [125,126]. It was hypothesized that a subset of DR patients might exhibit peripheral distribution of retinal lesions, unavailable for visualization in fundus photography [50,127]. Moreover, one-third of retinal hemorrhages and/or microaneurysms, intraretinal microvascular abnormalities and new vessels elsewhere might be situated outside the ETDRS fields, and visible in UWF-SLO but not in 7-field ETDRS photography [126]. UWF-SLO has, as well, the potential of identifying peripheral retinal lesions and vitreous pathologic findings [128]. Another potential benefit could be the reduction in the rate of ungradable images due to better imaging technology [127]. In some of the UWF-SLO systems obtaining fluorescein angiography images is possible [129].
A study by Silva et al. showed that UWF-SLO may underdiagnose proliferative DR [50]. This was presumably associated with colour distortion from the machine and, therefore, requires significant magnification of the images to evaluate discrete retinal lesions. The recently released Clarus 500 and Clarus 700 (Carl Zeiss Meditec AG, Jena, Germany) capture “true-color” images that may potentially enable more accurate identification of DR lesions, although this has yet to be demonstrated in clinical trials [130]. Within the currently published studies, images obtained with Clarus were consistent with current UWF-SLO devices in assessing the severity of DR, with no statistically significant difference in patient or technician preference, and image acquisition time [131,132,133]. The Eidon confocal scanner (Centervue, Padova, Italy) couples confocal imaging with natural white-light illumination to obtain a true-colour image using a white LED (440–650 nm). The Spectralis (Heidelberg Engineering, Heidelberg, Germany) has a dedicated Spectralis MultiColor Module, which is not available in the standard version of the device and uses three laser wavelengths simultaneously to receive color images; thus, the basic version of device cannot be considered as optimized for DR screening. Potentially, UWF-SLO could be less susceptible to media opacities or decreased pupil diameter compared with conventional fundus photography [78]. However, in another study, all images of patients with proliferative DR were found ungradable due to glare associated with media opacities in a dense cataract or vitreous hemorrhage [51]. In the investigation by Aiello et al., UWF-SLO imaging in a clinical setting increased the frequency of DR identification nearly two-fold but the agreement with ETDRS 7-field imaging was moderate [134].
One disadvantage of the UWF-SLO technology compared to other approaches is that it is still more costly [78]. This issue could be critical to wide-spread use of UWF-SLO for DR screening, as a screening examination should be inexpensive. For example, expenditures on the English DR Screening Program which employed fundus cameras were approximately 85.6 million USD or 40 USD per person screened [106]. With UWF-SLO devices, which are significantly more expensive than fundus cameras, these costs could even be higher. Although the results of the English program are excellent, high costs preclude implementation of this strategy worldwide; in several studies emphasis is placed on new, cost-effective systems. On the other hand, Lois et al. showed that savings associated with UWF-SLO for DR assessments are greater than for 7-field photography mainly due to longer time to obtain and read images in the 7-field photography technique [30]. Future research may aim to clarify the association of peripheral diabetic lesions with the stage of DR [135]. One might discuss whether UWF-SLO is advisable for screening of high-risk DR or proliferative DR [136]. This aspect requires further validation [51].
4.8. Multimodal Imaging Techniques and Potential Future Directions
Multimodal imaging techniques employ several imaging methods to examine a particular finding. Quantitation of retinal thickness and precise topographic mapping of the retina have been useful in assessing retinal thickness in both non-clinically significant macular edema and clinically significant macular edema [137]. Optical coherence tomography (OCT) is more reproducible and more sensitive to follow changes in retinal thickness when compared to fundus photography [138]. Technically, it is possible to obtain simultaneous or immediately sequential fundus photographs and OCT images [139]. Such devices are commercially available, e.g. in the Maestro2 (Topcon Corporation, Tokyo, Japan) or the Revo FC (Optopol Technology Sp. z o.o., Zawiercie, Poland) [140]. Importantly, adding OCT to the assessment of maculopathy improves the sensitivity and specificity of detecting clinically significant macular edema as well as any maculopathy (i.e., exudates only) [52]. Both of the aforementioned devices also allow obtaining OCT-angiography images. Nevertheless, current limitations of OCT angiography include a small field of view, projection and motion artifact, and inability to assess flow and filling speeds or vascular competence by assessing dye leakage [141]. OCT can also be combined with UWF-SLO imaging [52].
Other technical advantages may play a role in multimodal DR assessment in the future [141]. Enhanced depth imaging OCT or swept-source OCT could allow improved choroidal visualization [142]. Choroidal thickness was shown to be altered in patients with diabetes and diabetic choroidopathy; it was suggested that a change in choroidal thickness may precede any retinopathy [143,144,145]. Adaptive optics allow a noninvasive acquisition of images of the retina with cellular-level resolution and assessment of individual photoreceptor cells [146]. Hyperspectral imaging might be a promising way to measure oxygenation in the retinal blood vessels; this is important because hyperglycaemia is known to increase retinal oxygen consumption [147,148].
Author Contributions
P.K.: concept and design, data collection, data analysis and interpretation, drafting the article; R.T.: conception of the work, critical revision of the article; R.K.: conception of the work, critical revision of the article. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for this study.
Institutional Review Board Statement
Not applicable.
Acknowledgments
Tuuminen reports non-financial support from Bayer, and personal fees from Novartis, outside the submitted work. Khoramnia reports grants, personal fees, and non-financial support from Alimera, Alcon, Bayer, Johnson&Johnson, Hoya, Novartis, Physiol, Rayner, Roche, personal fees, and non-financial support from Allergan, Kowa, Ophtec, Oculentis/Teleon, Santen, and Acufocus, outside the submitted work. None of the authors have a proprietary interest.
Conflicts of Interest
Kanclerz reports non-financial support from Visim and Optopol Technology. Tuuminen reports non-financial support from Bayer, and personal fees from Novartis, outside the submitted work. Khoramnia reports grants, personal fees, and non-financial support from Alimera, Alcon, Bayer, Johnson&Johnson, Hoya, Novartis, Physiol, Rayner, Roche, personal fees, and non-financial support from Allergan, Kowa, Ophtec, Oculentis/Teleon, Santen, and Acufocus, outside the submitted work. The authors have neither proprietary nor commercial interests in any medications or materials discussed.
Appendix A. Search Strategy
Literature searches of the PubMed and Web of Science databases were conducted in 30 June 2021; the search strategies are as follows. Specific limited update searches were conducted after 30 June 2021. Reference lists of the included studies were also considered as a source of publications.
Appendix A.1. PubMed Search (Publication Date 1/10/11–06/30/2021)
((“diabetes”[Title]) OR (“diabetic”[Title])) AND ((“retinopathy”[Title]) OR (“macular edema”[Title]) OR (“macular oedema”[Title])) AND ((“screening”[Title]) OR (“imaging”[Title]) OR (“fundus”[Title]) OR (“photography”[Title]) OR (“scanning laser ophthalmoscopy”[Title])). 1339 references.
Appendix A.2. Web of Science Search (Publication Date 1/10/11–6/30/2021)
(TI=(“diabetes”) OR TI=(“diabetic”)) AND (TI=(“retinopathy”) OR TI=(“macular edema”)) AND (TI=(“screening”) OR TI=(“imaging”) OR TI=(“fundus”) OR TI=(“photography”) OR TI=(“scanning laser ophthalmoscopy”)) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years. 2051 references.
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [CrossRef]
- World Health Organization. Global Report on Diabetes 2016. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 15 March 2021).
- International Diabetes Federation (IDF) IDF Diabetes Atlas 7th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 13 October 2017).
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of Diabetic Retinopathy, Diabetic Macular Edema and Related Vision Loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Diabetes Care and Research in Europe: The Saint Vincent Declaration. Diabet. Med. 1990, 7, 360. [CrossRef]
- Javitt, J.C.; Aiello, L.P. Cost-Effectiveness of Detecting and Treating Diabetic Retinopathy. Ann. Intern. Med. 1996, 124, 164–169. [Google Scholar] [CrossRef]
- Rohan, T.E.; Frost, C.D.; Wald, N.J. Prevention of Blindness by Screening for Diabetic Retinopathy: A Quantitative Assessment. BMJ 1989, 299, 1198–1201. [Google Scholar] [CrossRef]
- Schachat, A.P.; Hyman, L.; Leske, M.C.; Connell, A.M.; Hiner, C.; Javornik, N.; Alexander, J. Comparison of Diabetic Retinopathy Detection by Clinical Examinations and Photograph Gradings. Barbados (West Indies) Eye Study Group. Arch. Ophthalmol. 1993, 111, 1064–1070. [Google Scholar] [CrossRef]
- Augustin, A.J.; Bopp, S.; Fechner, M.; Holz, F.; Sandner, D.; Winkgen, A.-M.; Khoramnia, R.; Neuhann, T.; Warscher, M.; Spitzer, M.; et al. Three-Year Results from the Retro-IDEAL Study: Real-World Data from Diabetic Macular Edema (DME) Patients Treated with ILUVIEN (0.19 Mg Fluocinolone Acetonide Implant). Eur. J. Ophthalmol. 2020, 30, 382–391. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-Up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Folk, J.C.; Han, D.P.; Walker, J.D.; Williams, D.F.; Russell, S.R.; Massin, P.; Cochener, B.; Gain, P.; Tang, L.; et al. Automated Analysis of Retinal Images for Detection of Referable Diabetic Retinopathy. JAMA Ophthalmol. 2013, 131, 351–357. [Google Scholar] [CrossRef]
- Bragge, P.; Gruen, R.L.; Chau, M.; Forbes, A.; Taylor, H.R. Screening for Presence or Absence of Diabetic Retinopathy: A Meta-Analysis. Arch. Ophthalmol. 2011, 129, 435–444. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Lu, Y.; Dou, X.; Ye, B.; Cai, Z.; Pu, Z.; Mou, L. Single-Field Non-Mydriatic Fundus Photography for Diabetic Retinopathy Screening: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2019, 62, 61–67. [Google Scholar] [CrossRef]
- Pugh, J.A.; Jacobson, J.M.; Van Heuven, W.A.; Watters, J.A.; Tuley, M.R.; Lairson, D.R.; Lorimor, R.J.; Kapadia, A.S.; Velez, R. Screening for Diabetic Retinopathy. The Wide-Angle Retinal Camera. Diabetes Care 1993, 16, 889–895. [Google Scholar] [CrossRef]
- Harding, S.P.; Broadbent, D.M.; Neoh, C.; White, M.C.; Vora, J. Sensitivity and Specificity of Photography and Direct Ophthalmoscopy in Screening for Sight Threatening Eye Disease: The Liverpool Diabetic Eye Study. BMJ 1995, 311, 1131–1135. [Google Scholar] [CrossRef]
- Joannou, J.; Kalk, W.J.; Mahomed, I.; Ntsepo, S.; Berzin, M.; Joffe, B.I.; Raal, F.J.; Sachs, E.; van der Merwe, M.T.; Wing, J.R. Screening for Diabetic Retinopathy in South Africa with 60 Degrees Retinal Colour Photography. J. Intern. Med. 1996, 239, 43–47. [Google Scholar] [CrossRef]
- Stellingwerf, C.; Hardus, P.L.; Hooymans, J.M. Two-Field Photography Can Identify Patients with Vision-Threatening Diabetic Retinopathy: A Screening Approach in the Primary Care Setting. Diabetes Care 2001, 24, 2086–2090. [Google Scholar] [CrossRef]
- Olson, J.A.; Strachan, F.M.; Hipwell, J.H.; Goatman, K.A.; McHardy, K.C.; Forrester, J.V.; Sharp, P.F. A Comparative Evaluation of Digital Imaging, Retinal Photography and Optometrist Examination in Screening for Diabetic Retinopathy. Diabet. Med. 2003, 20, 528–534. [Google Scholar] [CrossRef]
- Scanlon, P.H.; Malhotra, R.; Thomas, G.; Foy, C.; Kirkpatrick, J.N.; Lewis-Barned, N.; Harney, B.; Aldington, S.J. The Effectiveness of Screening for Diabetic Retinopathy by Digital Imaging Photography and Technician Ophthalmoscopy. Diabet. Med. 2003, 20, 467–474. [Google Scholar] [CrossRef]
- Scanlon, P.H.; Malhotra, R.; Greenwood, R.H.; Aldington, S.J.; Foy, C.; Flatman, M.; Downes, S. Comparison of Two Reference Standards in Validating Two Field Mydriatic Digital Photography as a Method of Screening for Diabetic Retinopathy. Br. J. Ophthalmol. 2003, 87, 1258–1263. [Google Scholar] [CrossRef]
- Lawrence, M.G. The Accuracy of Digital-Video Retinal Imaging to Screen for Diabetic Retinopathy: An Analysis of Two Digital-Video Retinal Imaging Systems Using Standard Stereoscopic Seven-Field Photography and Dilated Clinical Examination as Reference Standards. Trans. Am. Ophthalmol. Soc. 2004, 102, 321–340. [Google Scholar] [PubMed]
- Murgatroyd, H.; Ellingford, A.; Cox, A.; Binnie, M.; Ellis, J.D.; MacEwen, C.J.; Leese, G.P. Effect of Mydriasis and Different Field Strategies on Digital Image Screening of Diabetic Eye Disease. Br. J. Ophthalmol. 2004, 88, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Aptel, F.; Denis, P.; Rouberol, F.; Thivolet, C. Screening of Diabetic Retinopathy: Effect of Field Number and Mydriasis on Sensitivity and Specificity of Digital Fundus Photography. Diabetes Metab. 2008, 34, 290–293. [Google Scholar] [CrossRef]
- Molina Fernández, E.; Valero Moll, M.S.; Pedregal González, M.; Calvo Lozano, J.; Sánchez Ramos, J.L.; Díaz Rodríguez, E.; Uceda Torres, R. Validation of the electronic mailing of retinographs of diabetic patients in order to detect retinopathy in primary care. Aten. Primaria 2008, 40, 119–123. [Google Scholar] [CrossRef]
- Neubauer, A.S.; Rothschuh, A.; Ulbig, M.W.; Blum, M. Digital Fundus Image Grading with the Non-Mydriatic Visucam(PRO NM) versus the FF450(plus) Camera in Diabetic Retinopathy. Acta Ophthalmol. 2008, 86, 177–182. [Google Scholar] [CrossRef]
- Baeza, M.; Orozco-Beltrán, D.; Gil-Guillen, V.F.; Pedrera, V.; Ribera, M.C.; Pertusa, S.; Merino, J. Screening for Sight Threatening Diabetic Retinopathy Using Non-Mydriatic Retinal Camera in a Primary Care Setting: To Dilate or Not to Dilate? Int. J. Clin. Pract. 2009, 63, 433–438. [Google Scholar] [CrossRef]
- Sengupta, S.; Sindal, M.D.; Baskaran, P.; Pan, U.; Venkatesh, R. Sensitivity and Specificity of Smartphone-Based Retinal Imaging for Diabetic Retinopathy: A Comparative Study. Ophthalmol Retina 2019, 3, 146–153. [Google Scholar] [CrossRef]
- Lois, N.; Cook, J.A.; Wang, A.; Aldington, S.; Mistry, H.; Maredza, M.; McAuley, D.; Aslam, T.; Bailey, C.; Chong, V.; et al. Evaluation of a New Model of Care for People with Complications of Diabetic Retinopathy: The EMERALD Study. Ophthalmology 2021, 128, 561–573. [Google Scholar] [CrossRef]
- Williams, R.; Nussey, S.; Humphry, R.; Thompson, G. Assessment of Non-Mydriatic Fundus Photography in Detection of Diabetic Retinopathy. Br. Med. J. 1986, 293, 1140–1142. [Google Scholar] [CrossRef]
- Peters, A.L.; Davidson, M.B.; Ziel, F.H. Cost-Effective Screening for Diabetic Retinopathy Using a Nonmydriatic Retinal Camera in a Prepaid Health-Care Setting. Diabetes Care 1993, 16, 1193–1195. [Google Scholar] [CrossRef]
- Siu, S.C.; Ko, T.C.; Wong, K.W.; Chan, W.N. Effectiveness of Non-Mydriatic Retinal Photography and Direct Ophthalmoscopy in Detecting Diabetic Retinopathy. Hong Kong Med. J. 1998, 4, 367–370. [Google Scholar] [PubMed]
- Taylor, D.J.; Fisher, J.; Jacob, J.; Tooke, J.E. The Use of Digital Cameras in a Mobile Retinal Screening Environment. Diabet. Med. 1999, 16, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.E.; Cavallerano, J.D.; Cavallerano, A.A.; Clermont, A.C.; Birkmire-Peters, D.; Aiello, L.P.; Aiello, L.M. Joslin Vision Network Research Team Stereo Nonmydriatic Digital-Video Color Retinal Imaging Compared with Early Treatment Diabetic Retinopathy Study Seven Standard Field 35-Mm Stereo Color Photos for Determining Level of Diabetic Retinopathy. Ophthalmology 2001, 108, 572–585. [Google Scholar] [CrossRef]
- Maberley, D.; Cruess, A.F.; Barile, G.; Slakter, J. Digital Photographic Screening for Diabetic Retinopathy in the James Bay Cree. Ophthalmic Epidemiol. 2002, 9, 169–178. [Google Scholar] [CrossRef]
- Lin, D.Y.; Blumenkranz, M.S.; Brothers, R.J.; Grosvenor, D.M. The Sensitivity and Specificity of Single-Field Nonmydriatic Monochromatic Digital Fundus Photography with Remote Image Interpretation for Diabetic Retinopathy Screening: A Comparison with Ophthalmoscopy and Standardized Mydriatic Color Photography. Am. J. Ophthalmol. 2002, 134, 204–213. [Google Scholar] [CrossRef]
- Perrier, M.; Boucher, M.C.; Angioi, K.; Gresset, J.A.; Olivier, S. Comparison of Two, Three and Four 45 Degrees Image Fields Obtained with the Topcon CRW6 Nonmydriatic Camera for Screening for Diabetic Retinopathy. Can. J. Ophthalmol. 2003, 38, 569–574. [Google Scholar] [CrossRef]
- Herbert, H.M.; Jordan, K.; Flanagan, D.W. Is Screening with Digital Imaging Using One Retinal View Adequate? Eye 2003, 17, 497–500. [Google Scholar] [CrossRef][Green Version]
- Phiri, R.; Keeffe, J.E.; Harper, C.A.; Taylor, H.R. Comparative Study of the Polaroid and Digital Non-Mydriatic Cameras in the Detection of Referrable Diabetic Retinopathy in Australia. Diabet. Med. 2006, 23, 867–872. [Google Scholar] [CrossRef]
- Lopez-Bastida, J.; Cabrera-Lopez, F.; Serrano-Aguilar, P. Sensitivity and Specificity of Digital Retinal Imaging for Screening Diabetic Retinopathy. Diabet. Med. 2007, 24, 403–407. [Google Scholar] [CrossRef]
- Vujosevic, S.; Benetti, E.; Massignan, F.; Pilotto, E.; Varano, M.; Cavarzeran, F.; Avogaro, A.; Midena, E. Screening for Diabetic Retinopathy: 1 and 3 Nonmydriatic 45-Degree Digital Fundus Photographs vs. 7 Standard Early Treatment Diabetic Retinopathy Study Fields. Am. J. Ophthalmol. 2009, 148, 111–118. [Google Scholar] [CrossRef]
- Gupta, V.; Bansal, R.; Gupta, A.; Bhansali, A. Sensitivity and Specificity of Nonmydriatic Digital Imaging in Screening Diabetic Retinopathy in Indian Eyes. Indian J. Ophthalmol. 2014, 62, 851. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E.; Rajalakshmi, R.; Prathiba, V.; Anjana, R.M.; Ranjani, H.; Narayan, K.M.V.; Olsen, T.W.; Mohan, V.; Ward, L.A.; Lynn, M.J.; et al. Comparison among Methods of Retinopathy Assessment (CAMRA) Study: Smartphone, Nonmydriatic, and Mydriatic Photography. Ophthalmology 2015, 122, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, R.; Arulmalar, S.; Usha, M.; Prathiba, V.; Kareemuddin, K.S.; Anjana, R.M.; Mohan, V. Validation of Smartphone Based Retinal Photography for Diabetic Retinopathy Screening. PLoS ONE 2015, 10, e0138285. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Morescalchi, F.; Costagliola, C.; Delcassi, L.; Semeraro, F. Comparison of Smartphone Ophthalmoscopy with Slit-Lamp Biomicroscopy for Grading Diabetic Retinopathy. Am. J. Ophthalmol. 2015, 159, 360–364.e1. [Google Scholar] [CrossRef] [PubMed]
- Toy, B.C.; Myung, D.J.; He, L.; Pan, C.K.; Chang, R.T.; Polkinhorne, A.; Merrell, D.; Foster, D.; Blumenkranz, M.S. Smartphone-based dilated fundus photography and near visual acuity testing as inexpensive screening tools to detect referral warranted diabetic eye disease. Retina 2016, 36, 1000–1008. [Google Scholar] [CrossRef]
- Kim, T.N.; Myers, F.; Reber, C.; Loury, P.J.; Loumou, P.; Webster, D.; Echanique, C.; Li, P.; Davila, J.R.; Maamari, R.N.; et al. A Smartphone-Based Tool for Rapid, Portable, and Automated Wide-Field Retinal Imaging. Transl. Vis. Sci. Technol. 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, R.; Subashini, R.; Anjana, R.M.; Mohan, V. Automated Diabetic Retinopathy Detection in Smartphone-Based Fundus Photography Using Artificial Intelligence. Eye 2018, 32, 1138–1144. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Noble, J.; Aiello, L.M.; Aiello, L.P. Nonmydriatic Ultrawide Field Retinal Imaging Compared with Dilated Standard 7-Field 35-Mm Photography and Retinal Specialist Examination for Evaluation of Diabetic Retinopathy. Am. J. Ophthalmol. 2012, 154, 549–559.e2. [Google Scholar] [CrossRef]
- Szeto, S.K.H.; Wong, R.; Lok, J.; Tang, F.; Sun, Z.; Tso, T.; Lam, T.C.H.; Tham, C.C.; Ng, D.S.; Cheung, C.Y. Non-Mydriatic Ultrawide Field Scanning Laser Ophthalmoscopy Compared with Dilated Fundal Examination for Assessment of Diabetic Retinopathy and Diabetic Macular Oedema in Chinese Individuals with Diabetes Mellitus. Br. J. Ophthalmol. 2019, 103, 1327–1331. [Google Scholar] [CrossRef]
- Manjunath, V.; Papastavrou, V.; Steel, D.H.W.; Menon, G.; Taylor, R.; Peto, T.; Talks, J. Wide-Field Imaging and OCT vs Clinical Evaluation of Patients Referred from Diabetic Retinopathy Screening. Eye 2015, 29, 416–423. [Google Scholar] [CrossRef]
- Horton, M.B.; Silva, P.S.; Cavallerano, J.D.; Aiello, L.P. Operational Components of Telemedicine Programs for Diabetic Retinopathy. Curr. Diab. Rep. 2016, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.B.; Silva, P.S.; Cavallerano, J.D.; Aiello, L.P. Clinical Components of Telemedicine Programs for Diabetic Retinopathy. Curr. Diab. Rep. 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Obuchowski, N.A.; Graham, R.J.; Baker, M.E.; Powell, K.A. Ten Criteria for Effective Screening: Their Application to Multislice CT Screening for Pulmonary and Colorectal Cancers. AJR Am. J. Roentgenol. 2001, 176, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Edwards, R.T. Diabetic Retinopathy Screening: A Systematic Review of the Economic Evidence. Diabet. Med. 2010, 27, 249–256. [Google Scholar] [CrossRef]
- Burns-Cox, C.J.; Hart, J.C. Screening of Diabetics for Retinopathy by Ophthalmic Opticians. Br. Med. J. 1985, 290, 1052–1054. [Google Scholar] [CrossRef][Green Version]
- Hulme, S.A.; Tin-U, A.; Hardy, K.J.; Joyce, P.W. Evaluation of a District-Wide Screening Programme for Diabetic Retinopathy Utilizing Trained Optometrists Using Slit-Lamp and Volk Lenses. Diabet. Med. 2002, 19, 741–745. [Google Scholar] [CrossRef]
- Kleinstein, R.N.; Roseman, J.M.; Herman, W.H.; Holcombe, J.; Louv, W.C. Detection of Diabetic Retinopathy by Optometrists. J. Am. Optom. Assoc. 1987, 58, 879–882. [Google Scholar]
- Schmid, K.L.; Swann, P.G.; Pedersen, C.; Schmid, L.M. The Detection of Diabetic Retinopathy by Australian Optometrists. Clin. Exp. Optom. 2002, 85, 221–228. [Google Scholar] [CrossRef]
- Reenders, K.; de Nobel, E.; van den Hoogen, H.; van Weel, C. Screening for Diabetic Retinopathy by General Practitioners. Scand. J. Prim. Health Care 1992, 10, 306–309. [Google Scholar] [CrossRef]
- Verma, L.; Prakash, G.; Tewari, H.K.; Gupta, S.K.; Murthy, G.V.S.; Sharma, N. Screening for Diabetic Retinopathy by Non-Ophthalmologists: An Effective Public Health Tool. Acta Ophthalmol. Scand. 2003, 81, 373–377. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Kessler, S.D.; Richie, K.A. Comparison between Ophthalmoscopy and Fundus Photography in Determining Severity of Diabetic Retinopathy. Ophthalmology 1985, 92, 62–67. [Google Scholar] [CrossRef]
- Forrest, R.D.; Jackson, C.A.; Yudkin, J.S. Screening for Diabetic Retinopathy—Comparison of a Nurse and a Doctor with Retinal Photography. Diabetes Res. 1987, 5, 39–42. [Google Scholar]
- Taylor, R.; Lovelock, L.; Tunbridge, W.M.; Alberti, K.G.; Brackenridge, R.G.; Stephenson, P.; Young, E. Comparison of Non-Mydriatic Retinal Photography with Ophthalmoscopy in 2159 Patients: Mobile Retinal Camera Study. BMJ 1990, 301, 1243–1247. [Google Scholar] [CrossRef]
- Sundling, V.; Gulbrandsen, P.; Straand, J. Sensitivity and Specificity of Norwegian Optometrists’ Evaluation of Diabetic Retinopathy in Single-Field Retinal Images—A Cross-Sectional Experimental Study. BMC Health Serv. Res. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Yogesan, K.; Kumar, S.; Goldschmidt, L.; Cuadros, J. Teleophthalmology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 9783540705154. [Google Scholar]
- Lee, V.S.; Kingsley, R.M.; Lee, E.T.; Lu, M.; Russell, D.; Asal, N.R.; Bradford, R.H., Jr.; Wilkinson, C.P. The Diagnosis of Diabetic Retinopathy. Ophthalmology 1993, 100, 1504–1512. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Neider, M.W.; Hubbard, L.D.; Meuer, S.M.; Brothers, R.J. Diabetic Retinopathy as Detected Using Ophthalmoscopy, a Nonmydriatic Camera and a Standard Fundus Camera. Ophthalmology 1985, 92, 485–491. [Google Scholar] [CrossRef]
- Emanuele, N.; Klein, R.; Moritz, T.; Davis, M.D.; Glander, K.; Anderson, R.; Reda, D.; Duckworth, W.; Abraira, C. VADT Study Group Comparison of Dilated Fundus Examinations with Seven-Field Stereo Fundus Photographs in the Veterans Affairs Diabetes Trial. J. Diabetes Complicat. 2009, 23, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kinyoun, J.L.; Martin, D.C.; Fujimoto, W.Y.; Leonetti, D.L. Ophthalmoscopy versus Fundus Photographs for Detecting and Grading Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1888–1893. [Google Scholar]
- Diabetic Retinopathy Study Report Number 6. Design, Methods, and Baseline Results. Report Number 7. A Modification of the Airlie House Classification of Diabetic Retinopathy. Prepared by the Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 1981, 21, 1–226.
- Allen, L. Ocular fundus photography: Suggestions for achieving consistently good pictures and instructions for stereoscopic photography. Am. J. Ophthalmol. 1964, 57, 13–28. [Google Scholar] [CrossRef]
- United States. Public Health Service Symposium on the Treatment of Diabetic Retinopathy; United States: Washington, DC, USA, 1969. [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Li, H.K.; Hubbard, L.D.; Danis, R.P.; Esquivel, A.; Florez-Arango, J.F.; Krupinski, E.A. Monoscopic versus Stereoscopic Retinal Photography for Grading Diabetic Retinopathy Severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3184–3192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, N.V.; Vigil, E.M.; Hassan, M.; Halim, M.S.; Baluyot, S.C.; Guzman, H.A.; Afridi, R.; Do, D.V.; Sepah, Y.J. Comparison of Montage with Conventional Stereoscopic Seven-Field Photographs for Assessment of ETDRS Diabetic Retinopathy Severity. Int. J. Retina Vitreous 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Kernt, M.; Hadi, I.; Pinter, F.; Seidensticker, F.; Hirneiss, C.; Haritoglou, C.; Kampik, A.; Ulbig, M.W.; Neubauer, A.S. Assessment of Diabetic Retinopathy Using Nonmydriatic Ultra-Widefield Scanning Laser Ophthalmoscopy (Optomap) Compared with ETDRS 7-Field Stereo Photography. Diabetes Care 2012, 35, 2459–2463. [Google Scholar] [CrossRef]
- Rasmussen, M.L.; Broe, R.; Frydkjaer-Olsen, U.; Olsen, B.S.; Mortensen, H.B.; Peto, T.; Grauslund, J. Comparison between Early Treatment Diabetic Retinopathy Study 7-Field Retinal Photos and Non-Mydriatic, Mydriatic and Mydriatic Steered Widefield Scanning Laser Ophthalmoscopy for Assessment of Diabetic Retinopathy. J. Diabetes Complicat. 2015, 29, 99–104. [Google Scholar] [CrossRef]
- Rudnisky, C.J.; Tennant, M.T.S.; de Leon, A.R.; Hinz, B.J.; Greve, M.D.J. Benefits of Stereopsis When Identifying Clinically Significant Macular Edema via Teleophthalmology. Can. J. Ophthalmol. 2006, 41, 727–732. [Google Scholar] [CrossRef][Green Version]
- Sapkota, R.; Chen, Z.; Zheng, D.; Pardhan, S. The Profile of Sight-Threatening Diabetic Retinopathy in Patients Attending a Specialist Eye Clinic in Hangzhou, China. BMJ Open Ophthalmol. 2019, 4, e000236. [Google Scholar] [CrossRef]
- Scanlon, P.H. Update on Screening for Sight-Threatening Diabetic Retinopathy. Ophthalmic Res. 2019, 62, 218–224. [Google Scholar] [CrossRef]
- Davies, E.G.; Petty, R.G.; Kohner, E.M. Long Term Effectiveness of Photocoagulation for Diabetic Maculopathy. Eye 1989, 3 Pt 6, 764–767. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ferris, F.L., 3rd; Csaky, K.G.; Murphy, R.P.; Agrón, E.; Thompson, D.J.S.; Reed, G.F.; Schachat, A.P. The Long-Term Effects of Laser Photocoagulation Treatment in Patients with Diabetic Retinopathy: The Early Treatment Diabetic Retinopathy Follow-up Study. Ophthalmology 2003, 110, 1683–1689. [Google Scholar] [CrossRef]
- Elman, M.J.; Qin, H.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Ferris, F.L., 3rd; Glassman, A.R.; Maturi, R.K.; Melia, M.; Diabetic Retinopathy Clinical Research Network. Intravitreal Ranibizumab for Diabetic Macular Edema with Prompt versus Deferred Laser Treatment: Three-Year Randomized Trial Results. Ophthalmology 2012, 119, 2312–2318. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Toby Prevost, A.; Vasconcelos, J.C.; Riddell, A.; Murphy, C.; Kelly, J.; Bainbridge, J.; Tudor-Edwards, R.; Hopkins, D.; Hykin, P.; et al. Clinical Efficacy of Intravitreal Aflibercept versus Panretinal Photocoagulation for Best Corrected Visual Acuity in Patients with Proliferative Diabetic Retinopathy at 52 Weeks (CLARITY): A Multicentre, Single-Blinded, Randomised, Controlled, Phase 2b, Non-Inferiority Trial. Lancet 2017, 389, 2193–2203. [Google Scholar] [PubMed]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., 3rd; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. Retina 1994, 14, 286–287. [Google Scholar] [CrossRef]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk Factors for Incidence and Progression of Retinopathy in Type II Diabetes over 6 Years from Diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Nathan, D.M. Preventing Diabetic Retinopathy Progression. Ophthalmology 2016, 123, 1840–1842. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.P.; Garg, S.K.; Jackson, W.E.; Thomas, M.A.; Harris, S.; Marshall, G.; Crews, M.J. Blood Pressure and Retinopathy in Type I Diabetes. Ophthalmology 1990, 97, 155–159. [Google Scholar] [CrossRef]
- Kohner, E.M.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Matthews, D.R.; Uk Prospective Diabetes Study ukpds Group. Relationship between the Severity of Retinopathy and Progression to Photocoagulation in Patients with Type 2 Diabetes Mellitus in the UKPDS (UKPDS 52). Diabet. Med. 2001, 18, 178–184. [Google Scholar] [PubMed]
- Benbassat, J.; Polak, B.C.P. Reliability of Screening Methods for Diabetic Retinopathy. Diabet. Med. 2009, 26, 783–790. [Google Scholar] [CrossRef]
- Natarajan, S.; Jain, A.; Krishnan, R.; Rogye, A.; Sivaprasad, S. Diagnostic Accuracy of Community-Based Diabetic Retinopathy Screening With an Offline Artificial Intelligence System on a Smartphone. JAMA Ophthalmol. 2019, 137, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P. Methods for Achieving Adequate Pupil Size in Cataract Surgery. Curr. Opin. Ophthalmol. 2020, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P.; Huerva, V.; Ascaso, F.J.; Tuuminen, R. Diabetes and Phacoemulsification Cataract Surgery: Difficulties, Risks and Potential Complications. J. Clin. Med. Res. 2019, 8, 716. [Google Scholar] [CrossRef]
- Hreidarsson, A.B. Pupil Motility in Long-Term Diabetes. Diabetologia 1979, 17, 145–150. [Google Scholar] [CrossRef]
- Smith, S.A.; Smith, S.E. Evidence for a Neuropathic Aetiology in the Small Pupil of Diabetes Mellitus. Br. J. Ophthalmol. 1983, 67, 89–93. [Google Scholar] [CrossRef][Green Version]
- Alio, J.; Hernandez, I.; Millan, A.; Sanchez, J. Pupil Responsiveness in Diabetes Mellitus. Ann. Ophthalmol. 1989, 21, 132–137. [Google Scholar]
- Huber, M.J.; Smith, S.A.; Smith, S.E. Mydriatic Drugs for Diabetic Patients. Br. J. Ophthalmol. 1985, 69, 425–427. [Google Scholar] [CrossRef]
- Williams, G.A.; Scott, I.U.; Haller, J.A.; Maguire, A.M.; Marcus, D.; McDonald, H.R. Single-Field Fundus Photography for Diabetic Retinopathy Screening: A Report by the American Academy of Ophthalmology. Ophthalmology 2004, 111, 1055–1062. [Google Scholar] [CrossRef]
- Panwar, N.; Huang, P.; Lee, J.; Keane, P.A.; Chuan, T.S.; Richhariya, A.; Teoh, S.; Lim, T.H.; Agrawal, R. Fundus Photography in the 21st Centur—A Review of Recent Technological Advances and Their Implications for Worldwide Healthcare. Telemed. J. E. Health 2016, 22, 198–208. [Google Scholar] [CrossRef]
- Molina-Casado, J.M.; Carmona, E.J.; García-Feijoó, J. Fast Detection of the Main Anatomical Structures in Digital Retinal Images Based on Intra- and Inter-Structure Relational Knowledge. Comput. Methods Programs Biomed. 2017, 149, 55–68. [Google Scholar] [CrossRef]
- Lau, H.C.; Voo, Y.O.; Yeo, K.T.; Ling, S.L.; Jap, A. Mass Screening for Diabetic Retinopathy—A Report on Diabetic Retinal Screening in Primary Care Clinics in Singapore. Singapore Med. J. 1995, 36, 510–513. [Google Scholar]
- Scanlon, P.H. The English National Screening Programme for Diabetic Retinopathy 2003–2016. Acta Diabetol. 2017, 54, 515–525. [Google Scholar] [CrossRef]
- Massin, P.; Aubert, J.-P.; Erginay, A.; Bourovitch, J.C.; BenMehidi, A.; Audran, G.; Bernit, B.; Jamet, M.; Collet, C.; Laloi-Michelin, M.; et al. Screening for Diabetic Retinopathy: The First Telemedical Approach in a Primary Care Setting in France. Diabetes Metab. 2004, 30, 451–457. [Google Scholar] [CrossRef]
- Quellec, G.; Bazin, L.; Cazuguel, G.; Delafoy, I.; Cochener, B.; Lamard, M. Suitability of a Low-Cost, Handheld, Nonmydriatic Retinograph for Diabetic Retinopathy Diagnosis. Transl. Vis. Sci. Technol. 2016, 5, 16. [Google Scholar] [CrossRef]
- Tran, K.; Mendel, T.A.; Holbrook, K.L.; Yates, P.A. Construction of an Inexpensive, Hand-Held Fundus Camera through Modification of a Consumer “Point-and-Shoot” Camera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7600–7607. [Google Scholar] [CrossRef]
- Teichman, J.C.; Sher, J.H.; Ahmed, I.I.K. From iPhone to eyePhone: A Technique for Photodocumentation. Can. J. Ophthalmol. 2011, 46, 284–286. [Google Scholar] [CrossRef]
- Lord, R.K.; Shah, V.A.; San Filippo, A.N.; Krishna, R. Novel Uses of Smartphones in Ophthalmology. Ophthalmology 2010, 117. [Google Scholar] [CrossRef]
- Haddock, L.J.; Kim, D.Y.; Mukai, S. Simple, Inexpensive Technique for High-Quality Smartphone Fundus Photography in Human and Animal Eyes. J. Ophthalmol. 2013, 2013, 518479. [Google Scholar] [CrossRef]
- Bastawrous, A. Smartphone Fundoscopy. Ophthalmology 2012, 119, 432–433.e2. [Google Scholar] [CrossRef]
- Toslak, D.; Ayata, A.; Liu, C.; Erol, M.K.; Yao, X. Wide-field smartphone fundus video camera based on miniaturized indirect ophthalmoscopy. Retina 2018, 38, 438–441. [Google Scholar] [CrossRef]
- Vilela, M.A.; Valença, F.M.; Barreto, P.K.; Amaral, C.E.; Pellanda, L.C. Agreement between Retinal Images Obtained via Smartphones and Images Obtained with Retinal Cameras or Fundoscopic Exams—Systematic Review and Meta-Analysis. Clin. Ophthalmol. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Myung, D.; Jais, A.; He, L.; Blumenkranz, M.S.; Chang, R.T. 3D Printed Smartphone Indirect Lens Adapter for Rapid, High Quality Retinal Imaging. J. Mob. Technol. Med. 2014, 3, 9–15. [Google Scholar] [CrossRef]
- Ludwig, C.A.; Murthy, S.I.; Pappuru, R.R.; Jais, A.; Myung, D.J.; Chang, R.T. A Novel Smartphone Ophthalmic Imaging Adapter: User Feasibility Studies in Hyderabad, India. Indian J. Ophthalmol. 2016, 64, 191–200. [Google Scholar]
- Sharma, A.; Subramaniam, S.D.; Ramachandran, K.I.; Lakshmikanthan, C.; Krishna, S.; Sundaramoorthy, S.K. Smartphone-Based Fundus Camera Device (MII Ret Cam) and Technique with Ability to Image Peripheral Retina. Eur. J. Ophthalmol. 2016, 26, 142–144. [Google Scholar] [CrossRef]
- Maamari, R.N.; Keenan, J.D.; Fletcher, D.A.; Margolis, T.P. A Mobile Phone-Based Retinal Camera for Portable Wide Field Imaging. Br. J. Ophthalmol. 2014, 98, 438–441. [Google Scholar] [CrossRef]
- Nazari Khanamiri, H.; Nakatsuka, A.; El-Annan, J. Smartphone Fundus Photography. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Adam, M.K.; Brady, C.J.; Flowers, A.M.; Juhn, A.T.; Hsu, J.; Garg, S.J.; Murchison, A.P.; Spirn, M.J. Quality and Diagnostic Utility of Mydriatic Smartphone Photography: The Smartphone Ophthalmoscopy Reliability Trial. Ophthalmic Surg. Lasers Imaging Retina 2015, 46, 631–637. [Google Scholar] [CrossRef]
- Kim, Y.; Chao, D.L. Comparison of Smartphone Ophthalmoscopy vs Conventional Direct Ophthalmoscopy as a Teaching Tool for Medical Students: The COSMOS Study. Clin. Ophthalmol. 2019, 13, 391–401. [Google Scholar] [CrossRef]
- Friberg, T.R.; Pandya, A.; Eller, A.W. Non-Mydriatic Panoramic Fundus Imaging Using a Non-Contact Scanning Laser-Based System. Ophthalmic Surg. Lasers Imaging 2003, 34, 488–497. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Tsui, I.; Sadda, S.R. Ultra-Wide-Field Imaging in Diabetic Retinopathy. Vis. Res. 2017, 139, 187–190. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Manivannan, A.; Gupta, A.K.; Hipwell, J.; Forrester, J.V.; Sharp, P.F. Fundus Imaging in Patients with Cataract: Role for a Variable Wavelength Scanning Laser Ophthalmoscope. Br. J. Ophthalmol. 1995, 79, 892–899. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Soliman, A.Z.; Aiello, L.M.; Aiello, L.P. Peripheral Lesions Identified by Mydriatic Ultrawide Field Imaging: Distribution and Potential Impact on Diabetic Retinopathy Severity. Ophthalmology 2013, 120, 2587–2595. [Google Scholar] [CrossRef]
- Price, L.D.; Au, S.; Chong, N.V. Optomap Ultrawide Field Imaging Identifies Additional Retinal Abnormalities in Patients with Diabetic Retinopathy. Clin. Ophthalmol. 2015, 9, 527–531. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Haddad, N.M.N.; Tolls, D.; Thakore, K.; Patel, B.; Sehizadeh, M.; Tolson, A.M.; Sun, J.K.; Aiello, L.P. Comparison of Nondiabetic Retinal Findings Identified With Nonmydriatic Fundus Photography vs Ultrawide Field Imaging in an Ocular Telehealth Program. JAMA Ophthalmol. 2016, 134, 330–334. [Google Scholar] [CrossRef]
- Wessel, M.M.; Aaker, G.D.; Parlitsis, G.; Cho, M.; D’Amico, D.J.; Kiss, S. Ultra-Wide-Field Angiography Improves the Detection and Classification of Diabetic Retinopathy. Retina 2012, 32, 785–791. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Prathiba, V.; Arulmalar, S.; Usha, M. Review of Retinal Cameras for Global Coverage of Diabetic Retinopathy Screening. Eye 2021, 35, 162–172. [Google Scholar] [CrossRef]
- Lim, W.S.; Grimaldi, G.; Nicholson, L.; Basheer, K.; Rajendram, R. Widefield Imaging with Clarus Fundus Camera vs Slit Lamp Fundus Examination in Assessing Patients Referred from the National Health Service Diabetic Retinopathy Screening Programme. Eye 2021, 35, 299–306. [Google Scholar] [CrossRef]
- Hirano, T.; Imai, A.; Kasamatsu, H.; Kakihara, S.; Toriyama, Y.; Murata, T. Assessment of Diabetic Retinopathy Using Two Ultra-Wide-Field Fundus Imaging Systems, the Clarus® and OptosTM Systems. BMC Ophthalmol. 2018, 18, 332. [Google Scholar] [CrossRef]
- Chen, A.; Dang, S.; Chung, M.M.; Ramchandran, R.S.; Bessette, A.P.; DiLoreto, D.A.; Kleinman, D.M.; Sridhar, J.; Wykoff, C.C.; Kuriyan, A.E. Quantitative Comparison of Fundus Images by 2 Ultra-Widefield Fundus Cameras. Ophthalmol. Retina 2021, 5, 450–457. [Google Scholar] [CrossRef]
- Aiello, L.P.; Odia, I.; Glassman, A.R.; Melia, M.; Jampol, L.M.; Bressler, N.M.; Kiss, S.; Silva, P.S.; Wykoff, C.C.; Sun, J.K.; et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging With Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 65–73. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Wang, K.; Khadamy, J.; Sadda, S.R. Ultra-Wide-Field Imaging in Diabetic Retinopathy; an Overview. J. Curr. Ophthalmol. 2016, 28, 57–60. [Google Scholar] [CrossRef]
- Kanclerz, P.; Hecht, I.; Tuuminen, R. Re: Lois et Al.: Evaluation of a New Model of Care for People with Complications of Diabetic Retinopathy: The EMERALD Study (Ophthalmology. 2021;128:561-573). Ophthalmology 2021, 128, e45–e46. [Google Scholar] [CrossRef]
- Strøm, C.; Sander, B.; Larsen, N.; Larsen, M.; Lund-Andersen, H. Diabetic Macular Edema Assessed with Optical Coherence Tomography and Stereo Fundus Photography. Investig. Ophthalmol. Vis. Sci. 2002, 43, 241–245. [Google Scholar]
- Davis, M.D.; Bressler, S.B.; Aiello, L.P.; Bressler, N.M.; Browning, D.J.; Flaxel, C.J.; Fong, D.S.; Foster, W.J.; Glassman, A.R.; Hartnett, M.E.R.; et al. Comparison of Time-Domain OCT and Fundus Photographic Assessments of Retinal Thickening in Eyes with Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1745–1752. [Google Scholar] [CrossRef]
- Salas, M.; Drexler, W.; Levecq, X.; Lamory, B.; Ritter, M.; Prager, S.; Hafner, J.; Schmidt-Erfurth, U.; Pircher, M. Multi-Modal Adaptive Optics System Including Fundus Photography and Optical Coherence Tomography for the Clinical Setting. Biomed. Opt. Express 2016, 7, 1783–1796. [Google Scholar] [CrossRef]
- Kocaoglu, O.P.; Uhlhorn, S.R.; Hernandez, E.; Juarez, R.A.; Will, R.; Parel, J.-M.; Manns, F. Simultaneous Fundus Imaging and Optical Coherence Tomography of the Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Pakzad-Vaezi, K. Multimodal Imaging of Diabetic Retinopathy. Curr. Opin. Ophthalmol. 2018, 29, 566–575. [Google Scholar] [CrossRef]
- Querques, G.; Lattanzio, R.; Querques, L.; Del Turco, C.; Forte, R.; Pierro, L.; Souied, E.H.; Bandello, F. Enhanced Depth Imaging Optical Coherence Tomography in Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6017–6024. [Google Scholar] [CrossRef]
- Regatieri, C.V.; Branchini, L.; Carmody, J.; Fujimoto, J.G.; Duker, J.S. Choroidal Thickness in Patients with Diabetic Retinopathy Analyzed by Spectral-Domain Optical Coherence Tomography. Retina 2012, 32, 563–568. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.H.; Joe, S.G.; Kim, J.-G.; Yoon, Y.H. Changes in Choroidal Thickness in Relation to the Severity of Retinopathy and Macular Edema in Type 2 Diabetic Patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3378–3384. [Google Scholar] [CrossRef]
- Nagaoka, T.; Kitaya, N.; Sugawara, R.; Yokota, H.; Mori, F.; Hikichi, T.; Fujio, N.; Yoshida, A. Alteration of Choroidal Circulation in the Foveal Region in Patients with Type 2 Diabetes. Br. J. Ophthalmol. 2004, 88, 1060–1063. [Google Scholar] [CrossRef]
- Nesper, P.L.; Scarinci, F.; Fawzi, A.A. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS ONE 2017, 12, e0169926. [Google Scholar] [CrossRef]
- Tiedeman, J.S.; Kirk, S.E.; Srinivas, S.; Beach, J.M. Retinal Oxygen Consumption during Hyperglycemia in Patients with Diabetes without Retinopathy. Ophthalmology 1998, 105, 31–36. [Google Scholar] [CrossRef]
- Cole, E.D.; Novais, E.A.; Louzada, R.N.; Waheed, N.K. Contemporary Retinal Imaging Techniques in Diabetic Retinopathy: A Review. Clin. Experiment. Ophthalmol. 2016, 44, 289–299. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).