An Image-Based Workflow for Objective Vessel Wall Enhancement Quantification in Intracranial Aneurysms
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Image Acquisition
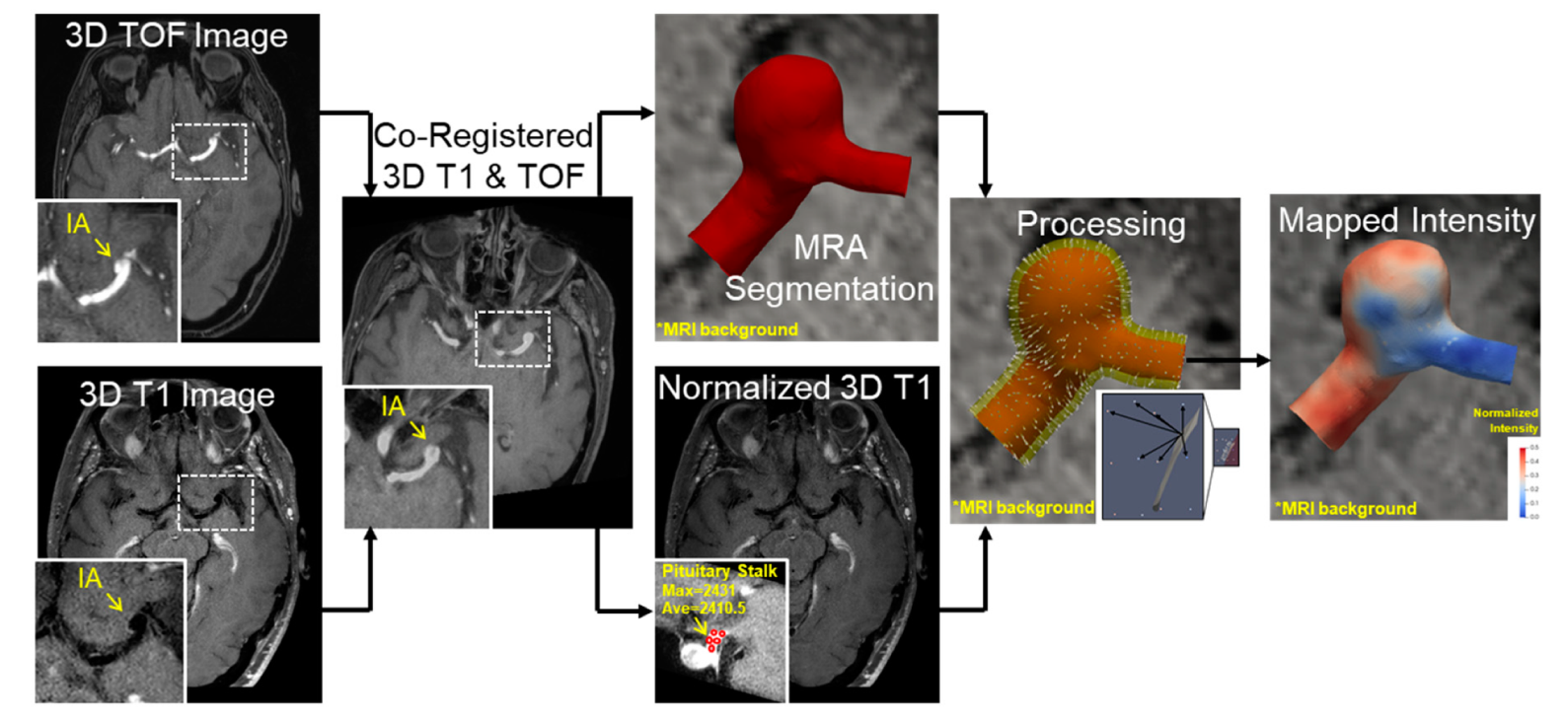
2.3. VWE Quantification Pipeline
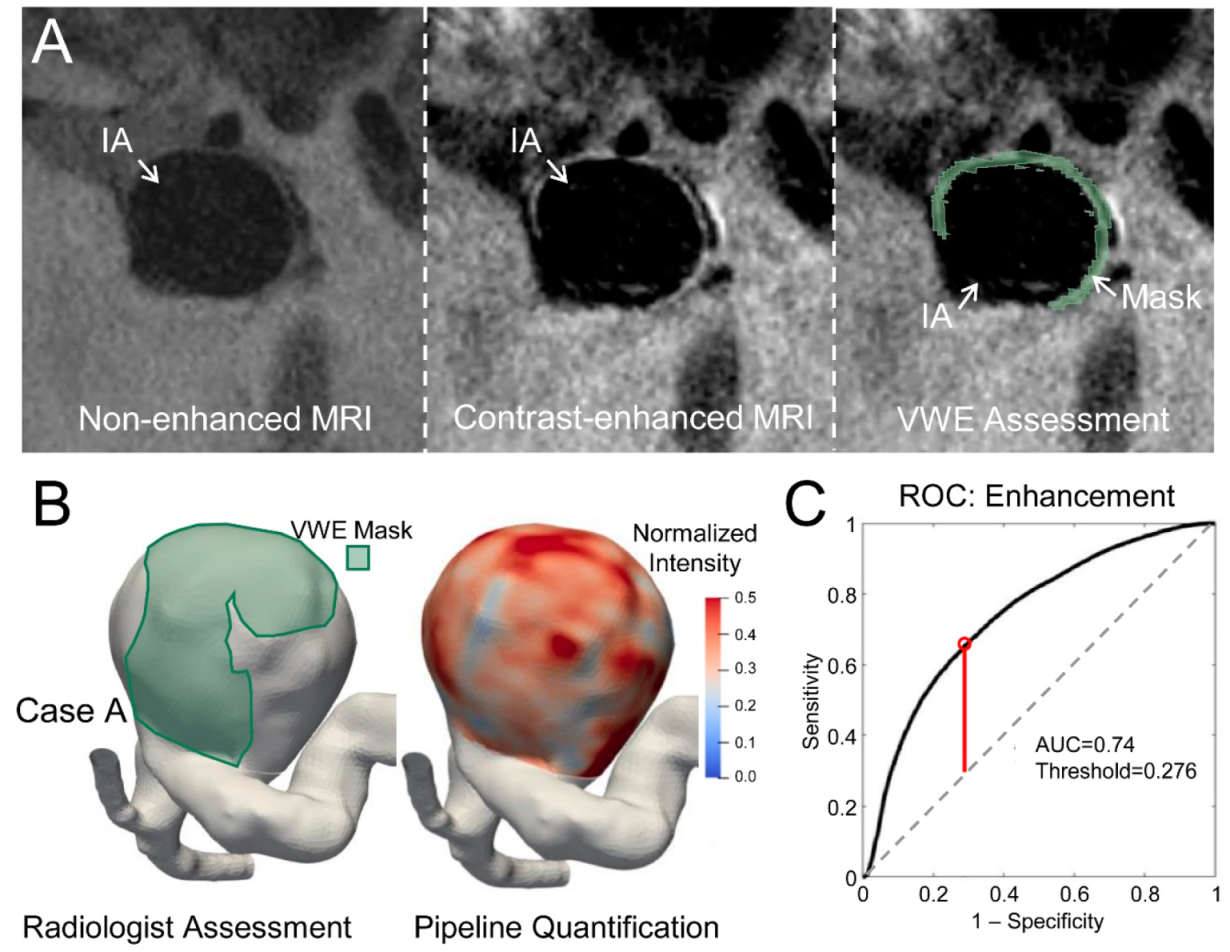
2.4. Identification of Enhancing Regions
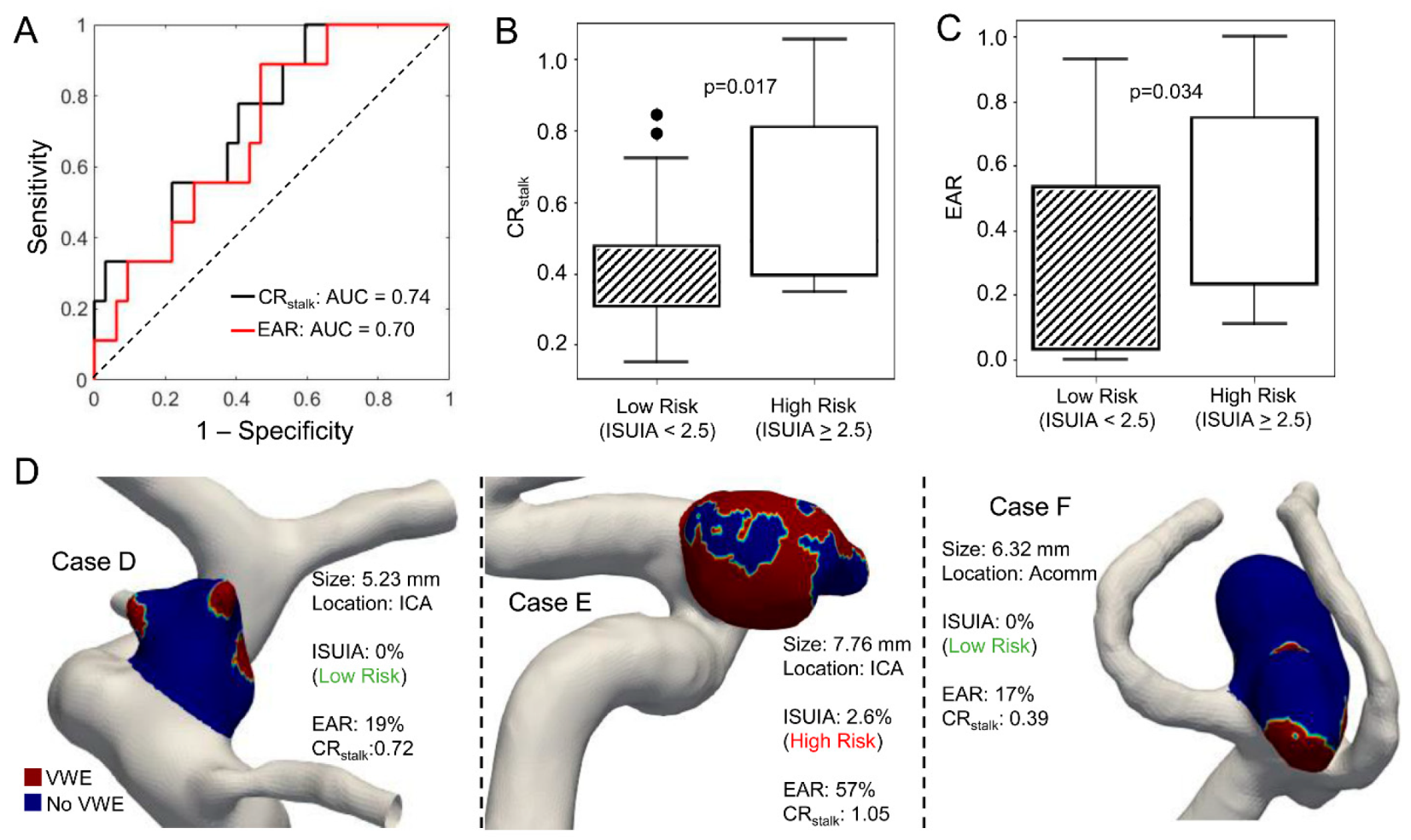
2.5. Enhancement Area Ratio as an Indicator of Risk
3. Results
3.1. Patient Characteristics
3.2. Determining Optimal Intensity Threshold
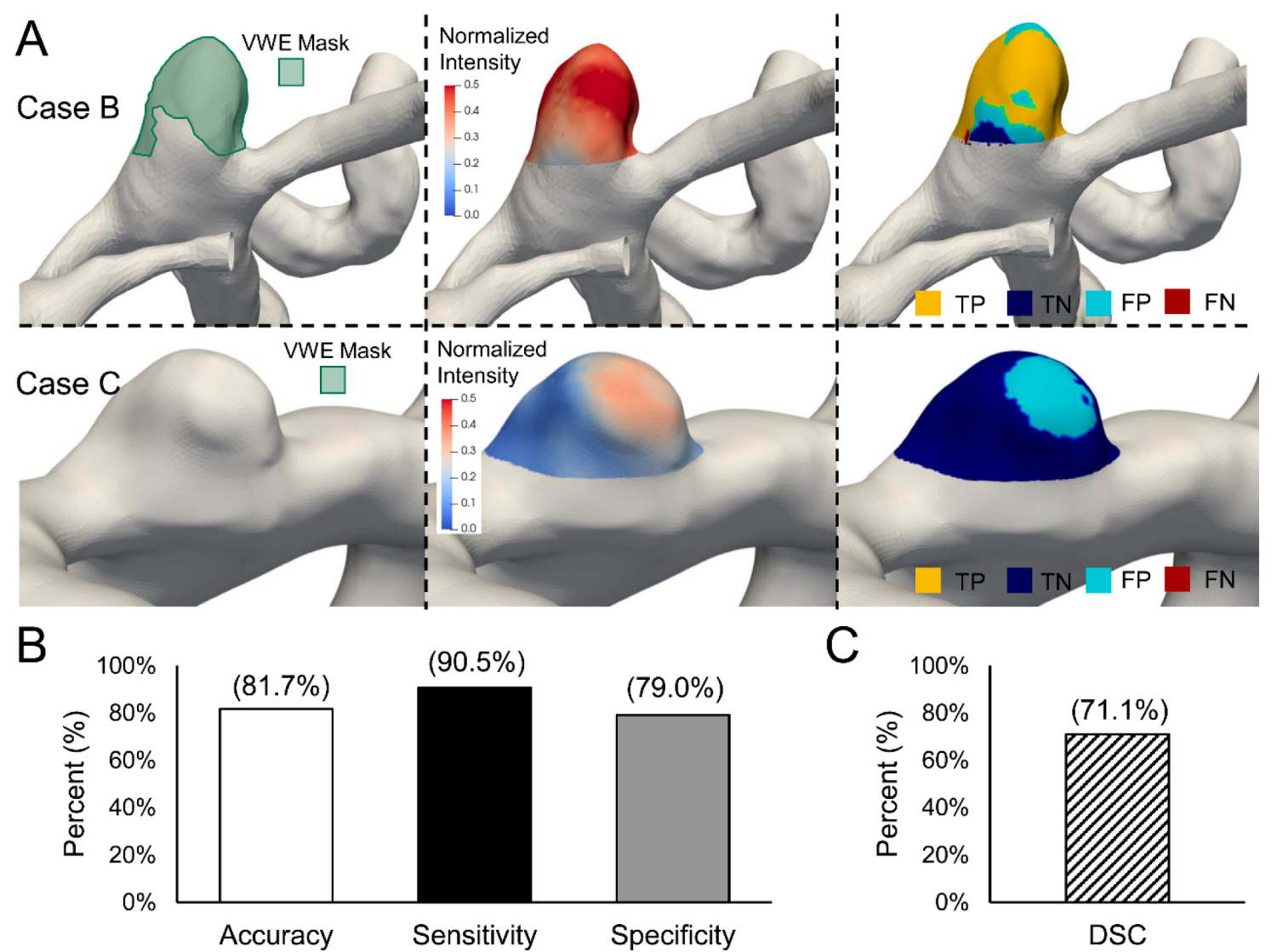
3.3. Validation of the Intensity Threshold
3.4. CRstalk and Enhancement Area Ratio as Indicators of Aneurysm Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Texakalidis, P.; Hilditch, C.A.; Lehman, V.; Lanzino, G.; Pereira, V.M.; Brinjikji, W. Vessel wall imaging of intracranial aneurysms: Systematic review and meta-analysis. World Neurosurg. 2018, 117, 453–458.e1. [Google Scholar] [CrossRef]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Dumont, A.S.; Kung, D.K.; Magnotta, V.A.; Young, W.L.; Hashimoto, T.; Winn, H.R.; Heistad, D. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: A pilot study. Stroke J. Cereb. Circ. 2012, 43, 3258–3265. [Google Scholar] [CrossRef] [Green Version]
- Edjlali, M.; Gentric, J.C.; Regent-Rodriguez, C.; Trystram, D.; Hassen, W.B.; Lion, S.; Nataf, F.; Raymond, J.; Wieben, O.; Turski, P.; et al. Does aneurysmal wall enhancement on vessel wall mri help to distinguish stable from unstable intracranial aneurysms? Stroke J. Cereb. Circ. 2014, 45, 3704–3706. [Google Scholar] [CrossRef] [Green Version]
- Shimonaga, K.; Matsushige, T.; Ishii, D.; Sakamoto, S.; Hosogai, M.; Kawasumi, T.; Kaneko, M.; Ono, C.; Kurisu, K. Clinicopathological insights from vessel wall imaging of unruptured intracranial aneurysms. Stroke J. Cereb. Circ. 2018, 49, 2516–2519. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Yang, Q.; Wang, D.-D.; Guan, S.-C.; Zhang, H.-Q. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology 2016, 58, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.S.; Zanaty, M.; Nakagawa, D.; Kung, D.K.; Jabbour, P.; Samaniego, E.A.; Hasan, D. Magnetic resonance vessel wall imaging in human intracranial aneurysms. Stroke J. Cereb. Circ. 2019, 50. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Von Der Brelie, C.; Trick, D.; Riedel, C.; Lindner, T.; Madjidyar, J.; Jansen, O.; Synowitz, M.; Flüh, C. Vessel wall enhancement in unruptured intracranial aneurysms: An indicator for higher risk of rupture? High-resolution mr imaging and correlated histologic findings. AJNR Am. J. Neuroradiol. 2018, 39, 1617–1621. [Google Scholar] [CrossRef]
- Petridis, A.K.; Filis, A.; Chasoglou, E.; Fischer, I.; Dibué-Adjei, M.; Bostelmann, R.; Steiger, H.J.; Turowski, B.; May, R. Aneurysm wall enhancement in black blood mri correlates with aneurysm size. Black blood mri could serve as an objective criterion of aneurysm stability in near future. Clin. Pract. 2018, 8, 1089. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Qi, T.; He, S.; Li, Z.; Ou, S.; Zhang, G.; Liu, X.; Huang, Z.; Liang, F. Low wall shear stress is associated with local aneurysm wall enhancement on high-resolution mr vessel wall imaging. AJNR Am. J. Neuroradiol. 2018, 39, 2082–2087. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.O.; Arana, V.T.; Rubbert, C.; Cornelius, J.F.; Fischer, I.; Bostelmann, R.; Mijderwijk, H.-J.; Turowski, B.; Steiger, H.-J.; May, R.; et al. Association between aneurysm hemodynamics and wall enhancement on 3d vessel wall mri. J. Neurosurg. 2021, 134, 565–575. [Google Scholar] [CrossRef]
- Larsen, N.; Flüh, C.; Saalfeld, S.; Voß, S.; Hille, G.; Trick, D.; Wodarg, F.; Synowitz, M.; Jansen, O.; Berg, P. Multimodal validation of focal enhancement in intracranial aneurysms as a surrogate marker for aneurysm instability. Neuroradiology 2020, 62, 1627–1635. [Google Scholar] [CrossRef]
- Kolega, J.; Poppenberg, K.E.; Lim, H.-W.; Gutierrez, L.C.; Veeturi, S.S.; Siddiqui, A.H.; Rajabzadeh-Oghaz, H.; Tutino, V.M. Identification of intima-to-media signals for flow-induced vascular remodeling using correlative gene expression analysis. Sci. Rep. 2021, 11, 16142. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, M.; Oh, C.; Raju, A.; Choudhri, A. Physiologic pineal region, choroid plexus, and dural calcifications in the first decade of life. AJNR Am. J. Neuroradiol. 2014, 36, 575–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roa, J.; Zanaty, M.; Osorno, C.; Ishii, D.; Bathla, G.; Ortega-Gutierrez, S.; Hasan, D.M.; Samaniego, E.A. Objective quantification of contrast enhancement of unruptured intracranial aneurysms: A high-resolution vessel wall imaging validation study. J. Neurosurg. 2021, 134, 862–869. [Google Scholar] [CrossRef]
- Harteveld, A.A.; Denswil, N.P.; Van Hecke, W.; Kuijf, H.; Vink, A.; Spliet, W.G.; Daemen, M.J.; Luijten, P.R.; Zwanenburg, J.J.; Hendrikse, J.; et al. Data on vessel wall thickness measurements of intracranial arteries derived from human circle of willis specimens. Data Brief 2018, 19, 6–12. [Google Scholar] [CrossRef]
- Kleinloog, R.; Korkmaz, E.; Zwanenburg, J.J.M.; Kuijf, H.J.; Visser, F.; Blankena, R.; Post, J.A.; Ruigrok, Y.M.; Luijten, P.R.; Regli, L.; et al. Visualization of the aneurysm wall: A 7.0-tesla magnetic resonance imaging study. Neurosurgery 2014, 75, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Wiebers, D.O. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. Scipy 1.0: Fundamental algorithms for scientific computing in python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Omodaka, S.; Endo, H.; Niizuma, K.; Fujimura, M.; Inoue, T.; Endo, T.; Sato, K.; Sugiyama, S.-I.; Tominaga, T. Circumferential wall enhancement in evolving intracranial aneurysms on magnetic resonance vessel wall imaging. J. Neurosurg. 2019, 131, 1262–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omodaka, S.; Endo, H.; Niizuma, K.; Fujimura, M.; Inoue, T.; Sato, K.; Sugiyama, S.-I.; Tominaga, T. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using mr imaging. Am. J. Neuroradiol. 2016, 37, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.-X.; Wen, L.; Lei, S.; Ran, Q.; Yin, J.-B.; Gong, Z.-L.; Zhang, D. Wall enhancement ratio and partial wall enhancement on mri associated with the rupture of intracranial aneurysms. J. Neurointerv. Surg. 2017, 10, 566–570. [Google Scholar] [CrossRef]
- Hartman, J.B.; Watase, H.; Sun, J.; Hippe, D.S.; Kim, L.; Levitt, M.; Sekhar, L.; Balu, N.; Hatsukami, T.; Yuan, C.; et al. Intracranial aneurysms at higher clinical risk for rupture demonstrate increased wall enhancement and thinning on multicontrast 3d vessel wall mri. Br. J. Radiol. 2019, 92, 20180950. [Google Scholar] [CrossRef] [PubMed]
- Matsushige, T.; Shimonaga, K.; Mizoue, T.; Hosogai, M.; Hashimoto, Y.; Kaneko, M.; Ono, C.; Ishii, D.; Sakamoto, S.; Kurisu, K. Focal aneurysm wall enhancement on magnetic resonance imaging indicates intraluminal thrombus and the rupture point. World Neurosurg. 2019, 127, e578–e584. [Google Scholar] [CrossRef]
- Krupa, K.; Bekiesinska-Figatowska, M. Artifacts in magnetic resonance imaging. Pol. J. Radiol. 2015, 80, 93–106. [Google Scholar] [PubMed] [Green Version]
- Challa, V.; Han, H.-C. Spatial variations in wall thickness, material stiffness and initial shape affect wall stress and shape of intracranial aneurysms. Neurol. Res. 2007, 29, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Roa, J.A.; Zhang, H.; Koscik, T.; Ortega-Gutierrez, S.; Bathla, G.; Sonka, M.; Derdeyn, C.; Magnotta, V.; Hasan, D. Increased contrast enhancement of the parent vessel of unruptured intracranial aneurysms in 7t mr imaging. J. Neurointerv. Surg. 2020, 12, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeturi, S.S.; Pinter, N.K.; Monteiro, A.; Baig, A.A.; Rai, H.H.; Waqas, M.; Siddiqui, A.H.; Rajabzadeh-Oghaz, H.; Tutino, V.M. An Image-Based Workflow for Objective Vessel Wall Enhancement Quantification in Intracranial Aneurysms. Diagnostics 2021, 11, 1742. https://doi.org/10.3390/diagnostics11101742
Veeturi SS, Pinter NK, Monteiro A, Baig AA, Rai HH, Waqas M, Siddiqui AH, Rajabzadeh-Oghaz H, Tutino VM. An Image-Based Workflow for Objective Vessel Wall Enhancement Quantification in Intracranial Aneurysms. Diagnostics. 2021; 11(10):1742. https://doi.org/10.3390/diagnostics11101742
Chicago/Turabian StyleVeeturi, Sricharan S., Nandor K. Pinter, Andre Monteiro, Ammad A. Baig, Hamid H. Rai, Muhammad Waqas, Adnan H. Siddiqui, Hamidreza Rajabzadeh-Oghaz, and Vincent M. Tutino. 2021. "An Image-Based Workflow for Objective Vessel Wall Enhancement Quantification in Intracranial Aneurysms" Diagnostics 11, no. 10: 1742. https://doi.org/10.3390/diagnostics11101742
APA StyleVeeturi, S. S., Pinter, N. K., Monteiro, A., Baig, A. A., Rai, H. H., Waqas, M., Siddiqui, A. H., Rajabzadeh-Oghaz, H., & Tutino, V. M. (2021). An Image-Based Workflow for Objective Vessel Wall Enhancement Quantification in Intracranial Aneurysms. Diagnostics, 11(10), 1742. https://doi.org/10.3390/diagnostics11101742





