Multiple Chromatographic Analysis of Urine in the Detection of Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Principle of HPLC
2.3. Chemicals
2.4. Equipment and Conditions
2.5. Data Processing and Statistical Analysis
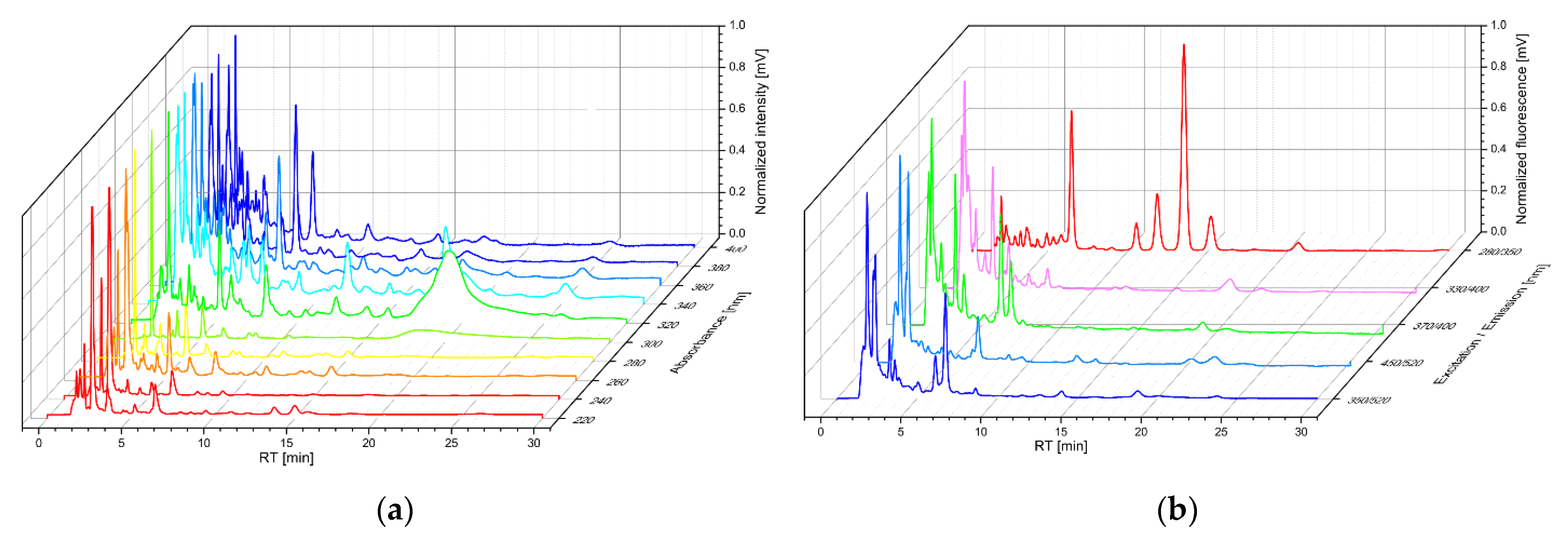
3. Results
3.1. ROC (Receiver Operating Characteristic) Analysis
3.2. Logistic Regression Analysis
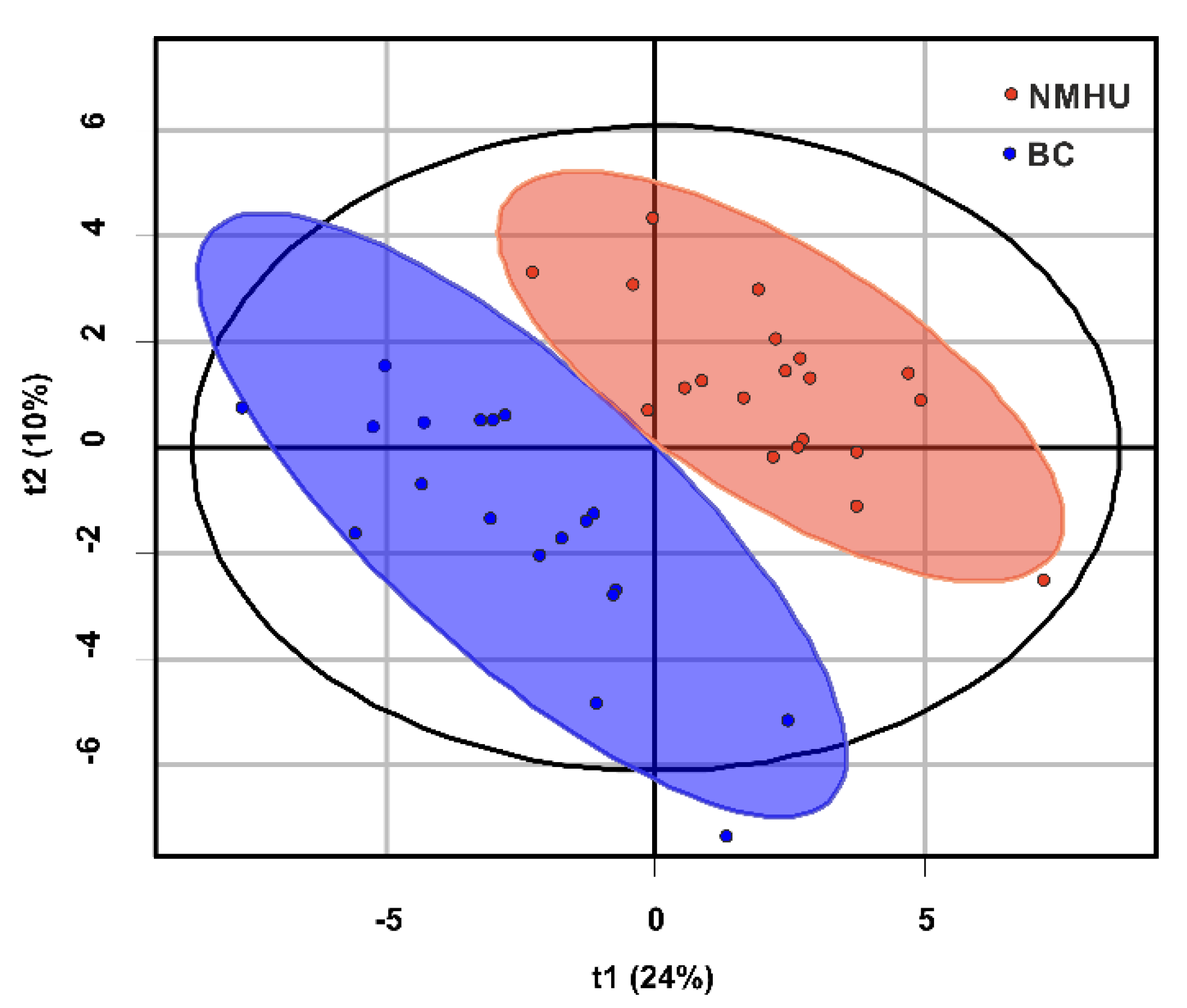
3.3. Discriminant Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Available online: http://gco.iarc.fr/ (accessed on 24 March 2019).
- Schulster, M. Bladder Cancer Academy 2019 Selected Summaries. Rev. Urol. 2019, 21, 23–28. [Google Scholar] [PubMed]
- Alberice, J.V.; Amaral, A.F.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Márquez, M.; García, A.; Malats, N.; Barbas, C. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J. Chromatogr. A 2013, 1318, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Blick, C.G.; Nazir, S.A.; Mallett, S.; Turney, B.W.; Onwu, N.N.; Roberts, I.S.; Crew, J.P.; Cowan, N.C. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: Results for 778 patients from a hospital haematuria clinic. BJU Int. 2012, 110, 84–94. [Google Scholar] [CrossRef]
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leão, R.; Máximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Caires, H.R.; Oliveira, M.J.; Fraga, A.; Vasconcelos, M.H.; Ribeiro, R. Urinary Biomarkers in Bladder Cancer: Where Do We Stand and Potential Role of Extracellular Vesicles. Cancers 2020, 12, 1400. [Google Scholar] [CrossRef]
- Loras, A.; Trassierra, M.; Sanjuan-Herráez, D.; Martínez-Bisbal, M.C.; Castell, J.V.; Quintás, G.; Ruiz-Cerdá, J.L. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci. Rep. 2018, 8, 9172. [Google Scholar] [CrossRef]
- Shao, C.H.; Chen, C.L.; Lin, J.Y.; Chen, C.J.; Fu, S.H.; Chen, Y.T.; Chang, Y.S.; Yu, J.S.; Tsui, K.H.; Juo, C.G.; et al. Metabolite marker discovery for the detection of bladder cancer by comparative metabolomics. Oncotarget 2017, 8, 38802–38810. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Liu, X.; Sun, H.; Guo, Z.; Zheng, G.; Zhang, Y.; Sun, W. UPLC-MS based urine untargeted metabolomic analyses to differentiate bladder cancer from renal cell carcinoma. BMC Cancer 2019, 19, 1195. [Google Scholar] [CrossRef]
- Amara, C.S.; Vantaku, V.; Lotan, Y.; Putluri, N. Recent advances in the metabolomic study of bladder cancer. Expert Rev. Proteom. 2019, 16, 315–324. [Google Scholar] [CrossRef]
- Pashos, C.L.; Botteman, M.F.; Laskin, B.L.; Redaelli, A. Bladder cancer: Epidemiology, diagnosis, and management. Cancer Pract. 2002, 10, 311–322. [Google Scholar] [CrossRef]
- Kupiec, T. Quality-control analytical methods: High-performance liquid chromatography. Int. J. Pharm. Compd. 2004, 8, 223–227. [Google Scholar]
- Zvarik, M.; Martinicky, D.; Hunakova, L.; Lajdova, I.; Sikurova, L. Fluorescence characteristics of human urine from normal individuals and ovarian cancer patients. Neoplasma 2013, 60, 533–537. [Google Scholar] [CrossRef]
- Kollarik, B.; Zvarik, M.; Bujdak, P.; Weibl, P.; Rybar, L.; Sikurova, L.; Hunakova, L. Urinary fluorescence analysis in diagnosis of bladder cancer. Neoplasma 2017, 65, 234–241. [Google Scholar] [CrossRef]
- Maas, M.; Bedke, J.; Stenzl, A.; Todenhöfer, T. Can urinary biomarkers replace cystoscopy? World J. Urol. 2019, 37, 1741–1749. [Google Scholar] [CrossRef]
- Oge, O.; Kozaci, D.; Gemalmaz, H. The BTA stat test is nonspecific for hematuria: An experimental hematuria model. J. Urol. 2002, 167, 1318–1320. [Google Scholar] [CrossRef]
- Gupta, N.P.; Sharma, N.; Kumar, R. Nuclear matrix protein 22 as adjunct to urine cytology and cystoscopy in follow-up of superficial TCC of urinary bladder. Urology 2009, 73, 592–597. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Rizwani, W.; Ross, S.; Bart Grossman, H.; Rosser, C.J. Urinary BTA: Indicator of bladder cancer or of hematuria. World J. Urol. 2012, 30, 869–873. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, S.I.; Kim, D.J.; Jeong, T.Y. Usefulness of the NMP22BladderChek Test for Screening and Follow-up of Bladder Cancer. Korean J. Urol. 2010, 51, 88–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlake, A.; Crispen, P.L.; Cap, A.P.; Atkinson, T.; Davenport, D.; Preston, D.M. NMP-22, urinary cytology, and cystoscopy: A 1 year comparison study. Can J. Urol. 2012, 19, 6345–6350. [Google Scholar] [PubMed]
- Darwiche, F.; Parekh, D.J.; Gonzalgo, M.L. Biomarkers for non-muscle invasive bladder cancer: Current tests and future promise. Indian J. Urol. 2015, 31, 273–282. [Google Scholar] [PubMed]
- Jin, X.; Yun, S.J.; Jeong, P.; Kim, I.Y.; Kim, W.J.; Park, S. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget 2014, 5, 1635–1645. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Non-Malignant Hematuria (NMHU) | Bladder Cancer (BC) |
|---|---|---|
| Age (mean ± SD) | 65.2 ± 8.2 | 65.4 ± 5.4 |
| No. of subject | 24 | 23 |
| Training set | 20 | 19 |
| Test set | 4 | 4 |
| Cancer Grade | ||
| G1 | - | 11 |
| G2 | - | 3 |
| G3 | - | 9 |
| Cancer Stage | ||
| Ta | - | 10 |
| T1 | - | 9 |
| T2 | - | 4 |
| Wavelength (nm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absorption | 220 | 240 | 260 | 280 | 300 | 320 | 340 | 360 | 380 | 400 |
| Excitation/ Emission | 280/350 | 330/400 | 370/440 | 450/520 | 370/520 | |||||
| Excitation/Emission (Wavelength (nm)) | p-Value | RT (min) (SD) | Area/Area of Creatinine × 10−3 Median (IQR) | |
|---|---|---|---|---|
| Non-Malignant Hematuria (NMHU) | Bladder Cancer (BC) | |||
| 370/440 | 0.0151 | 1.83 | 8.13 | 12.96 |
| (0.012) | (5.03–14.35) | (11.47–18.83) | ||
| 370/440 | 0.0021 | 2.37 | 8.43 | 11.98 |
| (0.036) | (6.17–11.06) | (10.25–18.13) | ||
| 450/520 | 0.0026 | 1.87 | 0.93 | 1.98 |
| (0.013) | (0.75–1.92) | (1.36–3.50) | ||
| 450/520 | 0.0015 | 2.28 | 0.78 | 1.46 |
| (0.008) | (0.55–1.21) | (1.07–2.23) | ||
| 370/520 | 0.0057 | 1.87 | 7.84 | 14.63 |
| (0.014) | (6.04–13.32) | (10.18–17.24) | ||
| 370/520 | 0.0002 | 2.32 | 9.21 | 15.80 |
| (0.051) | (7.80–10.93) | (11.29–20.23) | ||
| Wavelength (nm) | p-Value | RT (min) (SD) | Area/Area of Creatinine × 10−3 Median (IQR) | |
|---|---|---|---|---|
| Non-Malignant Hematuria (NMHU) | Bladder Cancer (BC) | |||
| 240 | 0.0057 | 1.77 | 26.09 | 31.75 |
| (0.024) | (22.82–29.17) | (26.50–39.79) | ||
| 240 | 0.0177 | 4.62 | 10.95 | 17.01 |
| (0.033) | (8.10–14.24) | (9.85–46.14) | ||
| 260 | 0.0283 | 4.63 | 12.70 | 17.91 |
| (0.037) | (8.47–15.85) | (13.46–25.52) | ||
| 280 | 0.328 | 10.53 | 3.46 | 4.82 |
| (0.045) | (2.36–4.89) | (3.09–9.83) | ||
| 320 | 0.0012 | 1.76 | 2.15 | 3.48 |
| (0.025) | (1.78–2.64) | (2.75–4.31) | ||
| 340 | 0.0090 | 1.78 | 7.90 | 13.18 |
| (0.022) | (6.62–11.50) | (10.73–14.78) | ||
| 340 | 0.0501 | 2.19 | 4.88 | 9.36 |
| (0.024) | (4.16–10.29) | (5.72–10.90) | ||
| 360 | 0.0128 | 1.77 | 4.66 | 7.38 |
| (0.020) | (3.07–6.37) | (4.86–10.10) | ||
| 380 | 0.0164 | 1.77 | 1.41 | 2.75 |
| (0.019) | (1.01–2.91) | (1.87–4.58) | ||
| 380 | 0.0087 | 2.19 | 1.25 | 1.74 |
| (0.007) | (1.08–1.54) | (1.30–2.36) | ||
| 400 | 0.0052 | 1.78 | 0.89 | 1.60 |
| (0.019) | (0.89–0.89) | (0.92–2.88) | ||
| 400 | 0.0057 | 2.19 | 0.72 | 1.08 |
| (0.007) | (0.64–0.93) | (0.79–1.28) | ||
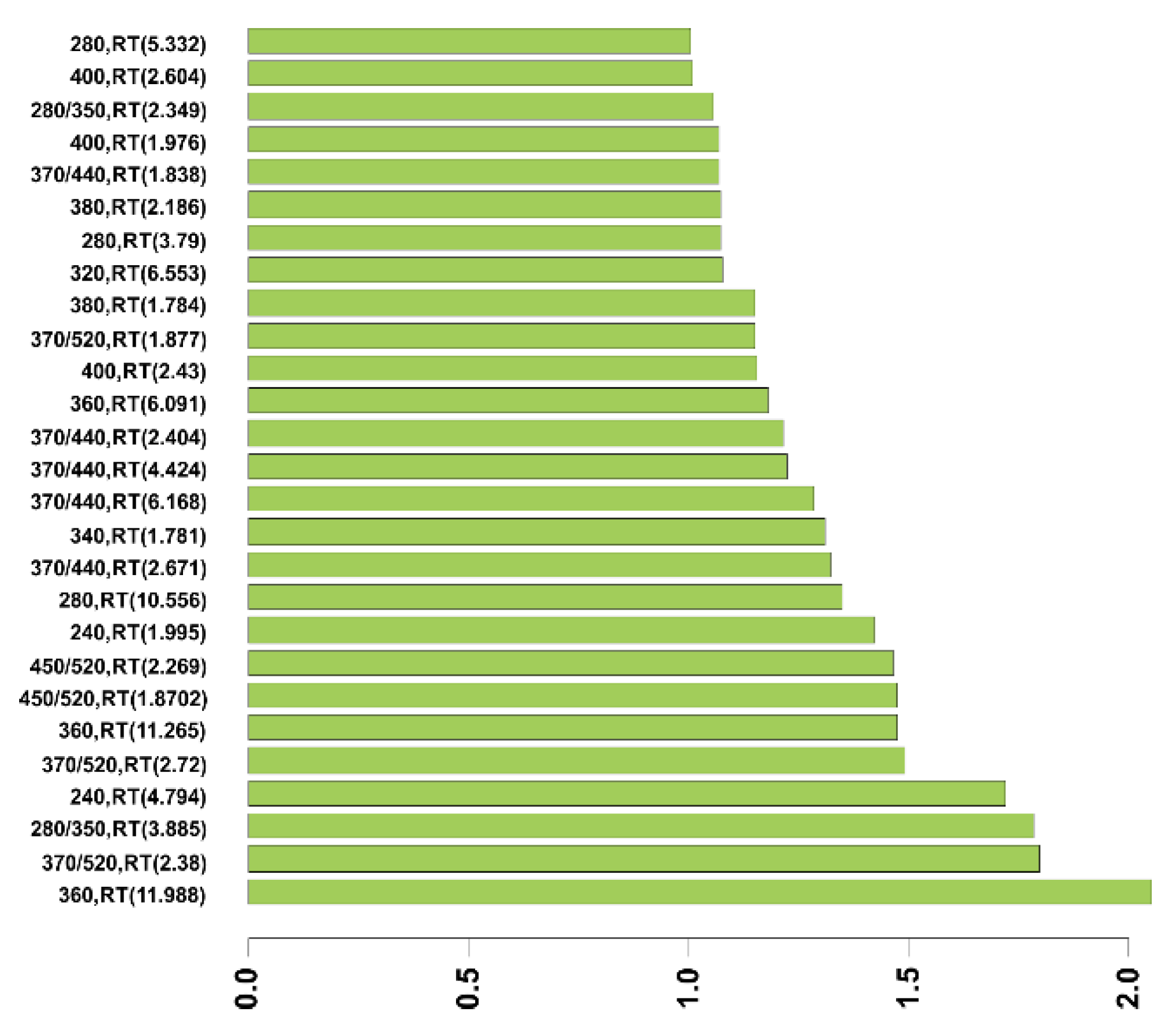
| Peak (RT (min)) | Wavelength (ex/em (nm)) | Cut-Off Value | Specificity (%) (CI) | Sensitivity (%) (CI) | NPV (%) | PPV (%) | AUC |
|---|---|---|---|---|---|---|---|
| F (1.83) | 370/440 | 0.007 | 94.7 | 50 | 64 | 91 | 0.726 |
| (84.2–100) | (30–75) | ||||||
| F (2.37) | 370/440 | 0.010 | 84.2 | 70 | 73 | 82 | 0.782 |
| (68.4–100) | (50–90) | ||||||
| F (1.87) | 450/520 | 0.001 | 94.7 | 60 | 69 | 92 | 0.776 |
| (84.2–100) | (40–80) | ||||||
| F (2.28) | 450/520 | 0.001 | 73.7 | 80 | 78 | 76 | 0.789 |
| (52.6–94.7) | (60–95) | ||||||
| F (1.87) | 370/520 | 0.010 | 84.2 | 70 | 73 | 82 | 0.755 |
| (68.4–100) | (50–90) | ||||||
| F (2.32) | 370/520 | 0.012 | 73.7 | 90 | 88 | 78 | 0.824 |
| (52.6–89.5) | (75–100) | ||||||
| A (1.77) | 240 | 0.029 | 63.2 | 80 | 75 | 70 | 0.755 |
| (42.1–84.2) | (60–95) | ||||||
| A (4.62) | 240 | 0.016 | 63.2 | 85 | 80 | 71 | 0.721 |
| (42.1–84.2) | (69.9–100) | ||||||
| A (4.63) | 260 | 0.015 | 68.4 | 75 | 72 | 71 | 0.705 |
| (47.4–89.5) | (55–90) | ||||||
| A (10.53) | 280 | 0.003 | 100 | 35 | 59 | 100 | 0.700 |
| (100–100) | (15–55) | ||||||
| A (1.76) | 320 | 0.003 | 78.9 | 85 | 83 | 81 | 0.795 |
| (57.9–94.7) | (70–100) | ||||||
| A (1.78) | 340 | 0.011 | 78.9 | 75 | 75 | 79 | 0.742 |
| (57.9–94.7) | (55–95) | ||||||
| A (2.19) | 340 | 0.005 | 89.5 | 55 | 65 | 85 | 0.684 |
| (73.7–100) | (35–80) | ||||||
| A (1.77) | 360 | 0.007 | 63.2 | 80 | 75 | 70 | 0.732 |
| (42.1–84.2) | (60–95) | ||||||
| A (1.77) | 380 | 0.001 | 89.5 | 55 | 65 | 85 | 0.724 |
| (73.7–100) | (30–75) | ||||||
| A (2.19) | 380 | 0.002 | 68.4 | 80 | 76 | 73 | 0.734 |
| (47.4–89.5) | (60–95) | ||||||
| A (1.78) | 400 | 0.001 | 89.5 | 60 | 68 | 86 | 0.758 |
| (73.7–100) | (35–80) | ||||||
| A (2.19) | 400 | 0.001 | 68.4 | 90 | 87 | 75 | 0.755 |
| (47.4–89.5) | (75–100) |
| Peak (RT [min]) | F(1.83) | F(2.37) | F(1.87) | F(2.28) | F(1.87) | F(2.32) | A(1.77) | A(4.62) | A(4.63) | A(10.53) | A(1.76) | A(1.78) | A(2.19) | A(1.77) | A(1.77) | A(2.19) | A(1.78) | A(2.19) | Sensitivity (%) | Specificity (%) | Log-likelihood | AIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 27.5 | |||||||||||||||||||||
| 1 | ■ | 74 | 85 | 35.07 | 41.83 | |||||||||||||||||
| 2 | ■ | ■ | 89 | 90 | 39.80 | 33.70 | ||||||||||||||||
| 3 | ■ | ■ | ■ | 84 | 90 | 41.65 | 33.70 | |||||||||||||||
| 4 | ■ | ■ | ■ | ■ | 84 | 90 | 43.70 | 31.14 | ||||||||||||||
| 5 | ■ | ■ | ■ | ■ | ■ | 89 | 100 | 45.65 | 28.53 | |||||||||||||
| 6 | ■ | ■ | ■ | ■ | ■ | ■ | 95 | 95 | 47.61 | 23.00 | ||||||||||||
| 7 | ■ | ■ | ■ | ■ | ■ | ■ | ■ | 100 | 100 | 50.78 | 16.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Džubinská, D.; Zvarík, M.; Kollárik, B.; Šikurová, L. Multiple Chromatographic Analysis of Urine in the Detection of Bladder Cancer. Diagnostics 2021, 11, 1793. https://doi.org/10.3390/diagnostics11101793
Džubinská D, Zvarík M, Kollárik B, Šikurová L. Multiple Chromatographic Analysis of Urine in the Detection of Bladder Cancer. Diagnostics. 2021; 11(10):1793. https://doi.org/10.3390/diagnostics11101793
Chicago/Turabian StyleDžubinská, Daniela, Milan Zvarík, Boris Kollárik, and Libuša Šikurová. 2021. "Multiple Chromatographic Analysis of Urine in the Detection of Bladder Cancer" Diagnostics 11, no. 10: 1793. https://doi.org/10.3390/diagnostics11101793
APA StyleDžubinská, D., Zvarík, M., Kollárik, B., & Šikurová, L. (2021). Multiple Chromatographic Analysis of Urine in the Detection of Bladder Cancer. Diagnostics, 11(10), 1793. https://doi.org/10.3390/diagnostics11101793





