Abstract
(1) Introduction: Telomerase reverse transcriptase (TERT) promoter mutations are associated with unfavorable clinical outcomes in papillary thyroid carcinomas (PTCs). Two substitution mutations, C228T (c.1-124C>T) and C250T (c.1-146C>T), make up most of the mutations and occur in a mutually exclusive manner. (2) Case presentation: A 72-year-old man was initially referred to a tertiary hospital for treatment of esophageal cancer. Preoperative imaging revealed a 3.2 cm thyroid nodule pathologically diagnosed as PTC on needle biopsy. The patient underwent thyroid lobectomy with esophagectomy and was finally diagnosed with synchronous solid variant PTC (SVPTC) and esophageal squamous cell carcinoma. Sanger sequencing using DNA from the thyroid tumor showed an indel mutation, c.1-132_1-124delinsT, composed of a deletion (c.1-132_1-125del) as well as a hotspot mutation (c.1-124C>T(C228T)) in the TERT promoter. (3) Conclusions: This is the first report of PTC harboring a novel deletion along with a hotspot mutation in the TERT promoter in a patient with synchronous esophageal squamous cell carcinoma.
1. Introduction
Papillary thyroid carcinoma (PTC) is the most common histologic type of thyroid cancer, and overall indolent tumor, with a cancer-specific mortality rate less than 5% [1]. However, the prognosis is significantly affected by disease stage (which is defined by patient age at diagnosis), tumor size, extrathyroidal extension, and distant metastases. In an effort to finding a biomarker that indicates aggressive biological behavior of the tumor, telomerase reverse transcriptase (TERT) promoter mutations have been suggested as a useful prognostic indicator of clinical aggressiveness and poor outcome in differentiated thyroid carcinomas [2,3].
TERT encodes the catalytic protein subunit of telomerase, which is crucial for maintaining telomeric length and is reactivated in many cancers [4]. Telomerase activity is determined by TERT expression, which is predominantly controlled at transcription levels, and the TERT promoter is considered to be the most important regulatory element [5]. Two of the most recurrent non-coding mutations, C228T (c.1-124G>A) and C250T (c.1-146G>A), create a de novo binding site (GGAA) for the E26 transformation-specific (ETS) family of transcription factors, specifically the multimeric GA-binding protein (GABP) in the TERT promoter [6].
These two TERT promoter mutations occur frequently in poorly differentiated thyroid carcinoma (43.2%) and anaplastic thyroid carcinoma [7]. Among three major variants of PTC, the tall cell variant shows highest prevalence of TERT promoter mutation, followed by conventional and follicular variants [7].
Solid variant PTC (SVPTC) is rare, comprising about 3% of the total PTC [8]. It has a less favorable prognosis than the classic papillary type; however, it is much better than for poorly differentiated carcinoma [8]. According to previous studies, TERT promoter mutation occurs in about 12% of SVPTC [9,10].
Here, we first report the co-occurrence of a novel deletion and hotspot mutation in the TERT promoter in solid variant PTC found in a patient with synchronous primary esophageal cancer.
2. Case Presentation
A 72-year-old man with dysphagia and pain behind the sternum was referred to a tertiary hospital for treatment of biopsy-proven squamous cell carcinoma of the esophagus. The patient had a 13-year medical history of diabetes, which was controlled, and a 30-year history of smoking a pack of cigarettes per day. He denied any remarkable family history or radiation exposure. Preoperative whole-body 18F-fluorodeosy-glucose positron tomography (PET/CT 18F-FDG) examination did not show any demonstrable abnormal FDG uptake in the esophagus. However, it revealed a hypermetabolic mass (SUVmax = 76.5) in the right thyroid as well as a focal hypermetabolic lesion (SUVmas = 7.7) in the left thyroid (Figure 1A). Ultrasound examination found 3.4 cm and 1.4 cm isoechoic nodules in the right and left thyroid lobes, respectively. The right lobe mass was distinctively accompanied by a 2.1 cm hypoechoic nodule with cystic changes and “nodule in nodule” appearance, and it was interpreted as an intermediate suspicious nodule (Figure 1B). The nodule of the left lobe was interpreted as a low suspicious nodule. To rule out the metastasis of esophageal cancer to thyroid, needle biopsy was performed on the right thyroid mass, which was diagnosed as papillary thyroid carcinoma. The patient underwent right thyroid lobectomy with central neck dissection for thyroid mass and McKeown esophagectomy with two-field lymph node dissection for esophageal cancer.
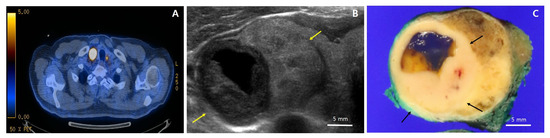
Figure 1.
18F-fluorodeosy-glucose positron tomography (FDG-PET), ultrasound, and gross photos. (A) FDG-PET showing a hypermetabolic mass (SUVmax = 76.5) in the right thyroid as well as a focal hypermetabolic lesion (SUVmas = 7.7) in the left thyroid; (B) Ultrasound of the right lobe showing a 3.4 cm isoechoic solid nodule with regular margins (yellow arrows), accompanied by a 2.1 cm hypoechoic solid nodule with cystic changes and “nodule in nodule” pattern. In contrast to the larger nodule, the smaller hypoechoic solid nodule is taller than it is wide; (C) Gross photo showing a tumor with the largest diameter of 3.2 cm circumscribed by a thin capsule; a distinctive 1.5 × 1.4 cm light yellowish to white homogeneous solid portion (black arrows) with partial cystic changes and smooth borders within a tan to brown mass.
The Institutional review board of the Samsung Medical Center has granted permission for this case report to be published on condition that no patient-identifiable data (including patient name and photograph) are included. The approval was obtained 15 September 2020 under the following number: SMC 2020-09-025. Informed written consent for publication was not asked for by the Institutional review board, since no data that can potentially and clearly identify the patient were found in the case description.
The thyroid gross mass measured 3.2 × 2.5 cm in the largest dimension and was circumscribed by a thin capsule. On serial section, a distinctive 1.5 × 1.4 cm light yellowish to white homogeneous solid portion with partial cystic changes and smooth borders was found within the tan to brown mass (Figure 1C). Histologically, the homogeneous solid portion showed trabecular and insular growth patterns (Figure 2B), which were predominantly composed of uniform cells with abundant pale eosinophilic cytoplasm (Figure 2C). Mitoses were occasional (1–2/10HPFs), and tumor necrosis was not found. The tumor area surrounding the solid portion showed highly irregular follicles with papillary infoldings and colloid scalloping, and tumor cells revealed a polygonal shape with abundant cytoplasm (Figure 2D,F). In the whole areas of the tumor, the neoplastic cells had the nuclear features characteristic of papillary carcinoma, including irregular nuclear contours, nuclear clearing, grooves, and pseudoinclusions (Figure 2C,E). Immunohistochemically, the tumor cells were positive for thyroglobulin (mouse monoclonal, clone 2H11+6E1, 1:400, Sigma-Aldrich, Rocklin, CA, USA), indicating thyroid origin (Figure 2F). As the tumor had more than 50% solid architecture (Figure 1A), it was finally diagnosed as solid variant PTC (SVPTC). Extrathyroidal extension, blood vessel invasion, and regional lymph node metastasis of the tumor were not identified. The esophagectomy specimen revealed a 2.5 × 1.0 cm ulcerative lesion in the lower third of the esophagus. Microscopically, the tumor was characterized as moderately differentiated squamous cell carcinoma extending to submucosa. The tumor metastasized to three regional lymph nodes.
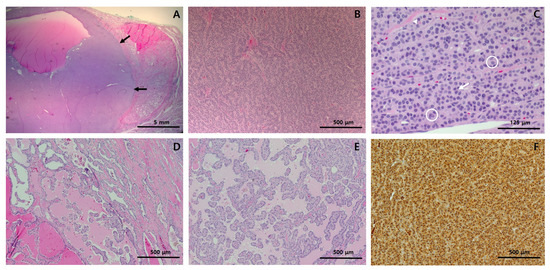
Figure 2.
Histological features and immunohistochemistry results of the right thyroid lobectomy specimen. (A) At low-power view, the solid portion (black arrows) constitutes more than half of the tumor; (B) At medium-power view, the solid portion showed trabecular and insular growth patterns; (C) At high-power view, the solid portion was found to be predominantly composed of uniform cells with abundant pale eosinophilic cytoplasm. They also had nuclear features characteristic of papillary carcinoma, including pseudoinclusions (circle) nuclear grooves (arrow), and irregular nuclear contour (arrow head). (D,E) The tumor area surrounding the solid portion showed highly irregular follicles, many of which have papillary infoldings and colloid scalloping. The tumor cells showed typical papillary carcinoma nuclear features. (F) Immunohistochemically, the tumor cells were positive for thyroglobulin, indicating thyroid origin. Staining method: (A–E), hematoxylin and eosin; (F), polymer method. Original magnification: (A), ×10; (B), ×100, (C), ×400; (D), ×100; (E), ×100; (F), ×100.
DNA from formalin-fixed, paraffin-embedded tissue from SVPTC was extracted. We carried out Sanger sequencing to identify TERT promoter mutations using a set of primers 5′-AGTGGATTCGCGGGCACAGA-3′ (forward) and 5′-AGCACCTCGCGGTAGTGG-3′ (reverse). The analysis revealed a novel indel mutation, c.1-132_1-124delinsT, composed of a deletion (c.1-132_c.1-125) along with the well-known substitution mutation (c.1-124(G>A)(C228T)) (Figure 3).
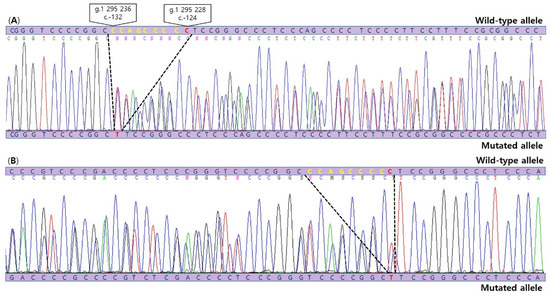
Figure 3.
Sanger sequencing chromatograms (A: forward sequence, B: reverse complement sequence) illustrating detection of the c.1-132_1-124delinsT, composed of a novel 8 bp deletion mutation (c.-132_c.-125del) (yellow) as well as the common C228T(c.1-124C>T) TERT promoter mutation (red) in SVPTC.
No evidence of recurrence was observed during seven months after the lobectomy. Complete thyroidectomy for the nodule of left lobe was performed afterwards, and it was finally diagnosed as follicular adenoma.
3. Discussion
TERT promoter mutations are identified in various types of cancers with different frequencies [11]. In thyroid cancer, TERT promoter mutations are more frequently found in poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma than PTCs [12]. As a result of its rarity, the frequency of TERT promoter mutations in SVPTC has not been well known, and only a few studies compared the genetic alterations among subvariants of PTC including SVPTC. According to these studies, the prevalence of TERT promoter mutations in SVPTC is slightly higher than that in conventional PTC (cPTC) but much lower than that of tall cell variant PTC [9,10].
Previous studies demonstrated that TERT promoter mutations in differentiated thyroid carcinomas was associated with some clinicopathological features, including older age at diagnosis, larger tumors, higher frequency of distant metastases, and worse tumor stage [2]. In our case, the older patient age (72 years old) and larger tumor size (3.2 cm) are relevant to the presence of the TERT promoter mutation. Although the clinical implication of this subtype is undetermined, a recent systemic review suggested that SVPTC should be regarded as an aggressive variant of PTC because of its higher propensity for vascular invasion and tumor recurrence [13]. Thus, a more aggressive approach, including total thyroidectomy accompanied by lymph node dissection, may be required.
Two mutations of the TERT core promoter, which cause a cytidine-to-thymidine (CT) dipyrimidine transition at chromosome 5 1,295,228 and 1,295,250 (−124 and −146bp from the ATG), c.1-124C>T(C228T) and c.1-146C>T(C250T), respectively, predominantly occur regardless of tumor type [14,15]. The C228T mutation is more prevalent than C250T among various malignancies including thyroid cancer [16,17]. The mutations occur in a mutually exclusive manner, suggesting their functional redundancy. Multiple studies provide evidence that the presence of C228T or C250T mutations promotes telomerase activity both in vivo and in vitro [6,18,19]. These mutations generate a de novo binding site for activating the ETS family of transcription factors, including GA-binding proteins (GABP), thereby activating TERT transcription and telomerase activity [6,20].
Although not a promoter lesion, one previous study reviewing 173 Hürthle cell carcinomas of thyroid cases found a 24 bp deletion in the 5′ UTR of exon 1 (c.-53_-29delAGCGCTGCGTCCTGCTGCGCACGT) of TERT in one sample [21]. A recent study identified two additional novel mutations in the thyroid tumor: c.-332C>T in one medullary thyroid carcinoma and c.-104_-83dup in one PTC [22]. They suggested these mutations might create additional ETS-binding sites. Our novel 8 bp deletion was found just upstream of the C228T mutation, and it does not seem to generate an additional transcriptional factor binding motif. Instead, the mutation deletes the Specificity Protein 1 (SP1) binding site (Figure 4). A previous study investigated the putative G-quadruplex-forming sequence (PQS) embracing 3 Sp1 binding sites, between −90 and −22 upstream of the transcription start site of TERT promoter [23]. The study suggested that the formation of a tandem G-quadruplex structure might produce a significant inhibitory effect by masking Sp1 binding sites, and it also confirmed the importance of loop size to G-quadruplex stability reported in several studies [24]. The mutated sequence in our case is included in one of the PQS, which is able to form stable G-quadruplex found between the −167 and −100 region of the TERT promoter [25] (Figure 4). The co-occurrence of deletion and point mutation in the PQS may have an effect on the stability of the G-quadruplex structure. The deletion mutation also affects spacing between pre-existing and de novo regulatory elements. In a previous study, the authors observed that hotspot mutant promoter activity fluctuated depending on the distance from the native ETS sites, and there was peak activity when two locations were separated by full helical turns of DNA, suggesting that TERT promoter mutation cooperates with native ETSs to recruit GABP [6]. In order to confirm the overall effects of deletion and point mutation of the TERT gene promoter, further studies should be performed through luciferase reporter assay.
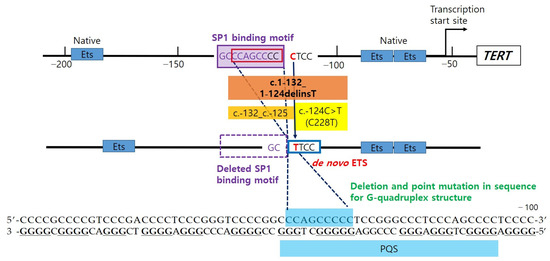
Figure 4.
c.1-124C>T creates de novo E26 transformation-specific (ETS), which cooperates with native ETS, to recruit activating transcription factor (GA-binding protein, GABP). c.1-132_1-125 deletion (8bp, 1.3 helix turn) eliminates the transcription factor SP1 binding site, which is identified using TFBIND (http://tfnbind.hgc.jp/), and it also closes the distance between native ETS and de novo ETS (C228T). In addition, the mutations are located in the putative quadruplex sequence (PQS) that are able to fold in G-quadruplex structure.
The co-occurrence of different substitution mutations of the TERT promoter has previously been reported. One previous PTC cohort study reported that mutations C229T (c.-125C>T) and C232T (c.-128C>T) were simultaneously found in one PTC sample [10]. Similarly, SNP and somatic hotspot mutations can co-exist in TERT promoter lesions. The rs2853669 common allele, a common T>C polymorphism at the −245 bp position from the ATG site, disrupts the pre-existing non-canonical ETS2 binding site in the proximal region of the TERT promoter, reducing the transcriptional activity of TERT [26]. Several studies reported that the prognostic effect of TERT promoter mutations was modified by the SNP in the TERT promoter. In the presence of a somatic TERT promoter mutation in the tumor, patients with the rs2853669 common allele showed poor prognosis in bladder cancer [27] and glioma [28], although conflicting evidence has been found for the latter [29]. However, the co-occurrence of different types of TERT mutations or variations has never been reported thus far.
A previous study evaluating two hotspot TERT promoter mutations in 313 esophageal squamous cell carcinoma samples identified 1.6% occurrence, showing the extremely low frequency compared to that of thyroid cancer (15–50%) [30]. Some studies identified a germline mutation in TERT, c.-57T>G in only two melanoma families worldwide, indicating that this inherited mutation is extremely rare [31,32]. These families were characterized by early age of melanoma onset (average 34 years) and evidence of susceptibility to other primary cancers (ovary, renal cell carcinoma, bladder, breast, and lung). Therefore, although we did not test for the TERT promoter mutation in tissue from esophageal cancer, it is unlikely that the TERT promoter mutation influences the occurrence of synchronous tumors.
The incidence of secondary cancer is 4.3% to 10.4% in patients with primary esophageal cancer [33,34,35,36]. Common secondary primary tumor sites include aerodigestive tract organs, such as the oral cavity, pharynx, larynx, lungs, and stomach [34], and the treatment and prognosis for these patients are determined primarily by the initial esophageal cancer [36]. The incidence of multiple primary cancers involving the esophagus and thyroid is low, and only less than 20 patients have been reported [37]. Most of these patients had advanced clinical staging of cancer. Histologically, esophageal cancer has been diagnosed as squamous cell carcinoma in all patients. Thyroid cancer has been evaluated as papillary carcinoma or follicular carcinoma.
In conclusion, this is the first report to describe the co-existing deletion and hotspot TERT promoter mutation of SVPTC in a patient with synchronous esophageal squamous cell carcinoma.
Author Contributions
Conceptualization, J.H.; methodology, J.-S.K.; validation, Y.L.O.; formal analysis, J.-S.K. and Y.L.O.; investigation, J.H.; resources, Y.L.O.; data curation; J.H. and J.-S.K.; writing—original draft preparation, J.H.; review and editing, J.-S.K.; visualization, J.H.; supervision, J.-S.K.; Project administration, J.H. and J.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Institutional review board of the Samsung Medical Center has granted permission for this case report to be published on condition that no patient-identifiable data (including patient name and photograph) are included. The approval was obtained 15 September 2020 under the following number: SMC 2020-09-025.
Informed Consent Statement
Informed written consent for publication was not asked for by the Institutional review board, since no data that can potentially and clearly identify the patient were found in the case description.
Conflicts of Interest
The authors declare no conflict of interest
References
- Orosco, R.K.; Hussain, T.; Brumund, K.T.; Oh, D.K.; Chang, D.C.; Bouvet, M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid 2015, 25, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Ren, Y.; O’Neill, C.; Gill, A.; Aniss, A.; Sywak, M.; Sidhu, S.; Delbridge, L.; Learoyd, D.; de Vathaire, F.; et al. TERT promoter mutations are a major indicator of recurrence and death due to papillary thyroid carcinomas. Clin. Endocrinol. 2016, 85, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Cukusić, A.; Skrobot Vidacek, N.; Sopta, M.; Rubelj, I. Telomerase regulation at the crossroads of cell fate. Cytogenet. Genome Res. 2008, 122, 263–272. [Google Scholar] [CrossRef]
- Bell, R.J.; Rube, H.T.; Kreig, A.; Mancini, A.; Fouse, S.D.; Nagarajan, R.P.; Choi, S.; Hong, C.; He, D.; Pekmezci, M.; et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015, 348, 1036–1039. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Erickson, L.A.; Nikiforova, M.N.; Caudill, C.M.; Lloyd, R.V. Solid variant of papillary thyroid carcinoma: Incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am. J. Surg. Pathol. 2001, 25, 1478–1484. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Lu, J.; Gao, J.; Ren, X.; Teng, L.; Duan, H.; Lin, Y.; Li, X.; Zhang, B.; et al. BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PLoS ONE 2016, 11, e0153319. [Google Scholar] [CrossRef]
- Gandolfi, G.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A.; Sancisi, V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur. J. Endocrinol. 2015, 172, 403–413. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Odate, T.; Duong, U.N.P.; Mochizuki, K.; Nakazawa, T.; Katoh, R.; Kondo, T. Prognostic importance of solid variant papillary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2018, 40, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, X.; Xu, D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.; Rube, H.T.; Xavier-Magalhães, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol. Cancer Res. 2016, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Alsaadi, R.; Murugan, A.K.; Sadiq, B.B. TERT Promoter Mutations in Thyroid Cancer. Horm. Cancer 2016, 7, 165–177. [Google Scholar] [CrossRef]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef]
- Arita, H.; Narita, Y.; Takami, H.; Fukushima, S.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013, 126, 939–941. [Google Scholar] [CrossRef]
- Wang, K.; Liu, T.; Liu, L.; Liu, J.; Liu, C.; Wang, C.; Ge, N.; Ren, H.; Yan, K.; Hu, S.; et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget 2014, 5, 1829–1836. [Google Scholar] [CrossRef]
- Mancini, A.; Xavier-Magalhães, A.; Woods, W.S.; Nguyen, K.T.; Amen, A.M.; Hayes, J.L.; Fellmann, C.; Gapinske, M.; McKinney, A.M.; Hong, C.; et al. Disruption of the β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer Cell 2018, 34, 513–528.e518. [Google Scholar] [CrossRef]
- Chindris, A.M.; Casler, J.D.; Bernet, V.J.; Rivera, M.; Thomas, C.; Kachergus, J.M.; Necela, B.M.; Hay, I.D.; Westphal, S.A.; Grant, C.S.; et al. Clinical and molecular features of Hürthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2015, 100, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Nikitski, A.V.; Nikiforova, M.N.; Nikiforov, Y.E. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019, 8, 5831–5839. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.L.; Ebbinghaus, S.W.; Hurley, L.H. Formation of a unique end-to-end stacked pair of G-quadruplexes in the hTERT core promoter with implications for inhibition of telomerase by G-quadruplex-interactive ligands. J. Am. Chem. Soc. 2009, 131, 10878–10891. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, A.; Balasubramanian, S. A sequence-independent study of the influence of short loop lengths on the stability and topology of intramolecular DNA G-quadruplexes. Biochemistry 2008, 47, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Micheli, E.; Martufi, M.; Cacchione, S.; De Santis, P.; Savino, M. Self-organization of G-quadruplex structures in the hTERT core promoter stabilized by polyaminic side chain perylene derivatives. Biophys. Chem. 2010, 153, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Hsu, N.Y.; Lee, L.W.; Ko, J.L. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter—Effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur. J. Cancer 2006, 42, 1466–1474. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef]
- Spiegl-Kreinecker, S.; Lötsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncology 2015, 17, 1231–1240. [Google Scholar] [CrossRef]
- Nencha, U.; Rahimian, A.; Giry, M.; Sechi, A.; Mokhtari, K.; Polivka, M.; Schmitt, Y.; Di Stefano, A.L.; Alentorn, A.; Labussière, M.; et al. TERT promoter mutations and rs2853669 polymorphism: Prognostic impact and interactions with common alterations in glioblastomas. J. Neuro-Oncol. 2016, 126, 441–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Chen, Z.; Hu, X.; Zhou, F.; He, J. Low frequency of TERT promoter somatic mutation in 313 sporadic esophageal squamous cell carcinomas. Int. J. Cancer 2014, 134, 493–494. [Google Scholar] [CrossRef]
- Harland, M.; Petljak, M.; Robles-Espinoza, C.D.; Ding, Z.; Gruis, N.A.; van Doorn, R.; Pooley, K.A.; Dunning, A.M.; Aoude, L.G.; Wadt, K.A.; et al. Germline TERT promoter mutations are rare in familial melanoma. Fam. Cancer 2016, 15, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Koide, N.; Yazawa, K.; Koike, S.; Adachi, W.; Amano, J. Oesophageal cancer associated with other primary cancers: A study of 31 patients. J. Gastroenterol. Hepatol. 1997, 12, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, S.; Onda, M.; Sasajima, K.; Takubo, K.; Miyashita, M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2000, 13, 226–230. [Google Scholar] [CrossRef]
- Shibuya, H.; Wakita, T.; Nakagawa, T.; Fukuda, H.; Yasumoto, M. The relation between an esophageal cancer and associated cancers in adjacent organs. Cancer 1995, 76, 101–105. [Google Scholar] [CrossRef]
- Poon, R.T.; Law, S.Y.; Chu, K.M.; Branicki, F.J.; Wong, J. Multiple primary cancers in esophageal squamous cell carcinoma: Incidence and implications. Ann. Thorac. Surg. 1998, 65, 1529–1534. [Google Scholar] [CrossRef]
- Cheng, P.; Xiang, Y.; Chen, E.; Zou, Z.; Zhang, X. Papillary thyroid microcarcinoma with synchronous asymptomatic advanced esophageal squamous cell carcinoma: A case report and review of the literature. Oncol. Lett. 2015, 9, 731–734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).