A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases
Abstract
:1. Introduction
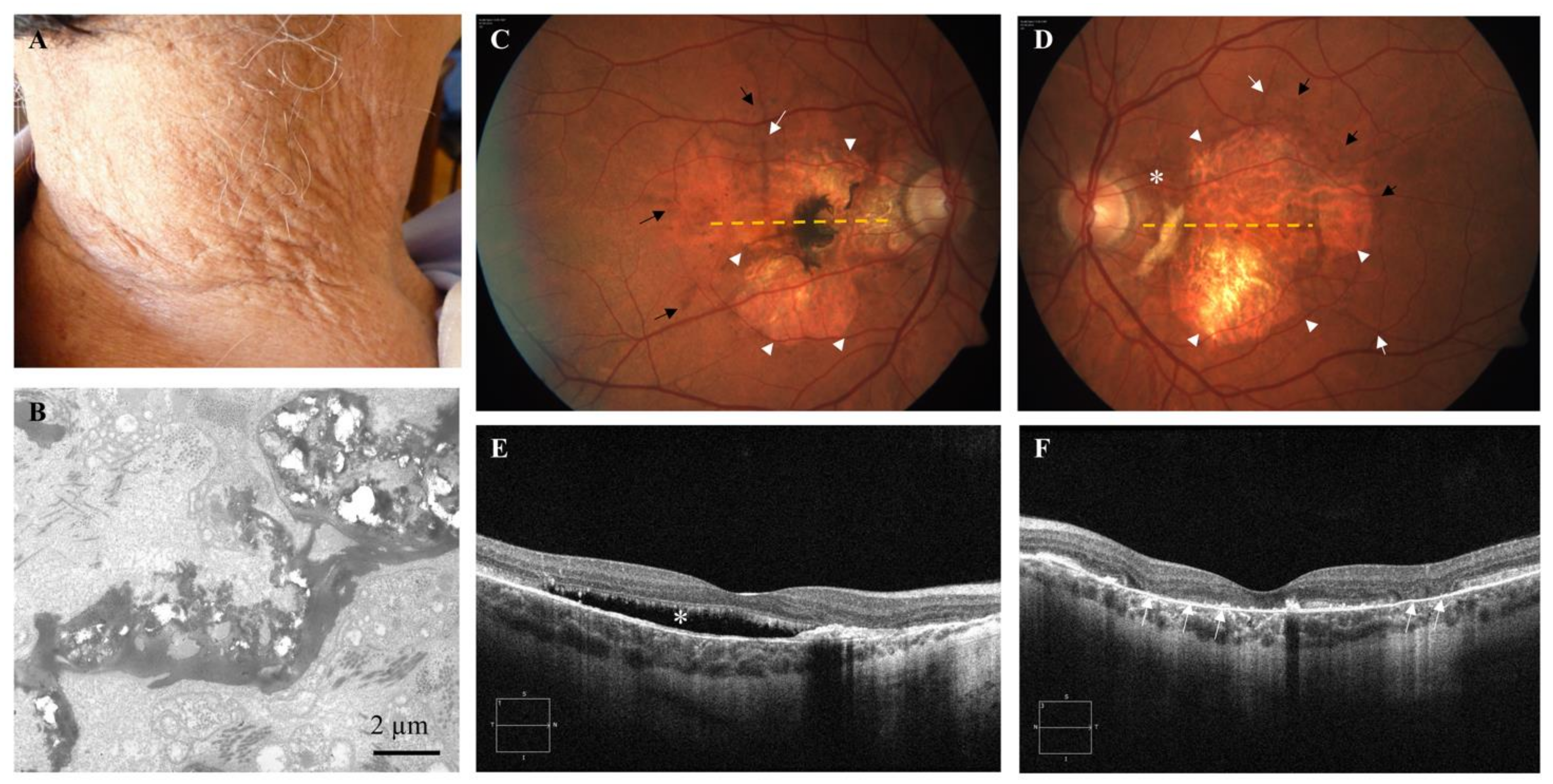
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boraldi, F.; Murro, V.; Lofaro, F.D.; Mucciolo, D.P.; Costa, S.; Pavese, L.; Quaglino, D. Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score. J. Clin. Med. 2021, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Marconi, B.; Bobyr, I.; Campanati, A.; Molinelli, E.; Consales, V.; Brisigotti, V.; Scarpelli, M.; Racchini, S.; Offidani, A. Pseudoxanthoma Elasticum and Skin: Clinical Manifestations, Histopathology, Pathomechanism, Perspectives of Treatment. Intractable Rare Dis. Res. 2015, 4, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Neidner, K.H. Cutaneous Manifestations. Clin. Dermatol. 1988, 6, 14–28. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Pavese, L.; Boraldi, F.; Quaglino, D.; Finocchio, L.; Sodi, A.; Virgili, G.; Giansanti, F. Adaptive Optics Imaging in Patients Affected by Pseudoxanthoma Elasticum. Am. J. Ophthalmol. 2020, 224, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Lefthériotis, G.; Omarjee, L.; Le Saux, O.; Henrion, D.; Abraham, P.; Prunier, F.; Willoteaux, S.; Martin, L. The Vascular Phenotype in Pseudoxanthoma Elasticum and Related Disorders: Contribution of a Genetic Disease to the Understanding of Vascular Calcification. Front. Genet. 2013, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Georgalas, I.; Tservakis, I.; Papaconstaninou, D.; Kardara, M.; Koutsandrea, C.; Ladas, I. Pseudoxanthoma Elasticum, Ocular Manifestations, Complications and Treatment. Clin. Exp. Optom. 2011, 94, 169–180. [Google Scholar] [CrossRef]
- Quaglino, D.; Boraldi, F.; Lofaro, F.D. The Biology of Vascular Calcification. Int. Rev. Cell Mol. Biol. 2020, 354, 261–353. [Google Scholar] [CrossRef]
- Le Saux, O.; Beck, K.; Sachsinger, C.; Silvestri, C.; Treiber, C.; Göring, H.H.; Johnson, E.W.; De Paepe, A.; Pope, F.M.; Pasquali-Ronchetti, I.; et al. A Spectrum of ABCC6 Mutations Is Responsible for Pseudoxanthoma Elasticum. Am. J. Hum. Genet. 2001, 69, 749–764. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Grange, D.K.; Armstrong, N.L.; Whelan, A.J.; Hurley, M.Y.; Rishavy, M.A.; Hallgren, K.W.; Berkner, K.L.; Schurgers, L.J.; Jiang, Q.; et al. Mutations in the GGCX and ABCC6 Genes in a Family with Pseudoxanthoma Elasticum-like Phenotypes. J. Investig. Dermatol. 2009, 129, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.-F.; et al. Generalized Arterial Calcification of Infancy and Pseudoxanthoma Elasticum Can Be Caused by Mutations in Either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Boraldi, F.; Lofaro, F.D.; Costa, S.; Moscarelli, P.; Quaglino, D. Rare Co-Occurrence of Beta-Thalassemia and Pseudoxanthoma Elasticum: Novel Biomolecular Findings. Front. Med. 2020, 6, 322. [Google Scholar] [CrossRef]
- Luo, H.; Faghankhani, M.; Cao, Y.; Uitto, J.; Li, Q. Molecular Genetics and Modifier Genes in Pseudoxanthoma Elasticum, a Heritable Multisystem Ectopic Mineralization Disorder. J. Investig. Dermatol. 2020, 141, 1148–1156. [Google Scholar] [CrossRef]
- Mahroo, O.A.; Fujinami, K.; Moore, A.T.; Webster, A.R. Retinal Findings in a Patient with Mutations in ABCC6 and ABCA4. Eye 2018, 32, 1542–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Statler, B.; Ramos, M.; DeBenedictis, M.J.; Babiuch, A.; Yuan, A.; Traboulsi, E.I. Hickam’s Dictum: Pseudoxanthoma Elasticum and Usher Syndrome in a Single Patient. Ophthalmic Genet. 2020, 41, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Romano, O.; Grilli, A.; Losi, L.; Moscarelli, P.; Bicciato, S.; Quaglino, D. Exome Sequencing and Bioinformatic Approaches Reveals Rare Sequence Variants Involved in Cell Signalling and Elastic Fibre Homeostasis: New Evidence in the Development of Ectopic Calcification. Cell Signal. 2019, 59, 131–140. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.L.; Grassmann, F.; Kellner, U.; Spital, G.; Rüther, K.; Jägle, H.; Hufendiek, K.; Rating, P.; Huchzermeyer, C.; Baier, M.J.; et al. Mutation Spectrum of the ABCA4 Gene in 335 Stargardt Disease Patients From a Multicenter German Cohort-Impact of Selected Deep Intronic Variants and Common SNPs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Kersten, E.; Geerlings, M.J.; Pauper, M.; Corominas, J.; Bakker, B.; Altay, L.; Fauser, S.; de Jong, E.K.; Hoyng, C.B.; den Hollander, A.I. Genetic Screening for Macular Dystrophies in Patients Clinically Diagnosed with Dry Age-related Macular Degeneration. Clin. Genet. 2018, 94, 569–574. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Losi, L.; Quaglino, D. Dermal Alterations in Clinically Unaffected Skin of Pseudoxanthoma Elasticum Patients. J. Clin. Med. 2021, 10, 500. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Sodi, A.; Boraldi, F.; Quaglino, D.; Virgili, G.; Giansanti, F. Pattern Dystrophy-like Changes and Coquille d’oeuf Atrophy in Elderly Patients Affected by Pseudoxanthoma Elasticum. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1881–1892. [Google Scholar] [CrossRef]
- Manes, G.; Meunier, I.; Avila-Fernández, A.; Banfi, S.; Le Meur, G.; Zanlonghi, X.; Corton, M.; Simonelli, F.; Brabet, P.; Labesse, G.; et al. Mutations in IMPG1 Cause Vitelliform Macular Dystrophies. Am. J. Hum. Genet. 2013, 93, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diakatou, M.; Manes, G.; Bocquet, B.; Meunier, I.; Kalatzis, V. Genome Editing as a Treatment for the Most Prevalent Causative Genes of Autosomal Dominant Retinitis Pigmentosa. Int. J. Mol. Sci. 2019, 20, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsybovsky, Y.; Molday, R.S.; Palczewski, K. The ATP-Binding Cassette Transporter ABCA4: Structural and Functional Properties and Role in Retinal Disease. Adv. Exp. Med. Biol. 2010, 703, 105–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliem, M.; Müller, P.L.; Birtel, J.; McGuinness, M.B.; Finger, R.P.; Herrmann, P.; Hendig, D.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence in Pseudoxanthoma Elasticum. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6159–6165. [Google Scholar] [CrossRef] [PubMed]
- Cremers, F.P.; van de Pol, D.J.; van Driel, M.; den Hollander, A.I.; van Haren, F.J.; Knoers, N.V.; Tijmes, N.; Bergen, A.A.; Rohrschneider, K.; Blankenagel, A.; et al. Autosomal Recessive Retinitis Pigmentosa and Cone-Rod Dystrophy Caused by Splice Site Mutations in the Stargardt’s Disease Gene ABCR. Hum. Mol. Genet. 1998, 7, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Mir, A.; Paloma, E.; Allikmets, R.; Ayuso, C.; del Rio, T.; Dean, M.; Vilageliu, L.; Gonzàlez-Duarte, R.; Balcells, S. Retinitis Pigmentosa Caused by a Homozygous Mutation in the Stargardt Disease Gene ABCR. Nat. Genet. 1998, 18, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Allikmets, R.; Shroyer, N.F.; Singh, N.; Seddon, J.M.; Lewis, R.A.; Bernstein, P.S.; Peiffer, A.; Zabriskie, N.A.; Li, Y.; Hutchinson, A.; et al. Mutation of the Stargardt Disease Gene (ABCR) in Age-Related Macular Degeneration. Science 1997, 277, 1805–1807. [Google Scholar] [CrossRef] [Green Version]
- Allikmets, R.; Singh, N.; Sun, H.; Shroyer, N.F.; Hutchinson, A.; Chidambaram, A.; Gerrard, B.; Baird, L.; Stauffer, D.; Peiffer, A.; et al. A Photoreceptor Cell-Specific ATP-Binding Transporter Gene (ABCR) Is Mutated in Recessive Stargardt Macular Dystrophy. Nat. Genet. 1997, 15, 236–246. [Google Scholar] [CrossRef]
- Zernant, J.; Schubert, C.; Im, K.M.; Burke, T.; Brown, C.M.; Fishman, G.A.; Tsang, S.H.; Gouras, P.; Dean, M.; Allikmets, R. Analysis of the ABCA4 Gene by Next-Generation Sequencing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8479–8487. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, L.G.; Fleckenstein, M.; Fiebig, B.S.; Schmitz-Valckenberg, S.; Bindewald-Wittich, A.; Keilhauer, C.N.; Renner, A.B.; Mackensen, F.; Mößner, A.; Pauleikhoff, D.; et al. A Subgroup of Age-Related Macular Degeneration Is Associated with Mono-Allelic Sequence Variants in the ABCA4 Gene. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2112–2118. [Google Scholar] [CrossRef] [Green Version]
- Westeneng-van Haaften, S.C.; Boon, C.J.F.; Cremers, F.P.M.; Hoefsloot, L.H.; den Hollander, A.I.; Hoyng, C.B. Clinical and Genetic Characteristics of Late-Onset Stargardt’s Disease. Ophthalmology 2012, 119, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
| Chr | Gene Symbol | Exonic Alteration | Exon | Gene Variant | Amino Acid Variant | ExAC | GnomAD | 1000G | dbSNP | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABCA4 | ns | 48 | c.6647C>T | p.Ala2216Val | / | / | / | / | [17] |
| 16 | ABCC6 | ns | 26 | c.3707T>C | p.Met1236Thr | / | / | / | / | This study |
| 6 | IMPG1 | ns | 14 | c.1945C>T | p.Leu649Phe | 0.004802 | 0.00695 | 0.0014 | rs118155926 | [18] |
| 12 | POC1B | ns | 3 | c.266T>C | p.Met89Thr | 0.000091 | 0.000032 | / | rs780961965 | This study |
| 19 | RAX2 | ns | 2 | c.87G>T | p.Arg29Ser | 0.000009 | / | / | rs778633054 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lofaro, F.D.; Mucciolo, D.P.; Murro, V.; Pavese, L.; Quaglino, D.; Boraldi, F. A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases. Diagnostics 2021, 11, 1800. https://doi.org/10.3390/diagnostics11101800
Lofaro FD, Mucciolo DP, Murro V, Pavese L, Quaglino D, Boraldi F. A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases. Diagnostics. 2021; 11(10):1800. https://doi.org/10.3390/diagnostics11101800
Chicago/Turabian StyleLofaro, Francesco Demetrio, Dario Pasquale Mucciolo, Vittoria Murro, Laura Pavese, Daniela Quaglino, and Federica Boraldi. 2021. "A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases" Diagnostics 11, no. 10: 1800. https://doi.org/10.3390/diagnostics11101800
APA StyleLofaro, F. D., Mucciolo, D. P., Murro, V., Pavese, L., Quaglino, D., & Boraldi, F. (2021). A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases. Diagnostics, 11(10), 1800. https://doi.org/10.3390/diagnostics11101800






